About Authors:
About Authors:
YASWANTH ALLAMNENI1*, NAVYA ALLAMNENI2, P DAYANANDA CHARY1, G VIJAY KUMAR3, ARUN KUMAR KALEKAR1, PAVAN KUMAR POTTURI1
1Research and Development Department, Natco Pharma Limited, Kothur, Mahaboobnagar,Andhra Pradesh – 509228.
2Department of Pharmaceutical Technology, Narasaraopeta Institute of Pharmaceutical Sciences, Narasaraopeta, Guntur, India.
3Principal, A.K.R.G college of Pharmacy, Nallagerla, West Godavari, Andhra Pradesh.
*yaswanthallamneni@gmail.com
ABSTRACT
Water is widely used as a raw material, ingredient, and solvent in the processing, formulation, and manufacture of pharmaceutical products, active pharmaceutical ingredients (APIs) and intermediates, compendial articles, and analytical reagents. This review discusses, primarily from a microbiological aspect, the review and evaluation of high purity water systems that are used for the manufacture of drug products and drug substances. It also includes a review of the design of the various types of systems and some of the problems that have been associated with these systems. As with other guides, it is not all-inclusive, but provides background and guidance for the review and evaluation of high purity water systems. The main objective of this review was to provide guidance to the industry on the pharmaceutical use of different grades of water in the manufacture of active pharmaceutical ingredients and medicinal products for human and veterinary use. This guidance is not intended to cover situations where, for example, medicinal products are prepared extemporaneously or where preparations are reconstituted or diluted with water prior to use by pharmacist (eg. Oral antibiotic mixtures) or in the case of veterinary products by the user (eg. Sheep dips).
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1283
IMPORTANT IN THIS ARTICLE:
INTRODUCTION
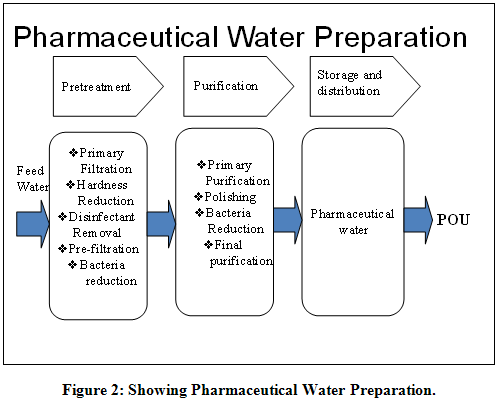
Water is the one of the major commodities used by the pharmaceutical industry. It may present as an excipient, or used for reconstitution of products, during synthesis, during production of finished product, or as a cleaning agent for rinsing vessels, equipment and primary packing materials etc. There are many different grades of water used for pharmaceutical purposes. Several are described in USP monographs that specify uses, acceptable methods of preparation, and quality attributes. These waters can be divided into two general types: bulk waters, which are typically produced on site where they are used; and packaged waters, which are produced, packaged, and sterilized to preserve microbial quality throughout their packaged shelf life. There are several specialized types of packaged waters, differing in their designated applications, packaging limitations, and other quality attributes. Different grades of water quality are required depending on the different pharmaceutical uses. Control of quality of water, in particular, the microbiological quality, is a major concern and the pharmaceutical industry devotes considerable resource to the development and maintenance of water purification systems which was shown in Table 1.
Table 1: Characteristics of Impurities in Water
|
Impurity Types |
Characteristics |
Types of Tests |
|
Microbiological |
Living, organic |
Sterility |
|
Microbiological |
Dead, organic |
BET |
|
Organic |
Non ionic |
TOC |
|
Inorganic |
Ionic |
Conductivity |
|
Particulate |
Insoluble |
Particle count |
|
Dissolved gases |
Ionic and non ionic |
Usually benign |
There are also other types of water for which there are no monographs. These are all bulk waters, with names given for descriptive purposes only. Many of these waters are used in specific analytical methods. These nonmonographed waters may not necessarily adhere strictly to the stated or implied modes of preparation or attributes. Waters produced by other means or controlled by other test attributes may equally satisfy the intended uses for these waters. It is the user's responsibility to ensure that such waters, even if produced and controlled exactly as stated, be suitable for their intended use. Wherever the term “water” is used within this compendium without other descriptive adjectives or clauses, the intent is that water of no less purity than Purified Water be used.
a. Purified Water is used as an excipient in the production of nonparenteral preparations and in other pharmaceutical applications, such as cleaning of certain equipment and nonparenteral product-contact components. Unless otherwise specified, Purified Wateris also to be used for all tests and assays for which water is indicated. Purified Watermust meet the requirements for ionic and organic chemical purity and must be protected from microbial contamination. The minimal quality of source or feed water for the production of Purified Wateris Drinking Water. This source water may be purified using unit operations that include deionization, distillation, ion exchange, reverse osmosis, filtration, or other suitable purification procedures. Purified water systems must be validated to reliably and consistently produce and distribute water of acceptable chemical and microbiological quality. Purified water systems that function under ambient conditions are particularly susceptible to the establishment of tenacious biofilms of microorganisms, which can be the source of undesirable levels of viable microorganisms or endotoxins in the effluent water.
b. Water for Injection is used as an excipient in the production of parenteral and other preparations where product endotoxin content must be controlled, and in other pharmaceutical applications, such as cleaning of certain equipment and parenteral product-contact components. The minimum quality of source or feed water for the generation ofWater for Injectionis Drinking Water as defined by the U.S. EPA, EU, Japan, or the WHO. This source water may be pre-treated to render it suitable for subsequent distillation (or whatever other validated process is used according to the monograph). The finished water must meet all of the chemical requirements for Purified Wateras well as an additional bacterial endotoxin specification. Since endotoxins are produced by the kinds of microorganisms that are prone to inhabit water, the equipment and procedures used by the system to purify, store, and distribute Water for Injectionmust be designed to minimize or prevent microbial contamination as well as remove incoming endotoxins from the starting water. Water for Injectionsystems must be validated to reliably and consistently produce and distribute this quality of water.
c. Water for Hemodialysis is used for hemodialysis applications, primarily the dilution of hemodialysis concentrate solutions. It is produced and used on-site and is made from EPA Drinking Water which has been further purified to reduce chemical and microbiological components. It may be packaged and stored in unreactive containers that preclude bacterial entry. The term “unreactive containers” implies that the container, especially its water contact surfaces, are not changed in any way by the water, such as by leaching of container-related compounds into the water or by any chemical reaction or corrosion caused by the water. The water contains no added antimicrobials and is not intended for injection.
d. Pure Steam is also sometimes referred to as “clean steam”. It is used where the steam or its condensate would directly contact official articles or article-contact surfaces such as during their preparation, sterilization, or cleaning where no subsequent processing step is used to remove any codeposited impurity residues.
[adsense:468x15:2204050025]
e. Sterile Purified Water is Purified Water, packaged and rendered sterile. It is used in the preparation of nonparenteral compendial dosage forms or in analytical applications requiring Purified Waterwhere access to a validated Purified Watersystem is not practical, where only a relatively small quantity is needed, where sterile Purified Wateris required, or where bulk packaged Purified Wateris not suitably microbiologically controlled.
f. Sterile Water for Injection is Water for Injection packaged and rendered sterile. It is used for extemporaneous prescription compounding and as a sterile diluent for parenteral products. It may also be used for other applications where bulk WaterforInjectionor PurifiedWateris indicated but where assess to a validated water system is either not practical or where only a relatively small quantity is needed. SterileWaterforInjectionis packaged in single-dose containers not larger than 1 L in size.
g. Bacteriostatic Water for Injection is sterile Water for Injection to which has been added one or more suitable antimicrobial preservatives. It is intended to be used as a diluent in the preparation of parenteral products, most typically for multi-dose products that require repeated content withdrawals. It may be packaged in single-dose or multiple-dose containers not larger than 30 mL.
h. Sterile Water for Irrigation is Water for Injection packaged and sterilized in single-dose containers of larger than 1 L in size that allows rapid delivery of its contents. It need not meet the requirement under small-volume injections. It may also be used in other applications which do not have particulate matter specifications, where bulk Water for Injectionor Purified Wateris indicated but where access to a validated water system is not practical, or where somewhat larger quantities than are provided as Sterile Water for Injectionare needed.
i. Sterile Water for Inhalation is Water for Injection that is packaged and rendered sterile and is intended for use in inhalators and in the preparation of inhalation solutions. It carries a less stringent specification for bacterial endotoxins than Sterile Water for Injection, and therefore, is not suitable for parenteral applications.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
QUALITY CONTROL TESTS FOR WATER
1. Descriptions and Solubility A Clear, Colorless, odorless liquid.
2. pH (at 25°C)
Rinse the pH electrode several times with water and at least twice with aqueous solution to be examined (purified water sample) at 250C ± 0.10C. Note the pH reading. The pH should be between 5 to 7.
3. Conductivity (at 25°C)
Rinse the conductivity cell several times with carbon dioxidefree water and at least twice with aqueous solution to be examined (purified water sample) at 250C ± 0.10C. Note the conductivity. The conductivity should not be more than 2.1 µS/cm
4. Total Organic Carbon
Perform the test on the Test Solution, and record the response, ru. The Test Solution meets the requirements if ru is not more than the limit response, rs – rw. This method also can be performed alternatively using on-line instrumentation that has been appropriately calibrated, standardized, and has demonstrated acceptable system suitability. The acceptability of such on-line instrumentation for quality attribute testing is dependent on its location(s) in the water system. These instrument location(s) and responses must reflect the quality of the water used.
5. Test for Nitrates
Take two fresh dried test tubes in ice, and label them as T1 and T2. Add 5 ml of sample to test tube (T1). Add 4.5 ml of sample and 0.5 ml of nitrate standard solution (2ppm of NO3) in test tube (T2). Add 0.4 ml of potassium chloride (100 g/l) solution, 0.1 ml of diphenylamine (0.1 g/100 ml) solution drop wise with shaking, 5ml of nitrogen-free sulphuric acid to both the tubes.
Transfer both the tubes on to a water-bath at 50ºC. After 15minutes, any blue colour in the test solution (T1) should not be more intense than that in a standard (T2), which meets the requirement to comply the test.
6. Test for Heavy Metals
Heat 200 ml of water sample in a glass-evaporating dish on a water bath until the volume is reduced to 20ml. Take 12 ml from the evaporated water, add 2 ml of buffer solution pH 3.5. Mix immediately. Add 1.2 ml of Thioacetamide (4 g in 100 ml) reagent. Prepare a standard in the same manner using a mixture of 10ml of lead standard solution (1 ppm Pb) and 2 ml of solution to be examined (water sample) and 1.2 ml of thioacetamide reagent. Prepare blank using a mixture of 10 ml of water and 2 ml of solution to be examined (Water sample). Compare to the blank, the standard shows a slight brown color. After two minutes, any brown color in the test solution is not more intense than that in the standard, which meets the requirement to comply the test.
7.Microbial Limit Test
7.1 Total Bacterial Count and Total Yeast and Mould Count {By Membrane Filtration}
Pipette 1ml each of purified water sample into a two sterile filtering apparatus and filter using 0.45 µm pore size filter paper under aseptic conditions. Rinse the filter paper with 100 ml peptone water. Transfer each membrane filter paper to the surface of the following agar medium: Soyabean Casein Digest Agar Medium or R2A Agar for Bacteria: Incubate the plate at 30 – 35°C for 5 days. Sabouraud Glucose Agar with Antibiotic Medium or Sabouraud Dextrose Agar with Antibiotic for Fungi: Incubate the plates at 20 to 25°C for 5 days. Perform the above test in duplicate and select the plates with highest number less than 100 colonies and report the number colony forming units per milliliter.
7.2 Test for Escherichia coli:
Transfer10 ml ofthe sample into 100 ml of Fluid lactose Medium, homogenize and incubate at 30ºC to 35ºC for 24-48 hours. After incubation, shake the container andtransfer 1 ml of the enrichment medium to 10 ml of Mac-Conkey Brothand incubate at 40°C to 45°C for 18 to 24 hours. Observe the medium for growth and if growth is present, sub culture a loopful onto Mac-Conkey Agar Medium and incubate at 30°C to 35°C for 24-72 hours. Growth of red generally non-mucoid colonies of gram-negative rods, sometimes surrounded by a reddish precipitation zone, indicates the possible presence of E.coli. Transfer the suspected colonies from the surface of Mac-Conkey Agar to the surface of Levine Eosin Methylene Blue Agar (EMB) and incubate at 30°C to 35°C for 24-48 hours. If colonies exhibiting metallic sheen under reflected light and blue black appearance under transmitted light indicates the presence of E.coli.
7.3 Test for Salmonella Species
Transfer 10 ml of the sample into 100 ml of Fluid Lactose Medium and incubate at 30ºC to 35ºC for 24-48 hours.After incubation,pipette 1ml each of the enrichment culture into two 2 tubes each containing 10 ml of Tetrathionate Bile Brilliant Green Bile Broth and 10ml of Rappaport Vassiliadis Salmonella Enrichment Broth respectively. Incubate Tetrathionate Brilliant Green Bile Broth at 40 to 45°C for 18 to 24 hours. Incubate Rappaport Vassiliadis Salmonella Enrichment Broth at 30 to 35°C for 18 to 24 hours.
After incubation, Subculture from both tubes onto the surface of two different agar media: Xylose, Lysine, Deoxycholate Agar and Brilliant Green Agar. Incubate at 30ºC to 35º C for 24 to 48 hours. The probable presence of salmonella is detected by the growth of culture. Transfer separately a few of the suspect colonies from the above mediums onto the surface of Triple Sugar Iron Agar in tubes, using surface and deep inoculation. The presence of salmonella is provisionally confirmed if in the deep inoculation but not in the surface cultures there is a change of color from red to yellow and usually a formation of gas, with or without production of hydrogen sulfide in the agar. Precise confirmation may be carried out by appropriate biochemical and serological tests.
7.4 Test for Pseudomonas aeruginosa
Transfer 10 ml of the sample into 100 ml of Soyabean Casein Digest Medium and incubate at 30ºC to 35ºC for 24-48 hours. After incubation, subculture a loopful of the sample onto the surface of Cetrimide Agar and incubate at 30 to 35° for 18 to 72 hours. If no growth of microorganism is detected, the product passes the test. After incubation if growth of the colonies as described in the below table appear, transfer the suspected colonies onto the surface of Pseudomonas agar for detection of flourescein and Pseudomonas agar for detection of Pyocyanin. Incubate at 30 to 35° for 72 hours. After incubation transfer suspect colonies from the above mediums into 100ml of Soyabean Casein Digest Medium (SCDM) and incubate at 40-45ºC for 18-24 hours. If growth occurs, it indicates the presence of Pseudomonas aeruginosa. Precise confirmation may be carried out by appropriate biochemical and serological tests.
7.5 Test for Staphylococcus aureus
Transfer 10 ml of the sample into 100 ml of Soyabean Casein Digest Medium and incubate at 30ºC to 35ºC for 24-48 hours. After incubation, subculture a loopful of the sample onto the surface of Baird Parker Agar and incubate at 30 to 35° for 18 to 72 hours. If no growth of microorganism is detected, the product passes the test. After incubation if growth of the colonies as described in the below table appear, Withthe aid of an inoculating loop, transfer representative suspect colonies from the agar surface (Baird Parker Agar medium) to individual tubes each containing 0.5ml of mammalian preferably rabbit or horse plasma with or without suitable additives. Incubate in water bath at 37°C examining the tubes at 3 hours and subsequently at suitable intervals of 24 hrs. Test positive and negative controls simultaneously with the unknown specimen. If no coagulation in any degree is observed the specimen meets the requirements of the test for absence of Staphylococcus aureus.

NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
QUALITY OF WATER FOR PHARMACEUTICAL USE
Validation and qualification of water purification, storage and distribution systems are a fundamental part of GMP and form an integral part of the GMP inspection. The grade of water used at different stages in the manufacture of the active pharmaceutical ingredients and pharmaceutical products should be discussed in the pharmaceutical dossier. The grade of water used should take account of the nature and intended uses of the finished product and the stage at which the water is used.
a. Use of Water as an Excipient in the Final Formulation
Water is commonly used excipient in medicinal products: the minimum quality of water depends on the intended use of the product. Table 1 showing that the list of sterile products in which water is used as an Excipient. WFI is required for those products intended for parentral administration and this includes solutions for haemofiltration and haemodiafiltration, and peritoneal dialysis. For convenience the pharmaceutical industry often uses WFI for the preparation of ophthalmic, sterile nasal or ear and cutaneous preparations. In such situations, highly purified water represents useful alternative with the added advantage of satisfying the industry’s need for large volumes.
Table 2: Use of water in Sterile Medicinal Products
|
Sl. No |
Name of The Sterile Medicinal Product |
Minimum acceptable quality of water |
|
1. |
Parenteral |
WFI |
|
2. |
Ophthalmic |
Purified Water |
|
3. |
Haemofiltration Solutions Haemodiafiltration Solutions |
WFI |
|
4. |
Peritoneal Dialysis Solutions |
WFI |
|
5. |
Nasal/Ear Preparations |
Purified Water |
|
6. |
Irrigation Solutions |
WFI |
|
7. |
Cutaneous Preparations |
Purified Water |
Table 2 summarises the main categories of non-sterile dosage forms. With the exception of some nebulizer preparations, purified water is the acceptable grade of water for all non-sterile products.
b. Water Used During Manufacturing of APIs and Medicinal Products
The acceptable grade of water will depend heavily on the stage at which it is to be used during manufacture, the subsequent processing steps and the nature of the final product.
c. Water for Cleaning or Rinsing of Equipment, Containers and Closures
In general, the final rinse used for equipment, containers and closures should use the same quality of water as used in the final stage of manufacture of the API or used as excipient in a medicinal product.
MICROBIOLOGICAL MONITORING OF PLANT WATER SYSTEMS
For WFI (Distilled Water), Purified Water and pre -treatment water, open sample valve and flush at least 1 litre of water. Collect flush water in a bucket or flush directly down the drain were possible. Where a Hose is attached to an outlet in production (the use point), the water should be sampled through that hose if possible. Without stopping the flow of water, throttle back the flow of water until a laminar flow is obtained and collect the required volume. Bioburden samples are to be collected in presterilised bottles. Spray the outlet port with 70% IPA after the sample is taken and leave to air dry. The time the sample was collected should be recorded on the container label and on the schedule form. Any observations or deviations should be recorded in the comments section in the schedule form. Bioburden samples must be refrigerated upon return to the laboratory and tested within 24 hrs of sampling.
Note: Distilled water should not be sampled when the temperature of the water exceeds 50oC. The exception could be Hot WFI. When sampling from the Hot Water Loop in the always wear heatproof gloves. All samples taken from outlets on Purified water systems (i.e. pre-treatment water and purified water) must be sampled on the same day in order to obtain a clear snapshot of the water quality.
a. Endotoxin Testing
Sufficient sample must be taken to enable a retest to be performed. Endotoxin testing will only be performed on WFI on routine basis. Label 7ml non- pyrogenic, sterile polystyrene vials with the outlet point, date and the time the samples are taken. Rinse the 7mL vial and cap once with the water to be tested before collecting the sample. Fill vial three -quarter full with water and cap. Where testing is conducted within 24 hours of collection, samples should be stored in refrigerator between 2 -8oC. Samples should be stored frozen if testing is not going to take place within 24hrs. Samples must be thawed out prior to testing.
b. Bioburden Testing
Sterilised equipment required for the tes t should be used no longer than 48 hours after autoclaving conduct the testing in the laminar flow cabinet. Under the Micro lab laminar flow cabinet, aseptically transfer the required volume, from the sterile bottle to sterilised filter cups. Filter the sample through a 0.45µm membrane filter, and rinse with 150mL of sterile Peptone water. Place the whole filter carefully onto an R2A plate. Include a negative control for each test session by filtering 150ml of Peptone water through a 0.45 µm membrane filter and place onto a R2A plate. Incubate all plates inverted at between 30oC - 35oC for 5 days and examine and count the number of colonies present. Bioburden and Bacterial Endotoxin Alert and Action Levels were mentioned in Table 4.
Table 3: Use of water in Sterile Medicinal Products
|
Sl. No |
Name of The Sterile Medicinal Product |
Minimum acceptable quality of water |
|
1. |
Oral Solutions |
Purified Water |
|
2. |
Cutaneous Preparations |
Purified Water |
|
3. |
Nebuliser Solutions |
Purified Water |
|
4. |
Nasal/Ear Preparations |
Purified Water |
|
5. |
Rectal/Vaginal Preparations |
Purified Water |
Table 4: Bioburden and Bacterial Endotoxin Alert and Action Levels
|
Type of water |
Bioburden Testing |
|
|
Alert Levels |
Action Levels |
|
|
Pre-Treatment Water |
1000 cfu/ml |
5000 cfu/ml |
|
Chiller Water |
100 cfu/ml |
1000 cfu/ml |
|
Reverse Osmosis Water (RO) |
10 cfu/ml |
100 cfu/ml |
|
Purified Water (PW) |
10 cfu/ml |
100 cfu/ml |
|
Water for Injection (WFI) |
1 cfu/ml |
10 cfu/ml |
|
Type of water |
Endotoxin Testing |
|
|
Pre-Treatment Water |
Not Applicable |
Not Applicable |
|
Chiller Water |
Not Applicable |
Not Applicable |
|
Reverse Osmosis Water (RO) |
Not Applicable |
Not Applicable |
|
Purified Water (PW) |
Not Applicable |
Not Applicable |
|
Water for Injection (WFI) |
0.125 EU/ml |
0.25 EU/ml |
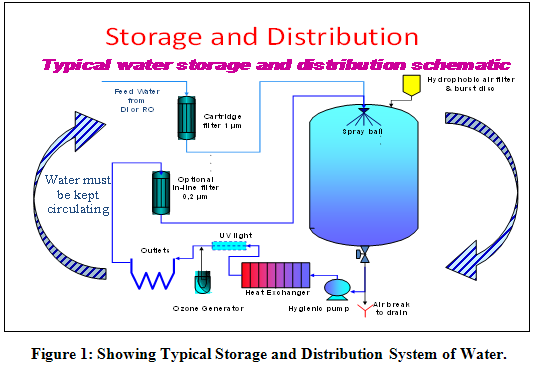
WATER SYSTEM DESIGN
Unless you buy packaged PW or WFI, you will need to produce it by purification of water from your local water supply. To produce it, you need to treat the water with a combination of purification steps (operations) that are designed to remove specific types of impurities. The combination and order of operations varies for every system. Factors that impact water system design includes required volume and quality(ies) of water peak, average, shutdown volumes, types of water, compendial requirements, temperature, uses of water, compendial ingredient, cleaning/rinsing/washing, humidification, redundancy, future capacity, source water, quality, seasonal changes, microbial control methods, green engineering, risk factors to the end product, cost, capital, available resources, operating costs.
1. Processes for Suspended Solids Removal includes multimedia filter, disposable cartridge filter, ultrafilter, microfilter, reverse osmosis, submicronfilter.
2. Processes for Conductivity Reduction includes reverse osmosis, ion exchange (regenerated on-site, regenerated off-site), continuous electrodeionization, distillation
3. Processes for TOC Reduction include Activated carbon, Organic scavenger resin, reverse osmosis, ultrafiltration, ultraviolet oxidation (185 nm or medium pressure), distillation, ozone.
4. Processes for Microbial Control includes residual disinfectant, ultraviolet light, reverse osmosis, distillation, ultrafiltration, submicron filtration, ozone, continuous heat.
A preventive maintenance program should be established to ensure that the water system remains in a state of control. The program should include (1) procedures for operating the system, (2) monitoring programs for critical quality attributes and operating conditions including calibration of critical instruments, (3) schedule for periodic sanitization, (4) preventive maintenance of components, and (5) control of changes to the mechanical system and to operating conditions.
a. Operating Procedures
Procedures for operating the water system and performing routine maintenance and corrective action should be written, and they should also define the point when action is required. The procedures should be well documented, detail the function of each job, assign who is responsible for performing the work, and describe how the job is to be conducted. The effectiveness of these procedures should be assessed during water system validation.
b. Monitoring Program
Critical quality attributes and operating parameters should be documented and monitored. The program may include a combination of in-line sensors or automated instruments (e.g., for TOC, conductivity, hardness, and chlorine), automated or manual documentation of operational parameters (such as flow rates or pressure drop across a carbon bed, filter, or RO unit), and laboratory tests (e.g., total microbial counts). The frequency of sampling, the requirement for evaluating test results, and the necessity for initiating corrective action should be included.
c. Sanitization
Depending on system design and the selected units of operation, routine periodic sanitization may be necessary to maintain the system in a state of microbial control. Technologies for sanitization are described above.
d. Preventive Maintenance
A preventive maintenance program should be in effect. The program should establish what preventive maintenance is to be performed, the frequency of maintenance work, and how the work should be documented.
e. Change Control
The mechanical configuration and operating conditions must be controlled. Proposed changes should be evaluated for their impact on the whole system. The need to requalify the system after changes are made should be determined. Following a decision to modify a water system, the affected drawings, manuals, and procedures should be revised.
CONCLUSION
There is no single design that is ever guaranteed to work. The capability to deliver safe water consistently and confidently is based on knowledge of inputs and outputs, good engineering practices and water system design, good monitoring/control program, and proper maintenance.Systems must be continuously or frequently sanitized for best microbial performance. Energy costs can be minimized through proper engineering.
ACKNOWLEDGEMENT
Authors wish to give thanks to Natco Pharma Ltd., Hyderabad for constant support and given literature to carry out this review. Also, we would like to thank Formulation Research and Development team leaders R. Amarnath, T. Ramaswamy Chowdary, Dr. V. Satyanarayana and K. Venkateswara Rao in Natco Pharma Ltd., Hyderabad for their constant support and suggestions. We also acknowledge the help provided by our colleagues in completion of the review.
REFERENCES
1. WHO, Joint training on GMP for biological products in Thailand, 2-11 September, 1997.
2. Chung Keel Lee, GMP and related topics, 13-15 October, 2003.
3. Chung Keel Lee, Current GMP for biological products and its practical implementation, 22-23 March, 2004.
4. FDA and WHO, GMP inspection workshop, 21-29 June, 2004.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE