 About Authors:
About Authors:
D. HariHaran*, M. Senthil kumar, M. Ashok Kumar, S. Dinesh & R.Jenish.
Annai Veilankanni’s College of Pharmacy,
81, V.G.P. Salai, Saidapet, Chennai-600015.
*haran_pharma@yahoo.com
ABSTRACT
The present study behind this work is to find to prepare sustained release tablets of Viloxazine by compression method. First of all to formulate Viloxazine sustained release tablets using the Ethylcellulose, Carbopol, Sodium Alginate, Hydroxy propyl methyl cellulose & Guar gum under ratio’s like 1:1, 1:1, 1:1,1:1,1:1 . Five batches were made in various polymers of ethylcellulose, carbopol, sodium alginate,hydroxy propyl methyl cellulose & guar gum is used by keeping the drug as constant. Then evaluation of Viloxazine sustained release tablets was carried out for characteristics like drug content in tablet, UV analysis. In vitro release starts from 1hr and up to 24hrs. It shows the percentage of gradual drug release as 17.80%, 27.65%, 35.12%, 45.16%, 51.20%, 57.42%, 61.30%, 66.32%, 72.08%, 77.15%, 81.32%, 84.48% & 98.20% against the label claim as 40mg.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1498
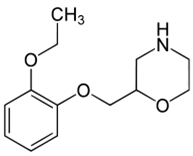
INTRODUCTION- Viloxazine HCl
Viloxazine HCl is chemically (RS)-2-[(2-ethoxyphenoxy) methyl] morpholine
HCL
Viloxazine Hydrochloride contains not less than 95.0 per cent and not more than 105.0 per cent of C13H19NO3, calculated on the anhydrous basis. It is White to off-white crystalline powder. Soluble in Acetic acid, Methanol and Water. Viloxazine Hydrochloride is used in the treatment of depression. It is a Nor-adrenaline reuptake inhibitor (NRI). It also weakly inhibits the dopamine re-uptake. It is also reported to have little affinity for Muscarinic, Histaminirgic or α1-adrenergic receptors. The drug is readily absorbed from the GIT. Following oral administration, it undergoes extensive first pass metabolism in the liver mainly to the active metabolite O-desmethyl Viloxazine. Peak plasma concentrations of Viloxazine and O-desmethyl Viloxazine appear about 2 and 4 hours after administration respectively. Protein bindings of Viloxazine e and O-desmethyl Viloxazine are low. (Approximately 25%). Formation of O-desmethyl Viloxazine is mediated by the CytochromeP-450 isoenzyme CYP2D6. Other metabolites include N-desmethyl Viloxazine and N, O-didesmethyl Viloxazine. The mean plasma elimination half life of Viloxazine and O-desmethyl Viloxazine 6 and 8 hrs respectively. Viloxazine is excreted predominantly in the form of its metabolites, either free or in conjugated form, about 2% is excreted in the faecus. Side effects include Nausea, Dizziness, dry mouth, Sexual dis-function, may cause sustained rise in BP, needs BP monitoring. It should be used with caution in those with history of myocardial infraction or unstable heart disease. It should also be used with caution in patients with a history of epilepsy and should be discontinued any patient developing a seizure. Viloxazine is known to increase plasma levels of phenytoinby an average of 37%.It is also known to significantly increase plasma levels of theophyllineand decrease its clearance from the body, sometimes resulting in accidental overdose of theophylline.
Viloxazine conventional formulationswere been developed and evaluated but no sustained release formulation are been developed. Here in this study Viloxazine sustained formulations are been developed with the help of ethylcellulose, carbopol, sodium alginate, hydroxy propyl methyl cellulose (HPMC K 100) and guar gum it is been evaluated with the help of U.V.
[adsense:468x15:2204050025]
REVIEW OF LITERATURE
* Pabon et al (1992)6, has studied the dissolution rates of mixed controlled release matrix tablets containing hydroxy propyl methyl cellulose and polyamide 12 for metaclopromide hydrochloride and a reference tablet using cellulose microcrystalline as excipient and has concluded that dissolution kinetics were markedly different for the different formulations studied depending upon the proportions of excipients.
* Sheu et al (1992)7, has studied the effects of the media on the dissolution of diclofenac sodium from voltaren and hydroxy propyl methyl cellulose matrix tablets and has concluded that drug release from hydroxy propyl methyl cellulose matrices was non-Fickian in all media and also concluded that diclofenac release from hydrophilic and hydrophobic tablets is dependent on the dissolution media.
* Pabon et al (1994)8, has studied the fracture and dissolution rates of matrix tablets prepared with hydroxy propyl methyl cellulose and polyamide 12 at different proportions using metaclopromide hydrochloride as model drug and has concluded that the dissolution rates was different for each formulation and depended on the specific polymer percentage.
* Dong et al (1996)9, has studied the effect of hydroxy propyl methyl cellulose on the mechanism of soluble drug release from hydrophilic matrix tablets prepared with captopril and albuterol sulfate and confirmed that the drugs were released by non-Fickian diffusion mechanism and concluded that the different contents of hydroxy propyl methyl cellulose in the matrices only affected Higuchi release patterns.
* Sung et al (1996)10, has studied the effect of hydroxy propyl methyl cellulose and lactose ratio and hydroxy propyl methyl cellulose viscosity grade on the release of a model drug, adinazolam mesylate and hydroxy propyl methyl cellulose from a hydroxy propyl methyl cellulose matrix tablet and concluded that with higher drug and polymer release rates found for formulations with lower polymer lactose ratio and lower polymer viscosity grades.
* Vazquez et al (1996)11, has studied the potential value of hydroxy propyl methyl cellulose mixture as gelling agents in matrix tablets for hydro soluble drugs and the relationship between the gelling agent viscosity and the kinetics of drug release from atenolol hydrophilic matrix tablets and has concluded that all drug s release was diffusion limited.
* Dortunc et al (1997)12, has studied the effect of polymer grade, influence of the additive magnesium stearate and lactose, particle size and pH of dissolution medium on the release of acetazolamide from a controlled release swellable tablets prepared by direct compression and found that hardness slightly increased with increasing the amount of hydroxy propyl methyl cellulose drug release decreased when the polymer amount was increased.
* Traconis et al (1997)13, has prepared the matrix tablets containing metronidazole and 10% of polymer, different proportions of sodium carboxy methyl cellulose plus hydroxy propyl methyl cellulose or ethyl cellulose plus hydroxy propyl methyl cellulose and studied the release of metronidazole from tablet in acidic medium and found that the addition of sodium carboxy methyl cellulose produced biphasic linear kinetics, before and after the transition point, increasing in concentration of sodium carboxy methyl cellulose resulted in decreasing dissolution rates.
* Relasco et al (1999)14, has studied the influence of drug : hydroxy propyl methyl cellulose ratio, drug and polymer particle size and compression force on the release of diclofenac sodium from hydroxy propyl methyl cellulose tablets and observed that the rate and mechanism of drug release matrices were mainly controlled by the drug: polymer ratio, the drug and polymer particle size influence on the drug release parameters and found that compressional force influence only on lag time.
* Heng et al (2001)15, has studied the influence of mean hydroxy propyl methyl cellulose particle size and number of polymer particles on the release of aspirin from swellable hydrophilic matrix tablets by preparing the tablets with different concentrations of different size fractions of hydroxy propyl methyl cellulose as well as lactose and magnesium stearate and has concluded that the release of aspirin from matrix tablet formulations increased markedly when hydroxy propyl methyl cellulose particle size was greater than 113µm and release rate was much less sensitive to changes in hydroxy propyl methyl cellulose particle size below 113µm.
* Rao et al (2001)16, has studied the effect of SBE 7M-beta-cyclodextrin as a solubilizing agent on prednisolone, a poorly water soluble drug, release from hydroxy propyl methyl cellulose matrix tablets and found that there is enhanced drug release to a control formulation, prepared using lactose instead of SBE 7M-beta-cyclodextrin.
* Shiva kumar et al (2001)17, has studied the effect of hydroxy propyl methyl cellulose and hydroxy ethyl cellulose on release of the cetostearyl alcohol embedded diclofenac sodium from tablets for controlled release and found that hydroxy propyl methyl cellulose and hydroxy ethyl cellulose was showing differences in their behavior in controlling the release of diclofenac sodium.
* Obadiat AA et al (2002)18, has studied the release of dextro methorphan hydrobromide DM-HBR) from matrix tablets containing sodium CMC and hydroxy propyl methyl cellulose and found that the results obtained indicated that the nonionic hydroxy propyl methyl cellulose had little retarding effect on drug release from the matrix, anionic sodium CMC significantly reduced the release rate of the drugs. An effect which was attributed to interaction between the drug and sodium CMC due to formation of insoluble complex. The pH affected both the solubility of the drug and die erosion rate of the matrix. Combination of both HPMC and sodium CMC in optimum proportions would result in a near zero order release.
* Reynolds et al (2002)19, has studied the effect of tablet surface area and/or volume on drug release from matrix tablets containing hydroxy propyl methyl cellulose using soluble drugs (Promethazine hydrochloride, Diphenhydramine hydrochloride, and proponalol hydrochloride) by comparing the drug release from tablets having similar values of surface area and/or volume within the same tablet shape and among different shapes and has concluded that surface area and/or volume is one of the key factor in controlling the drug release from hydroxy propyl methyl cellulose matrix tablets.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
* Sapna et al (2002)20, prepared venlafaxine hydrochloride sustained release tablets by using matrix system based on swellable and no swellable polymers such as hydroxy propyl methyl cellulose, ethyl cellulose, cellulose acetate and eudragit RSPO were selected. Combinations of non swellable polymers were also studied with hydroxy propyl methyl cellulose for sustained release up to 16 hours. The effect of drug to polymer in vitro release was studied.
* Al taani BM et al (2003)21, prepared pH dependent swellable and erodible-buffered matrix tablets by using hydrophilic polymers HPMC and pH dependent solubility polymer(eudragit L100-55) with different micro environment pHs. Diclofenac sodium was chosen as choice of drug. The swelling and erosion matrices containing only hydroxy propyl methyl cellulose and eudragit L100-55 were studied in phosphate buffer solution of pH similar to the microenvironment pHs of the matrices and found to be the drug release is linear as a function of time (amount of drug released was found to be higher in the medium of pH 7.4 than that of pH 5.9).
* Amaral MH et al (2003)22, has studied the effect of water soluble carriers on naproxen release from hydrophilic matrix tablets and found that the release profile of naproxen from hydroxy propyl methyl cellulose matrices remained unchanged when lactose and PEG-4000 were added to the same drug and polymer concentrations due to the higher water solubility of PEG-4000, the erosion of compressed mixtures of hydroxy propyl methyl cellulose k-100M/ PEG-4000(1:1)was higher than the erosion of tablets containing the same proportion of lactose.
* Chowdary KP et al (2003)23, have prepared mucoadhesive matrix tablets employing sodium CMC, hydroxy propyl methyl cellulose and EC using diltiazem as a model drug. Tablets formulated employing sodium CMC with 5% EC gave slow and complete release over a period of 12 hrs. These tablets exhibited good mucoadhesion in the intestine for 10-12 hrs in the X-Ray studies. Non-Fickian release was observed from most of the formulations ( A two layer tablet formulation, an immediately releasing layer consisting of diltiazem and croscarmellose sodium (A super disintegrate) and a matrix consisting of diltiazem, sodium CMC and EC as a second maintenance layer, gave release close to the theoretical sustained release (SR) needed for diltiazem.
* Gohel MC et al (2003)24, has prepared pH independent sustained release matrix tablets by using rate controlling polymers such as eudragit RSPO/ RlPO, HPMCK4M. Verapamil hydrochloride was chosen as model drug. Tablets were prepared by wet granulation method and succinic acid and KH were incorporated in the matrix in order to obtain pH independent drugs release. They found that the required release rate obtained with low level of eudragit RSPO/RLPO (30% w/w), low level of HPMC K4M (- 10% w/w), high level of PEG 4000 (15% w/w) and desired drug release pattern can be obtained by adopting a systemic formulation approach.
* Samani SM et al (2003)25, has studied the effect of polymer blends of hydroxy propyl methyl cellulose (viscosity grades 60 and 500mpas), carbopol-940 on release profile of diclofenac sodium from matrices and found that when hydroxy propyl methyl cellulose viscosity grade (60 mpas) at higher polymer drug ratio (more than 0.8:1). The Carbopol can extend the release time appreciably best release profile have fluctuations. When an appreciate blend of hydroxy propyl methyl cellulose and Carbopol was used the drug release became more uniform and it’s kinetic approached to zero order and release fluctuations were diminished.
* Vostalova L et al (2003)26, has formulated hydrophilic matrix tablets by using hydroxy propyl methyl cellulose and other cellulose derivatives and chosen well soluble diltiazem chloride and badly soluble ibuprofen as model drugs. Evaluated and found that the formulation and manufacture variables can influence the rate of drug release from matrix. Higher solubility of the drugs and lower solubility of compacts resulted in more rapid release of the active ingredient and lower concentrations of hydroxy propyl methyl cellulose accelerates release of both drugs.
* Bravo SA et al (2004)27, has formulated the swellable matrices for the controlled release of diclofenac sodium by using hydroxy propyl methyl cellulose and carboxy polymer (carbopol-934) as rate controlling polymers and studied for the influence of polymer content, the polymer ratio, the polymeric swelling behavior and pH changes on the release rate of diclofenac sodium. Combination of these two polymeric matrix formers resulted in near zero order release rate of diclofenac sodium. The diclofenac release from matrix tablets was pH dependent, being markedly reduced at lower pH (could be attributed to the poor solubility of diclofenac sodium at this pH values) in 0.1N HCl solution, HPMC controlled drug release. As the pH increases, both hydroxy propyl methyl cellulose and Carbomer controlled release occurs, as a result of Carbomer became ionized being able to interact with hydroxy propyl methyl cellulose to control drug release.
* Martinez Gonzelez et al (2004)28, has formulated matrix tablets using hydroxy propyl methyl cellulose as rate controlling polymer and 4-amino pyridine was chosen as model drug and studied for the influence of enteric coated lactose on the release profile from hydroxy propyl methyl cellulose matrix tablets were prepared by wet granulation method and found that decreasing release constant values were observed with increasing enteric coated lactose concentrations up to 9%. This is attributing to an increasing obstruction of the diffusion path by isolated enteric coated lactose particles that are insoluble in HCl and/or surrounded by a water filled space. After a critical enteric coated lactose concentrations, the water filled spaces surrounding enteric coated lactose particles percolate, producing opposite effect, increasing release constant as the enteric coated lactose proportion increases from 10-50%.
* Cao QR et al (2005)29, have studied the effect of pharmaceutical excipients on the in vitro release profiles and the release mechanism of monolithic hydroxy propyl methyl cellulose (4000Cps) matrix tablets (m-HPMC), in terms of mimicking the dual drug release character of bi layered tylenol-SR tablets was studied and acetaminophen was used as the model drug. The m-HPMC tablets were prepared using wet granulation method followed by direct compression. Release profile and swelling rate of m-HPMC tablets were found to be highly influenced by the types and amounts of pharma excipients incorporated. Starch -1500 and sodium lauryl sulphate played a key role in determining the dissolution rate of m-HPMC tablets. Additional excipients i.e. MCC and sodium di hydrogen phosphate used to tune the release profile of m-HPMC tablets. The effect of excipients on the drug release from HPMC based matrix tablets was found to be mainly due to a change in hydrophilic gel expansion and on physical interaction between the drug and HPMC.
* Genc L et al (2005)30, has prepared sustained release matrix tablets using different polymers such as hydroxy propyl methyl cellulose, carbopol-934 and eudragit-RL /PO by direct compression technique. Clarithromycin was chosen as model drug and evaluated for influence of polymer type and concentration on drug release from matrix were investigated by 23 factorial designs and found that tablets containing hydroxy propyl methyl cellulose, carbopol-934 and eudragit-RL/PO were found suitably to sustained drug release.
* Peng HL et al (2005)31, has formulated SR matrix tablets using carbopol-71G and m-HPMC and selected hydrochlorthiazide as model drug and studied for factors influencing dissolution rate. Tablets were prepared by wet granulation method. The factors affecting the drug release behaviors from the matrix tablets included the quantity of HPMC and carbopol-71G, tablet hardness, pH environment of the dissolution media paddle rotating speed.
* Srivathsva AK et al (2005)32, has formulated floating matrix tablets to prolong gastric residence time and to increase drug bioavailability and tablets prepared by direct compression method by using polymers such as hydroxy propyl methyl cellulose (K-15M and K-4M), guar gum and the sodium CMC- based matrix tablets showed greater swelling indices. The tablets exhibited controlled and prolonged drug release profile while floating over the dissolution medium.
* Pan WS et al (2005)33, has formulated acipimox sustained release matrix tablets by using hydroxy propyl methyl cellulose as a drug release retarding material and evaluated for the factors effecting the acipimox release rate from matrix including types and quantity of hydroxy propyl methyl cellulose and additives, and manufacturing procedures were evaluated and found that the types and quantity of hydroxy propyl methyl cellulose used in matrixes and the tablet surface area were closely associated with the acipimox release and the types and quantity of additives and manufacturing procedure(eg: dissolution apparatus rotating speed) showed low significant effect on acipimox release. The diffusion factor place a main role in the entire dissolution slow release of drugs obeys Non-Fickian diffusion mechanism.
* Vueba ML et al (2005)34, has studied the influence of different cellulose ether polymers such as release rate of ibuprofen from hydrophilic matrix tablets [methyl cellulose (MC 25), Hydroxy propyl cellulose (HPC) and HPMC (K 15M and K 100M)]. In addition the influence of diluents- lactose mono hydrate (LAC) and small be cyclodextrin(b-CD) was evaluated, polymers MC-25 and HPC were found to be unsuitable for the preparation of this kind of solid dosage forms, while hydroxy propyl methyl cellulose K-15M and K-100M showed to be advantageous. The highest MDT (mean dissolution time) obtained with hydroxy propyl methyl cellulose indicating higher drug retarding ability of the polymer. The release process was also found to be slightly influenced by the kind of diluents used.
* Harish NM et al (2006)35, has developed sustained matrix tablets by using hydroxy propyl methyl cellulose, eudragit RLPO as rate controlling polymers. Terbutaline sulphate was chosen as model drug. Tablets were prepared by wet granulation method and evaluated for flow properties of granules, compressibility index, swelling index and drug content and in vitro studies for drug release and found that granules showed that satisfactory flow properties, compressibility and drug content. The optimized formulation could extend release up to 12 hrs and these optimized formulations showed similar release profile as that of the marketed formulation.
* Haremath SN et al (2007)36, has formulated metformin hydrochloride matrix tablets by using different viscosity grades and ratios of hydroxy propyl methyl cellulose and evaluated mainly to assess the effect of drug polymer ratio and hydroxy propyl methyl cellulose viscosity grade and the release of drug from hydrophilic matrices and found that the lower viscosity grade polymer showed a rapid drug release from the matrices than the higher viscosity grades. The higher drug polymer ratio showed a greater drug release than the others.
MATERIALS AND METHODS
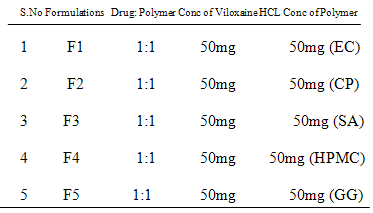
The instruments used for the study are eight stage dissolution apparatus model tdt-08l ELECTROLAB, UV-VIS double beam spectrophotometer (UV-1700 SHIMADZU). The drug used for the study is Viloxazine Hydrochloride and the polymers used for the study are ethylcellulose, carbopol, sodium alginate,hydroxy propyl methyl cellulose (HPMC K 100) and guar gum.
Formulation of Viloxazine SR tablets:
Preparation of Viloxazine SR tablets by using ethylcellulose, carbopol, sodium alginate, HPMC K 100 & guar gum which were prepared by wet granulation & compression method. During this procedure Viloxazine Hydrochloride SR tablets were prepared by wet granulation method where ethylcellulose, carbopol, sodium alginate,hydroxy propyl methyl cellulose (HPMC K 100), guar gum & Viloxazine Hydrochloride are combined together in which ethylcellulose, carbopol, sodium alginate,hydroxy propyl methyl cellulose (HPMC K 100), guar gum are carrier molecule and Viloxazine Hydrochloride is an Hydrophobic drug is mixed with Di calcium phosphate. Now this dry mix is added with ethylcellulose, carbopol, sodium alginate,hydroxy propyl methyl cellulose (HPMC K 100) & guar gum by using PVP K 90 as binder solution.
Steps involved in preparation of Viloxazine SR tablets (Formulation-I):
STEP I: Viloxazine Hydrochloride was passed through sieve no 40# then it was mixed with Dibasic calcium phosphate.
STEP II: The dry mix was added with ethyl cellulose & *.
STEP III: Then binder solution PVP K 90** was added to the above dry mass & dried at 600c for 30 minutes.
STEP IV: The above dried granules were passed through 30# to get uniform particle size. Then pre lubricated with talc, Aerosil & Then finally lubricated with magnesium stearate.
*For FII-FV in step II add carbopol, sodium alginate,hydroxy propyl methyl cellulose (HPMC K 100) & guar gum.
** Binder solution:- PVP K 90 was dissolved Isopropyl alcohol which is used as a binder solution.
Table No.1: The tabular column showing the ratio of drug & polymer concentration.
Drug Content Analysis: Twenty tablets of each formulation were weighed and powdered. The quantity of powder equivalent to 40mg of Viloxazine was transferred into a 100ml volumetric flask and extracted with distilled water. Then it was filtered and suitable dilutions were made and absorbance was measured by using Shimadzu UV-Visible spectrophotometer (UV-1601) at 273nm.
Dissolution study of the Viloxazine SR tablets was done by the six stage dissolution apparatus model tdt-08l ELECTROLAB, with USP specifications. The medium content of the dissolution was 900ml of phosphate buffer pH 6.8 was placed in a dissolution basket. The medium was allowed to equilibrate to the temperature 37± 0.5°C.The rpm maintained for this analysis was 100. The analysis was carried out for 24hours.
The percentage of drug release was analyzed by the help of UV-spectrophotometer for each formulation. All release rate was based upon the amount of drug released was calculated from the calibration curve.
Volume taken is 5ml from which suitable dilutions where made to get desired concentration of drug. The λmax of Viloxazine HCL was found to be 270nm.
The % of drug release of different formulations carried out at 270nm.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RESULTS & DISCUSSION
Formulations F-I to F-V were prepared as per table no 1. Granules were prepared by wet granulation method. The prepared granules were evaluated for bulk density, angle of repose and compressability.
Bulk density for all the formulations ranged between 0.43 to 0.54. the angle of repose for all formulations ranged between 25?.64 to 29?.25 the bulk density indicates good packing characters. The value of angle of repose (between 25?-30?) for all the formulation indicates good flow property. The value of Carr’s index for all the formulations ranged between 7.57-9.73. The value of Carr’s index (between 5-15%) indicates free flowing material. The granules were then compressed to tablets. The tablets were evaluated for uniformity of weight, hardness, friability, drug content and dissolution. The tablets prepared were white in color, oval shape. They were smooth, uniform and free from crack and chipping.
Thicknesses of all fabricated formulations are in the range of 3.2-3.6 mm. Weight variation was found with in specifications of I.P limits the hardness of all fabricated formulations were in the range of 80 –95N
Friability for all the formulations was in the range of 0.36-0.61. The value of hardness and percent friability indicates good handling property of prepared tablets. the results for the above parameters were found to be in the recommended range.
The results of drug content for all formulations were found to be between 39.40 mg - 40.57 mg per tablet with ±S.D.
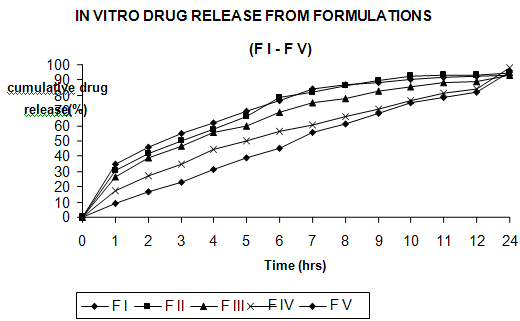
Formulations containing ethylcellulose, carbopol, sodium alginate,hydroxy propyl methyl cellulose (HPMC K 100) & guar gum showing following drug release profiles
FI and FII released more than 80% of drug at 7th hour, where as FIII, FIV and FV released more than 80% of drug at 9th, 11th and 12th hours respectively.
Whereas our selected formulations FIV releases 98.20% of drug in 24 hours respectively.
CONCLUSION
From the results and discussions, amongst the 5 different formulations designated as FI, FII, FIII,FIV andFV by using Ethylcellulose, Carbopol, Sodium Alginate, Hydroxy propyl methyl cellulose & Guar gum.
FI and FII released more than 80% of drug at 7th hour, where as FIII, FIV and FV released more than 80% of drug at 9th, 11th and 12th hours respectively.
The formulationFIV (HPMC K 100)was found to be the better formulation in terms of sustained release and maximum percentage drug release Compared to FIII (Sodium Alginate) & FV (Guar gum).
To conclude, hydroxyl propyl methyl cellulose at a concentration ratio of 1: 1 is suitable for preparing sustained release matrix tablets of Viloxazine hydrochloride.
ACKNOWLEDGEMENT
* We are thank full to our Principal Mr. M.Senthil kumar M.pharm, PhD., for his kind & cooperation to finish the work.
* We are thanking our beloved chairman Dr.S.Devaraj for his encouragement & for providing the facilities to complete the work.
* We thank all our staff & Friends for their support & help for successful completition.
DRUG CONTENT IN TABLETS
|
S.No |
Formulation |
Amount of drug in matrix tablets(mg) |
|||
|
Sample1 |
Sample2 |
Sample3 |
Mean |
||
|
1 |
I |
40.43 |
40.70 |
40.10 |
40.41 |
|
2 |
II |
40.32 |
40.45 |
40.22 |
40.33 |
|
3 |
III |
40.81 |
39.95 |
40.80 |
40.52 |
|
4 |
IV |
40.50 |
40.32 |
39.87 |
40.23 |
|
5 |
V |
40.25 |
40.09 |
40.11 |
40.15 |
In vitro drug release for formulation F IV
|
S.No |
Time(hrs) |
Absorbance |
Concentration (mcg/ml) |
Cumulative release(mg) |
Cumulative Percentage drug release±SD |
|
1 |
1 |
0.038 |
0.98 |
07.81 |
17.80 |
|
2 |
2 |
0.050 |
1.08 |
11. 34 |
27.65 |
|
3 |
3 |
0.065 |
1.15 |
14.30 |
35.12 |
|
4 |
4 |
0.087 |
1.48 |
18.83 |
45.16 |
|
5 |
5 |
0.103 |
1.97 |
20.92 |
51.20 |
|
6 |
6 |
0.130 |
2.43 |
23.50 |
57.42 |
|
7 |
7 |
0.148 |
2.76 |
24.87 |
61.30 |
|
8 |
8 |
0.165 |
3.12 |
27.25 |
66.32 |
|
9 |
9 |
0.184 |
3.54 |
29.08 |
72.08 |
|
10 |
10 |
0.202 |
3.90 |
31.12 |
77.15 |
|
11 |
11 |
0.218 |
4.21 |
32.89 |
81.32 |
|
12 |
12 |
0.227 |
4.42 |
34.20 |
84.48 |
|
13 |
24 |
0.282 |
5.45 |
39.48 |
98.20 |
REFERENCES
1. Tripathi KD. Drugs used in mental illness: antidepressants and antimanic drugs. In: Chein YW, Novel drug delivery system. 2nd ed.Marcel Dekker Inc. 1992; 1-10.
2. Joseph R Robinson. Sustained release drug delivery system. Remington pharmaceutical sciences. 18th ed. The edition Mac publishing company. 1990; 1682-1698.
3. Joseph R, Robinson, Vincent HL. Lee. Controlled drug delivery. 2nd edition, vol 29, 373-421, 1978.
4. Lee VH, Robinson JP. Sustained and controlled release drug delivery systems. Marcel Dekker: New York. 1978; 7-11.
5. Avaraham Yacob, Eva Halperin-Walgega. In: Oral sustained release formulations, design and evaluation; Pergamon press; 1988; 35-39.
6. Pabon CV, Frutos P, Lastres JL, Frutos G. In vitro study of mixed controlled release matrix tablets containing HPMC and polyamide 12. Drug Dev Ind Pharm; 1992; 18(21); 2163-2171.
7. Sheu MT, Chou HL, Kao CC, Liu CH, Sokokoski TD. Dissolution of diclofenac sodium from matrix tablets. Int J Pharm; 1992; 85(20); 57-63.
8. Pabon CV, Frutos P, Lastres JL, Frutos G. Comparison of dissolution data using the Gompertz function. Drug Dev Ind Pharm; 1994; 20(16); p 2509-2518.
9. DongZC, Jiang XT. Mechanism of soluble drug release from hydrophilic matrices, the effect of HPMC content. Acta Pharm Sinica; 1996; 31(1); p 43-47.
10. Sung KC, Nixon PR, Skoug JW, Ju TR, Patel MV. Effect of formulation variables on drug and polymer release from HPMC based matrix tablets. Int J Pharm; 1996; 142(27); p 53-60.
11. Vazquez MJ, Casalderrey M, Duro R, Gomez Amoza JL, Concheiro A. Atenolol release from hydrophilic matrix tablets with HPMC mixtures as gelling agent: effects of the viscosity of the HPMC mixture. Eur J Pharm Sci; 1996; 4(1); p 39-48.
12. Dortunc B, Gunal N. Release of acetazolamide from Swellable HPMC matrix tablets. Drug Dev Ind Pharm; 1997; 23(12); p 1245-1249.
13. Traconis N, Rodriguez R, Campos ME, Villafuerte L. Influence of admixed polymers on metronidazole release from HPMC tablets. Pharm Acta Helv; 1997; 72(1); p 131-138.
14. Velasco MV, Ford JL, Rowe P, Rajabi-Siahboomi AR. Influence of drug: HPMC ratio, drug and polymer particle size and compression force on the release of diclofenac sodium from HPMC tablets. J Controlled Release; 1999; 57(1); p 75-85.
15. Heng PW, Chan LW, Easterbrook MG, Li-X. Investigation of the influence of mean HPMC particle size and number of polymer particles on the release of aspirin from Swellable hydrophilic matrix tablets. J Controlled Release; 2001; 76(1-2); p 39-49.
16. Rao VM, Haslam JL, Stella VJ. Controlled and complete release of a model poorly water soluble drug, prednisolone, from HPMC matrix tablets using (SBE) 7m-beta-cyclodextrin as a solubilizing agent. J Pharm Sci; 201; 90(7); p 807-816.
17. Shivakumar HN, Nath BS, Desai BG. Effect of added HPMC and HEC on the release of cetostearyl alcohol embedded diclofenac sodium from tablets for controlled release. East Pharm; 2001; 44(2); p 117-119.
18. Obadiat AA, Rashdan LA, Najib NM. Release of dextromethorphan from matrix tablets containing sodium carboxy methyl cellulose and hydroxy propyl methyl cellulose. Acta Pharm Turc; 2002; 44(2); p 97-104.
19. Reynolds TD, Mitcell SA, Balwinski KM. Investigation of the effect of tablet surface area/volume on drug release from HPMC controlled release matrix tablets. Drug Dev Ind Pharm; 2002; 28(4); p 457-466.
20. Sapna N. Makhija, Pradeep R, Vaviya. Once daily sustained release tablets of venlafaxine, a novel antidepressant. Eur J Pharm Sci; 2002; 54; p 39-48. 9-15.
21. Al-Taani BM, Tashtoush BM. Effect of microenvironment pH of swellable and erodable buffered matrices on the release characteristics of diclofenac sodium. AAPS Pharm Sci Tech; 2003; 4(3); p NIL_ 0013.
22. Amaral MH, Lobo JM, Ferreira DC. Effect of water soluble carriers on naproxen release from hydrophilic matrix tablets. STP Pharma Sci; 2003; 13(3); p 191-194.
23. Chowdary KP, Suresh B, Sangeeta B, Reddy GK. Design and evaluation of diltiazem mucoadhesive tablets for oral controlled release. Saudi Pharm J; 2003; 11(4); p 201-205.
24. Gohel MC, Patel TP, Bariya SH. Studies in preparation and evaluation of pH independent sustained release matrix tablets of verapamil hydrochloride using directly compressible eudragits. Pharm Dev Technol; 2003; 8(4); p 323-333.
25. Samini SM, Montaseri H, Kazemi A. The effect polymer blends on release profile of diclofenac sodium from matrices. Eur J Pharm and Biopharm; 2003; 55(3); p 351-355.
26. Vostalova L, Rabiskova M, Medvecka G. The release of diltiazem chloride and ibuprofen from hydrophilic matrix tablets. Ceska Solv Farm; 2003; 52(6); p 295-298.
27. Bravo SA, Lamas MC, Salomon CJ. Swellable matrices for the controlled release of diclofenac sodium: Formulation and in vitro studies. Pharm Dev Technol; 2004; 9(1); p 75-83.
28. Martinez-Gonzalez I, Vilafuerte-Robles L. Influence of enteric coated lactose on the release profile of 4-aminopyridine from HPMC matrix tablets. Pharm Dev Technol; 2004; 9(2); p 145-153.
29. Cao QR, Choi YW, CUI JH, Lee BJ. Formulation, release characteristics and bioavailability of novel monolithic HPMC matrix tablets containing acetaminophen. J Controlled Release; 2005; 108(2-3); p 351-361.
30. Genc L, Kiran AM. In vitro evaluation of sustained release matrix tablets formulations of clarithromycin. Sci Pharm; 2005; 73(1); p 59-74.
31. Peng HL, Liu QF, Wang YM, Luo GA, Li BC. Evaluation of Carbopol 71G and HPMC as matrix materials in a sustained release formulation. Chin J New Drug; 2005; 14(8); p 1010-1014.
32. Srivathsava AK, Wadhwa S, Ridhurkar D, Mishra B. Oral sustained delivery of atenolol from floating matrix tablets- formulation and in vitro evaluation. Drug Dev Ind Pharm; 2005; 31(4-5); p 367-374.
33. Pan WS, Yang XG, Nie SF, Guo H, Zhang GH. Dissolution behavior and mechanism of acipimox sustained release tablets. Chin J New Drug; 2005; 14(4); p 440-444.
34. Vueba ML, de-Carvalho LA, Veiga F, Sousa JJ, Pina ME. Role of cellulose ether polymers on ibuprofen release from matrix tablets. Drug Dev Ind Pharm; 2005; 31(7); p 635-665.
35. Harish NM, Kiran AB, Rathnanand M, Shirwaikar A, Shenoy KRP. Sustained release matrix tablets of terbutaline sulphate; formulation and in vitro evaluation. Indian drugs; 2007; 44(3) p 233-235.









