About Authors:
Madhu dwivedi, Rishabha Malviya
Department of pharmaceutical technology
MIET,MEERUT
Introduction:
Natural polymer: A number of natural or partially modified polymers were screened for mucoadhesive properties by routinely measuring the force of detachment for swollen polymer films from pig intestinal mucosa in a saline medium. Suprisingly, hydroxypropyl- and carboxymethylcellulose showed almost no mucoadhesion, whereas the cationic polymer chitosan was fairly mucoadhesive in comparison to Polycarbophil as a reference substance. It is suggested that a strict difference be made between mucoadhesion of dry polymers on a wet tissue in air, and mucoadhesion of a swollen hydrogel in the presence of a third liquid phase. Cationic polymers should be further investigated with respect to possibly improved mucoadhesive properties in a neutral or slightly alkaline environment.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1217
Natural polymers are obtained in the form of macromolecules. The natural polymers demands in the households, agriculture, food industries and in packaging and it also help in reducing the environmental pollution and resulting in disposal in landfills. Natural polymers are act as an environment cleaner, renewable and help in recycling of global carbon. The natural gums are biodegradable, nontoxic and biocompatible in nature and swells when comes in contact with the aqueous media so it has been used in the preparation of sustained release or controlled release types of dosage form. In the present investigation it shows that plant polysaccharide have been useful for the construction of specific drug delivery systems.[1]The polysaccharide that is present in natural polymer is known as tamarind seed polysaccharide Gum is present in the tamarind seed and it is a hydrophilic polymer and had been used as gelling, thickening, suspending and emulsifying agents.[2,3,4,5] Gum consisted 65% of the seed components.[6,7,8] It is used as a thickening, stabilizing and gelling agents in food.[9]The gum can also be act as a binder in pharmaceutical tablets, as a humectants and emulsifier in the different types of formulations.[10] This shows that the Natural polymer is highly viscous, mucoadhesive and biocompatible in nature. Now the regular research is going on in field of natural occurring biocompatible polymeric material in designing of dosage form for oral controlled release administration. The Natural polymer has a widest scope in the pharmaceutical industries and it is act as a binder in tablet dosage form, ocular drug delivery system and in sustained release drug delivery systems. It is a novel mucoadhesive polymer, can be used in the delivery system for the ocular administration of hydrophilic and hydrophobic antibiotics[11] Polysaccharides are a class of biopolymers constituted by simple sugar monomers [12]. The monomers (monosaccharides) are linked together by O-glycosidic bonds that can be made to any of the hydroxyl groups of a monosaccharide, conferring polysaccharides the ability to form both linear and branched polymers. Differences in the monosaccharide composition, chain shapes and molecular weight dictate their physical properties including solubility, gelation and surface properties. These biological polymers can be obtained from different sources: microbial, animal and vegetal [13]. Several advantages can be derived from the use of these macromolecules. First of all, probably because of the chemical similarities with heparin, polysaccharides show good hemocompatibility properties. They are non-toxic, show interaction with living cells and, with few exceptions, have low costs in comparison with others biopolymers such as collagen [13] and [14]. These polysaccharidic polymers have been widely proposed as scaffold materials in tissue engineering applications as well as carriers for drug delivery systems as described in more detail in the following sections.
Collagen:Collagenis regarded by many as an ideal scaffold or matrix for tissue engineering as it is the major protein component of the extracellular matrix, providing support to connective tissues such as skin, tendons, bones, cartilage, blood vessels, and ligaments[6], [7], [8], [9] and [10]. In its native environment, collagen interacts with cells in connective tissues and transduces essential signals for the regulation of cell anchorage, migration, proliferation, differentiation, and survival. [11]Twenty-seven types of collagens have been identified to date, but collagen type I is the most abundant and the most investigated for biomedical applications. The different collagens are first synthesized as large precursor molecules known as procollagens [15]. After secretion of procollagen into the extracellular matrix, both C- and N-propeptides are cleaved and the molecules then self-assemble into fibrils (a detailed review can be found in [11]. Fibril-forming collagen molecules used in tissue engineering applications consist of three polypeptide chains of glycine-X-Y (Gly-X-Y) amino acid repeats twined around one another to form triple helices [16] and [17].
Gelatin: Gelatin is a natural polymer that is derived from collagen, and is commonly used for pharmaceutical and medical applications because of its biodegradability and biocompatibility in physiological environments as reviewed by Tabata and Mikos [18]and [19]. These characteristics have contributed to gelatin's safety as a component in drug formulations or as a sealant for vascular prostheses. [20]Moreover, gelatin has relatively low antigenicity because of being denatured in contrast to collagen which is known to have antigenicity due to its animal origin. Gelatin contains a large number of glycine, proline and 4-hydroxyproline residues.
Gelatin is a denatured protein obtained by acid and alkaline processing of collagen. As a result, two different types of gelatin can be produced depending on the method in which collagen is pre-treated, prior to the extraction process [20]. This pre-treatment affects also the electrical nature of collagen, producing gelatin with different isoelectric points. The alkaline process targets the amide groups of asparagine and glutamine and hydrolyses them into carboxyl groups, thus converting many of these residues to aspartate and glutamate. In contrast, acidic pre-treatment does little to affect the amide groups present. The result is that gelatin processed with an alkaline pre-treatment is electrically different from acidic-processed gelatin. This is due to hydrolysis of amide groups of collagen yields gelatin with a higher density of carboxyl groups present in the alkaline processed gelatin rendering it negatively charge and lowering its isoelectric point [18]. In contrast, the electrostatic nature of collagen is hardly modified through the acid process because of a less invasive reaction to amide groups of collagen. As a result, the isoelectric point of gelatin that is obtained with the acid process will remain similar to that of collagen [19]. By utilizing this technique, manufacturers now offer gelatin in a variety.
Silk fibroin: Silk is generally defined as protein polymers that are spun into fibers by some lepidoptera larvae such as silkworms, spiders, scorpions, mites and flies [21]. Spider silk is an intriguing biomaterial that is lightweight, extremely strong and elastic, and exhibits mechanical properties comparable to the best synthetic fibers produced by modern technology[22]. Spider silk is spun near ambient temperatures and pressures using water as the solvent, which gives rise to an environmentally safe, biodegradable material [22]. However, it is not possible to maintain domesticated spiders to produce massive amounts of silk. Therefore, the attention was turned to silk fibroin, a mass-producible natural polymer produced by silkworms, commonly used as a textile fiber. In the medical field, silk has long been used for surgical sutures [23].
Fibrin: Fibrin and fibrinogen have a well-established application in research in tissue engineering due to their innate ability to induce improved cellular interaction and subsequent scaffold remodelling compared to synthetic scaffolds. Furthermore, due to its biochemical characteristics, mainly in cellular interactions, fibrin-based materials also found applications in the field of drug delivery with special focus in cell delivery.
Chitosan: Chitosan is a cationic polymer obtained from chitin comprising copolymers of β(1→4)-glucosamine and N-acetyl-d-glucosamine. Chitin is a natural polysaccharide found particularly in the shell of crustacean, cuticles of insects and cell walls of fungi and is the second most abundant polymerized carbon found in nature. Chitosan, the fully or partially deacetylated form of chitin, due to its properties as attracted much attention in the tissue engineering and drug delivery fields with a wide variety of applications ranging from skin, bone, cartilage and vascular grafts to substrates for mammalian cell culture. It has been proved to be biologically renewable, biodegradable, biocompatible, non-antigenic, non-toxic and biofunctional[24].
Starch: Starch is one of the most promising natural polymers because of its inherent biodegradability, overwhelming abundance and renewability. It is composed of a mixture of glycans that plants synthesize and deposited in the chloroplasts as their principal food reserve. Starch is stored as insoluble granules composed of α-amylose (20–30%) and amylopectin (70–80%)[25]. α-Amylose is a linear polymer of several thousands of glucose residues linked by α(1→4) bonds. The α-glycosidic bonds of α-amylose cause it to adopt an helical conformation (left-handed helix) [25]. Amylopectin consists mainly of α(1→4)-linked glucose residues but it is a branched molecule with α(1→6) branch points every 24 to 30 glucose residues in average.
Amylopectin molecules contain up to 106 glucose residues, making them some of the largest molecules in nature [25]. Starch by itself is extremely difficult to process and is brittle when used without the addition of a plasticizer. In most applications, the semi-crystalline native starch granule structure is either destroyed or reorganized, or both [26]. Water is the usual plasticizer in starch processing, and the physical properties of starch are greatly influenced by the amount of water present [26]. Therefore, the use of other plasticizers, such as low molecular weight alcohols, especially for the production of thermoplastic starches, renders starch more processable [26]. Additionally, blending two or more chemically and physically dissimilar natural polymers has shown potential to overcome these difficulties. Over the years several materials have been blended with starch to improve its processability, including, but not restricted to, several synthetic polymers, such as polyethylene [27], polycaprolactone[28].
Alginate: Alginate is one of the most studied and applied polysaccharidic polymers in tissue engineering and drug delivery field. They are abundant in nature and are found as structural components of marine brown algae and as capsular polysaccharides in some soil bacteria. Commercial alginates are extracted from three species of brown algae. These include Laminaria hyperborean, Ascophyllum nodosum, and Macrocystis pyrifera in which alginate comprises up to 40% of the dry weight [29]. Bacterial alginates have also been isolated from Azotobacter vinelandii and several Pseudomonas species [29]. Alginates are naturally derived polysaccharide block copolymers composed of regions of sequential β-d-mannuronic acid monomers (M-blocks), regions of α-l-guluronic acid (G-blocks), and regions of interspersed M and G units [30]. The length of the M- and G-blocks and sequential distribution along the polymer chain varies depending on the source of the alginate. Alginates undergo reversible gelation in aqueous solution under mild conditions through interaction with divalent-cations such as Ca2+ that can cooperatively bind between the G-blocks of adjacent alginate chains creating ionic inter-chain bridges. This gentle property has led to their wide use as cell transplantation vehicles to grow new tissues and as wound dressings. Moreover, alginate as an anionic polymer with carboxyl end groups is a good mucoadhesive agent [29].
Dextran: Dextran is a branched, high molecular weight polymer of d-glucose, produced by different bacterial strains from sucrose via the action of dextransucrase enzyme [31], consisting of α(1→6)-linked d-glucose residues with some degree of branching via α(1→3) linkages. Dextran is readily available in a wide range of molecular weights along with several derivatives and it is biodegradable and biocompatible. These properties make it suitable for a whole range of applications, such as plasma-expanders and blood substitutes, since it binds to erythrocytes, platelets and vascular endothelium by reducing their aggregation and adhesiveness, respectively. Additionally, it has also been shown to be a bone healing promoter and also for dermal and subcutaneous augmentation and for drug delivery [13].
Polyhydroxyalkanoates: In nature, a special group of polyesters is produced by a wide variety of microorganisms as an internal carbon and energy storage, as part of their survival mechanism Poly(β-hydroxybutyrate) (PHB) was first mentioned in the scientific literature as early as 1901.
[adsense:468x15:2204050025]
Advantages of natural polymers:
The various advantages of natural plant base materials include:
1. Biodegradable:Biodegradable is the naturally available; they are produced byall living organisms.
2. Biocompatible and non-toxic: Basically, all of these plant materials are repeating sugar polysaccharides.
3. Low cost: cheaper to use as natural sources. the production cost is less compared with the synthetic material. In India and many other developing countries are dependent on agriculture and they are large amount of money investment on agricultures.
4. Environmental-friendly processing: There are many types of natural compounds obtained from different plant sources which are widely used in pharmaceutical industry and collected in large quantities due to the simple production processes involved.
5. Local availability (especially in developing countries): In India and similar developing countries, there is promotion for the production of plants as pharmaceutical excipients being done by government and it also provide the facilities for bulk production, like gum and mucilages because of there wide applications in industries.
6. They have better patient tolerance as well as public acceptance: There is less chance of side and adverse effects with natural materials compared with synthetic one. For example, povidone.
Disadvantages of natural polymer
1. Often antigenestic or rejection system.
2. Prediction at degradation rate is difficult.
3. Poor strength due to water absorption.
4. Questionable purity and high cost.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Tablet
The tablet is the widely used dosage form because of its convenience in terms of self-administration, low cost and ease in formulation. However, geriatric and pediatric patients experience difficulty in swallowing conventional tablets, which leads to poor patient compliance. “Mouth dissolve (MD)” tablets are novel types of tablets that disintegrate/ dissolve/disperse in saliva within 15 to 60 s, without the need of water. This characteristic advantage leads to their suitability for geriatric and pediatric patients at anytime, anywhere. The benefits, in terms of patient compliance, rapid onset of action, increased bioavailability, and good stability make these tablets popular as a dosage form of choice in the current market. Different technological techniques, such as freeze drying, moulding, direct compression, are currently employed to prepare the formulations of this type present in the pharmaceutical market. Beta vulgaris (Chenopodiaceae) is an important plant found in India. Pulp powder of this plant was used to prepare fast dispersible tablet. This type of natural plants plays an important role as pharmaceutical excipient. These are easily available, biodegradable and having low cost. Bio compatibility of these natural polymers promotes their use as in pharmaceutical formulations.
Present work used direct compression technique to prepare tablets. In present study Diclofenac sodium, a non-steroidal anti-inflammatory drug was selected as model drug. It is an acetic acid nonsteroidal antiinflammatory drug (NSAID) with analgesic and antipyretic properties. Diclofenac sodium is used to treat pain, dysmenorrhea, ocular inflammation, osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, and actinic keratosis.
Extraction and Characterization of Polymer
Preparation of Beta vulgaris pulp powder:
Beta vulgariswas purchased from local market of Meerut (Uttar Pradesh) India. The fruit was clean with water to remove dust from surface and further peel was removed. Pulp was cut into small pieces and put into grinder to form paste. This was further lyophilized to get solid porous mass. Size reduction was done and powder was collected. The collected powder was passed through 80 # sieve and stored in the air tight container for further study.[1]
Profile of Diclofenac[32]
Generic name: Diclofenac Sodium, Misoprostol
Brand Names: Voltaren, Cataflam, Voltaren-XR
Drug Type:
· Small Molecule
· Approved
IUPAC Name: 2-(2-(2,6-dichlorophenylamino)phenyl)acetic acid
Chemical Formula: C14H11Cl2NO2
Chemical Structure
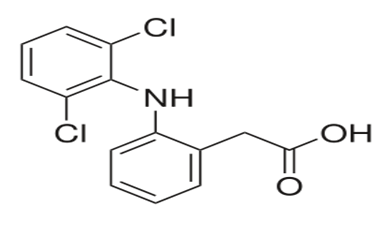
File:Diclofenac.svg
Average Molecular Weight: 296.148 g/mol
Protein Binding:99%
Melting Point: 280°C
Solubility: Soluble in water
Drug Category: Anti-inflammatory, pain relief
Indication:Diclofenac is used for musculoskeletal complaints, especially arthritis, rheumatoid arthritis, polymyositis, dermatomyositis, osteoarthritis, dental pain, TMJ, spondylarthritis, ankylosing spondylitis, gout attacks,[9]and pain managementin cases of kidney stones and gallstones. An additional indication is the treatment of acute migraines. Diclofenac is used commonly to treat mild to moderate post-operative or post-traumatic pain, particularly when inflammation is also present,[9] and is effective against menstrual pain and endometriosis.
Diclofenac suppositories:As long-term use of diclofenac and similar NSAIDs predisposes for peptic ulcer, many patients at risk for this complication are prescribed a combination (Arthrotec) of diclofenac and misoprostol, a synthetic prostaglandin analogue, to protect the gastric mucosa. An external, gel-based formulation containing 3% of diclofenac (Solaraze) is available for thetreatment of facial actinic keratosiswhich is caused by over-exposure to sunlight. Some countries have also approved the external use of diclofenac 1% gel to treat musculoskeletal conditions.Over-the-counter use against minor aches and pains and fever associated with common infections is also licensed in some countries, such as Australia and New Zealand. In many countries eye-drops are sold to treat acute and chronic non-bacterial inflammations of the anterior part of the eyes (e.g. postoperative states). A common brand name is Voltaren-ophta.
Mechanism of Action:
The exact mechanism of action is not entirely known, but it is thought that the primary mechanism responsible for its anti-inflammatory, antipyretic, and analgesic action is inhibition of prostaglandinsynthesis by inhibition of cyclooxygenase(COX). It also appears to exhibit bacteriostatic activity by inhibiting bacterial DNA synthesis.[7]Inhibition of COX also decreases prostaglandinsin the epithelium of the stomach, making it more sensitive to corrosion by gastric acid. This is also the main side effect of diclofenac. Diclofenac has a low to moderate preference to block the COX2-isoenzyme (approximately 10-fold) and is said to have therefore a somewhat lower incidence of gastrointestinal complaints than noted with indomethacin and aspirin. The action of one single dose is much longer (6 to 8 hours) than the very short half-life that the drug indicates. This could be partly because it persists for over 11 hours in synovial fluids. Diclofenac may also be a unique member of the NSAIDs. There is some evidence that diclofenac inhibits the lipoxygenase thus reducing formation of the leukotrienes(also pro-inflammatory autacoids). There is also speculationthat diclofenac may inhibit phospholipase A2as part of its mechanism of action. These additional actions may explain the high potency of diclofenac it is the most potent NSAID on a broad basis. Pharmacology of Diclofenac Sodium. Am J of Medicine Volume 80 April 28, 1986There are marked differences among NSAIDs in their selective inhibition of the two subtypes of cyclo-oxygenase, COX-1and COX-2. Much pharmaceutical drug design has attempted to focus on selective COX-2 inhibition as a way to minimize the gastrointestinal side effects of NSAIDs like aspirin. In practice, use of some COX-2 inhibitorswith their adverse effects has led to massive numbers of patient family lawsuits alleging wrongful death by heart attack, yet other significantly COX-selective NSAIDs such as diclofenac have been well-tolerated by most of the population
Biotransformation: Liver
Half Life: 1-2 hour
Contraindication:
Hypersensitivity against diclofenac
History of allergic reactions (bronchospasm, shock, rhinitis, urticaria) following the use of Aspirin or another NSAID
Third-trimester pregnancy
Active stomach and/or duodenal ulceration or gastrointestinal bleeding
Inflammative intestinal disorders such as Crohn's disease or ulcerative colitis
Severe insufficiency of the heart (NYHA III/IV)
Recently, a warning has been issued by FDA not to use to treat patients recovering from heart surgery
Severe liver insufficiency (Child-Pugh Class C)
Severe renal insufficiency (creatinine clearance <30 ml/min)
Caution in patients with preexisting hepatic porphyria, as diclofenac may trigger attacks
Caution in patients with severe, active bleeding such as cerebral hemorrhage
NSAIDs in general should be avoided during dengue fever.
Investigationaluses: Diclofenac is often used to treat chronic pain associated with cancer, particularly if inflammation is also present
Ø Fever due to malignant lymphogranulomatosis (Hodgkin's lymphoma) often responds to diclofenac. Treatment can be terminated as soon as the usual treatment with radiation and/or chemotherapy causes remission of fever.
Ø Diclofenac may prevent the development of Alzheimer's disease if given daily in small doses during many years.
Ø Diclofenac has been found to increase the blood pressure in patients with Shy-Drager syndrome and Diabetes Mellitus
Ø .Diclofenac has been found to be effective against all strains of multi drug resistant E. coli.
Ø Diclofenac has the capacity to treat uncomplicated urinary tract infections (UTI) caused by E. coli.[10]
Ø Effective In treating Salmonella infections in mice[11] and is under investigation for the treatment of tuberculosis[12]
Ø Diclofenac is an antiuricosuric
Side effects:
Cardiac
• Following the identification of increased risks of heart attacks with the selective COX-2 inhibitor rofecoxib in 2004, attention has focused on all the other members of the NSAIDs group, including diclofenac. Research results are mixed with a meta-analysis of papers and reports up to April 2006 suggesting a relative increased rate of heart disease of 1.63 compared to non-users.[14] Professor Peter Weissberg, Medical Director of the British Heart Foundation said, "However, the increased risk is small, and many patients with chronic debilitating pain may well feel that this small risk is worth taking to relieve their symptoms". Only Aspirin was found not to increase the risk of heart disease; however, this is known to have a higher rate of gastric ulceration than diclofenac.
A subsequent large study of 74,838 users of NSAIDs or coxibs, published in May 2006, found no additional cardiovascular risk from diclofenac use.[15] A very large study of 1,028,437 Danish users of various NSAIDs or coxibs, published online on June 8, 2010, found that "Use of the nonselective NSAID diclofenac and the selective cyclooxygenase-2 inhibitor rofecoxib was associated with an increased risk of cardiovascular death (odds ratio, 1.91; 95% confidence interval, 1.62 to 2.42; and odds ratio, 1.66; 95% confidence interval, 1.06 to 2.59 respectively), with a dose-dependent increase in risk." .[16]
• Diclofenac has similar COX-2 selectivity to celecoxib.[17] Perhaps related to this selectivity, a review of this constantly-changing topic by FDA Medical Officer David Graham concluded in September, 2006 that diclofenac does increase the risk of myocardial infarction.[18]
Gastrointestinal
• Gastrointestinal complaints are most often noted (see above). The development of ulceration and/or bleeding requires immediate termination of treatment with diclofenac. Most patients receive an ulcer-protective drug as prophylaxis during long-term treatment (misoprostol, ranitidine 150 mg at bedtime or omeprazole 20 mg at bedtime).
Hepatic
• Liver damage occurs infrequently, and is usually reversible. Hepatitis may occur rarely without any warning symptoms and may be fatal. Patients with osteoarthritis more often develop symptomatic liver disease than patients with rheumatoid arthritis. Liver function should be monitored regularly during long-term treatment. If used for the short term treatment of pain or fever, diclofenac has not been found to be more hepatotoxic than other NSAIDs.
• As of 12/2009 Endo, Novartis and FDA notified healthcare professionals to add new warnings and precautions about the potential for elevation in liver function tests during treatment with all products containing diclofenac sodium.[19]
• Cases of drug-induced hepatotoxicity have been reported in the first month, but can occur at any time during treatment with diclofenac. Postmarketing surveillance has reported cases of severe hepatic reactions, including liver necrosis, jaundice, fulminant hepatitis with and without jaundice, and liver failure. Some of these reported cases resulted in fatalities or liver transplantation.
• Physicians should measure transaminases periodically in patients receiving long-term therapy with diclofenac. Based on clinical trial data and postmarketing experiences, transaminases should be monitored within 4 to 8 weeks after initiating treatment with diclofenac.
Renal
• Studies in Pakistan showed that diclofenac caused acute kidney failure in vultures when they ate the carcasses of animals that had recently been treated with it (see below at Ecological problems). Species and individual humans that are drug sensitive are initially assumed to lack genes expressing specific drug detoxification enzymes.
• NSAIDs "are associated with adverse renal [kidney] effects caused by the reduction in synthesis of renal prostaglandins"[20] in sensitive persons or animal species, and potentially during long term use in non-sensitive persons if resistance to side effects decreases with age. Unfortunately this side effect can't be avoided merely by using a COX-2 selective inhibitor because, "Both isoforms of COX, COX-1 and COX-2, are expressed in the kidney... Consequently, the same precautions regarding renal risk that are followed for nonselective NSAIDs should be used when selective COX-2 inhibitors are administered."[20] However, diclofenac appears to have a different mechanism of renal toxicity.[13]
Other
• Bone marrow depression is noted infrequently (leukopenia, agranulocytosis, thrombopenia with/without purpura, aplastic anemia). These conditions may be life-threatening and/or irreversible, if detected too late. All patients should be monitored closely. Diclofenac is a weak and reversible inhibitor of thrombocytic aggregation needed for normal coagulation.
• Induces warm antibody hemolytic anemia by inducing antibodies to Rh antigens, Ibuprofen also does this.[21]
• Diclofenac may disrupt the normal menstrual cycle
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Lactose: Lactose is a carbohydrate, and as such a disaccharide. One Molecule of lactose consists of one molecule each of two other carbohydrates, i.e. galactose and glucose. These galactose and glucose moieties, as they are called, are linked together by means of what is known as a beta-(1,4) glucosidic linkage. The molecular structure of lactose is depicted below.
The official chemical name of lactose, as frequently encountered in regulatory documents such as the Pharmacopoeia is:
4-O-β-D-galactopyranosyl, D-glucopyranose.

A number of natural or partially modified polymers was screened for mucoadhesive properties by routinely measuring the force of detachment for swollen polymer films from pig intestinal mucosa in a saline medium. Suprisingly, hydroxypropyl- and carboxymethylcellulose showed almost no mucoadhesion, whereas the cationic polymer chitosan was fairly mucoadhesive in comparison to Polycarbophil as a reference substance. It is suggested that a strict difference be made between mucoadhesion of dry polymers on a wet tissue in air, and mucoadhesion of a swollen hydrogel in the presence of a third liquid phase. Cationic polymers should be further investigated with respect to possibly improved mucoadhesive properties in a neutral or slightly alkaline environment.Lactose is very stable from a chemical point of view. Except for some special cases, it has no tendency to react with the active ingredient or other components of a formulation. Some remarks on the chemical properties of lactose are useful, however
The low hygroscopicity of lactose supports its virtual chemical inertness. Most chemical reactions of lactose occur noticeably only in aqueous environments. Because lactose has no tendency to attract moisture, water in dry lactose preparations is normally not present in amounts sufficient for chemical reactions to proceed at a noticeable speed. The water of crystallisation is bound so tightly in the crystal lattice of the lactose that it is chemically inert.
α / β-isomer: In milk, lactose is present in two isomeric forms called α- and β-lactose. The molecular structures of α- and β-lactose differ in the orientation of a hydrogen and a hydroxyl group on carbon atom no. 1 in the glucose moiety. Both forms change into one another continuously. This phenomenon is called mutarotation. The velocity of mutarotation is determined by factors such as temperature, concentration, and pH (acidity) of the solution.
Lactose solutions seek a state of equilibrium between α and β form. At room temperature, the equilibrium results in a ratio of about 40% α-lactose and 60% β-lactose. The fact that two forms of lactose exist that differs in molecular structure has profound effects on various properties of lactose, such as its solid state properties, crystal morphology and solubility. [33-35]
List of Materials Used
|
Materials |
Suppliers |
|
Polyvinyl Pyrrolidone K30 |
Central Drug House, New Delhi |
|
Magnesium stearate |
Central Drug House, New Delhi |
|
Talc |
Central Drug House, New Delhi |
|
Lactose |
Central Drug House, New Delhi |
|
Sodium hydroxide |
Central Drug House, New Delhi |
|
Hydrochloric acid |
Central Drug House, New Delhi |
List of Equipments used
|
Equipment |
Model, Manufacturer & Country |
|
UV-Visible Spectrophotometer |
Pharmaspec-1700, Shimadzu, Japan |
|
FTIR- Spectrophotometer |
8400s, Shimadzu, Japan |
|
Dissolution Apparatus |
Lab India Disso Test Appartus, India |
|
Magnetic Starrier |
5MLH DX, Remi, India |
|
pH meter |
SE946-P, Systronics |
|
Electric Oven |
Ambassadar® Laboratory Electric Oven, New Delhi, India |
|
Friability Test Apparatus |
Electrolab- EF-2 Friability (USP) |
|
Hardness Tester |
Model:EL=500N, Electrolab |
Table: Formulations of matrix tablet: [36]
|
Excipient |
Formulation |
||
|
Batch 1 |
Batch 2 |
Batch 3 |
|
|
Drug |
500mg |
500mg |
500mg |
|
Polymer |
41.2mg |
82.4mg |
123.6mg |
|
Lactose |
1500mg |
1417.6mg |
1376.4mg |
|
Polyvinyl Pyrrolidone |
50mg |
50mg |
50mg |
|
Talc |
10mg |
10mg |
10mg |
PHYSIOCHEMICAL PROPERTIES:
Bulk density: Apparent bulk density (g/ml) was determined by placing pre-sieved bulk powder blend into a graduated cylinder via a large cylinder and measuring the volume and weight of powder blend.
Bulk density =weight of powder blend / volume of powder blend
Tapped density: It was determined by placing a graduated cylinder, containing a known mass of powder on mechanical tapping apparatus, which was operated for fixed number of taps (around 50). Using the weight of powder in a cylinder and its tapped volume, the tapped density was computed.
Tapped density =weight of powder blend/ tapped volume of powder blend
Carr’s index: It is an important parameter to study compressibility behavior of powder blend. Carr’s index was calculated, from the results of bulk density and tapped density.
Carr’s index = (bulk density-tapped density)/ tapped density
Bulkiness: It is reciprocal of bulk density, and calculated as follows-
Bulkiness= 1/bulk density
Angle of repose: For the measurement of angle of repose, a glass funnel was taken with its tip at a given height (H), above a piece of graph paper placed on a horizontal surface. Powder was poured through the funnel until the apex of the conical pile touched the tip of the funnel. The angle of repose was calculated with the formula; tan θ= H/R, where θ is the angle of repose and R is the radius of the conical pile.
Swelling index: The swelling index is defined as the volume (in milliliters) taken up by the swelling of 1 g of powder material under specified conditions. 1 gm of the pulp powder was introduced into a 25 ml glass-stoppered measuring cylinder. Twenty five milliliters of water was added and mixture was shaken thoroughly for 10 min. It was then allowed to stand for 24 h at room temperature. Then the volume occupied by the pulp powder was noted.
Weight variation: All prepared matrix tablets were evaluated for weight variation as per USP XXIV monograph. Twenty tablets of each batch were used to evaluate weight variation among tablets and standarddeviation was calculated.
Friability: Tablets of all batches were used to evaluate friability as per USP XXIV monograph. Friability testing was done by Roche friabilator with triplicate readings.
Hardness: Hardness of all batches was determined using Digital Force Gauge (Model:EL=500, Electrolab). The test was carried out in triplicate for all batches as per USP XXIV monograph for uncoated tablets.
Thickness: Thickness was measured by vernier caliper as per USP XXIV monograph. The readings were carried out in triplicate and average value was noted.
Drug content: The tablets were powdered, and 50 mg equivalent weight of Diclofenac Sodium in tablet powder was accurately weighted and transferred into a 100 ml volumetric flask. Initially, 10 ml of phosphate buffer (pH6.6) was added and shaken for 10 min. then, the volume was made up to 100 ml with buffer. Subsequently, the solution in volumetric flask was filtered and 1 ml of the filtrate was diluted and analyzed at 276 nm using ultraviolet/visible variable wavelength spectrophotometer at 276 nm (Shimadzu UV-2450, Japan). The drug content of the each sample was estimated from their standard curve.
In vitro dissolution study: Dissolution test was performed at 37°C using the paddle method at 100 rpm with 900phosphatebuffer (pH6.6) as a dissolutionIndia Disso 2000, India) was used. At predetermined intervals, 5 ml of the medium was sampled and filtered. The filtrate was analyzed by ultraviolet/visible variable wavelength spectrophotometer at 276 nm.

Charcterization parameters of cucurbita maxima pulp powder
Beta vulgarispulp powder was characterized as a pharmaceutical excipient in terms of micromeritic properties and flow behavior. Bulk density, tapped density, bulkiness and angle of repose all are found to be good to use this plant based material as a pharmaceutical excipient. Bulk density, tapped density, cars index, hausner’s ratio porosity and flow behavior (angle of repose) were found to be 0.51to.052, 0.52 to 0.053 ,0.84 to 0.045, 1.05 to 0.076,0.052 t0 0.02, 38.65 to 0.089 respectively .his pulp powder can be act as a good candidate for pharmaceutical preparations . Relative study of physical parameters of tablets of each batch of Beta vulgaris pulp powder reveals that the tablets compressed using pulp powder as disintegrant are quite harder, so can be easily handled. The variation in the hardness, weight variation, friability and thickness values of all the fabricated tablets were found to be 21.8 to 0.06, 211 to 0.03,0.62 to 0.82%,2.46 to 0.06 respectively in reference to average values for each parameter, were found within the official limits. Friability of tablets ranged from 0.62 to 0.82%, easily predict the fact that tablets were less friable and so provide ease of handling. Less weight variation and uniform drug content easily elicit the fact that this process of tablet formulation is reproducible and so easily adopted at industrial level. Findings of the results showed that as the concentration of pulp powder increases wetting time of tablets decreases in same proportion and so disintegrating time also go down in same manner.
|
Bulk density (mg/ml)
|
Tapped density (mg/ml)
|
Carr’s Index |
Hausner’s ratio |
Porosity |
Bulkiness (ml/mg)
|
Angle of repose (º) |
|
0.51 ±0.052 |
0.52 ±0.053 |
0.84 ±0.045 |
1.05 ±0.076 |
0.52 ±0.02 |
1.99 ±0.08 |
38.65 ±0.089 |
Evaluation parameters of tablet containing Diclofenac sodium as a model
|
Evaluation |
Batch1 |
Batch2 |
Batch3 |
|
Hardness(kg/cm^2) |
21.8 ±0.065 |
20.6 ±0.088 |
20.1 ±0.067 |
|
Friability (%) |
0.67 ±0.065 |
0.59 ±0.054 |
0.68 ±0.044 |
|
Thickness (mm) |
1.65 ±0.078 |
2.16 ±0.065 |
2.460 ±0.054 |
|
Diameter (mm) |
9.24 ±0.065 |
9.52 ±0.023 |
9.51 ±0.045 |
|
Disintegration (min) |
10.2 ±0.023 |
7.0 ±0.021 |
6.0 ±0.019 |
|
Weight Variation (mg) |
211 ± 0.067 |
210 ± 0.059 |
208 ±0.054 |
CONCLUSION
The comparative study of various parameters clearly states the fact that the naturally obtained Beta vulgaris pulp powder stands as a good candidate to act as disintegrant and it is possible to design promising Fast disintegrating tablet using this polymer. On the basis of results obtained it can be concluded that this polymer having good micromeritic properties and flow behavior and so may act as a pharmaceutical excipient.
REFERENCE:
1 J.C. Rodriguez-Cabello, J. Reguera, A. Girotti, M. Alonso and A.M. Testera, Developing functionality in elastin-like polymers by increasing their molecular complexity: the power of the genetic engineering approach, Prog. Polym. Sci. 30 (2005), pp. 1119–1145.
2.X.D. Guo, Q.X. Zheng, J.Y. Du, S.H. Yang, H. Wang, Z.W. Shao and E.J. Sun, Molecular tissue engineering: concepts, status and challenge, J. Wuhan Univ. Technol. 17 (2002), pp. 30–34.
3. H. Uludag, P. De Vos and P.A. Tresco, Technology of mammalian cell encapsulation, Adv. Drug Deliv. Rev. 42 (2000), pp. 29–64.
4. A. Chilkoti, T. Christensen and J.A. MacKay, Stimulus responsive elastin biopolymers: applications in medicine and biotechnology, Curr. Opin. Chem. Biol. 10 (2006), pp. 652–657.
5. A. Patel, B. Fine, M. Sandig and K. Mequanint, Elastin biosynthesis: the missing link in tissue-engineered blood vessels, Cardiovasc. Res. 71 (2006), pp. 40–49.
6. B. Chevallay and D. Herbage, Collagen-based biomaterials as 3D scaffolds for cell cultures: application for tissue engineering and gene therapy, Med. Biol. Eng. Comput. 38 (2000), pp. 211–218.
7. D.R. Eyre, Collagen: molecular diversity in the body's protein scaffold, Science 207 (1980), pp. 1315–1322.
8. P.D. Kemp, Tissue engineering and cell-populated collagen matrices, Methods Mol. Biol. 139 (2000), pp. 287–293.
9. C.H. Lee, A. Singla and Y. Lee, Biomedical applications of collagen, Int. J. Pharmacogn. 221 (2001), pp. 1–22
10. C. Wong Po Foo and D.L. Kaplan, Genetic engineering of fibrous proteins: spider dragline silk and collagen, Adv. Drug Deliv. Rev. 54 (2002), pp. 1131–1143.
11. Y. Chunlin, P.J. Hillas, J.A. Buez, M. Nokelainen, J. Balan, J. Tang, R. Spiro and J.W. Polarek, The application of recombinant human collagen in tissue engineering, BioDrugs 18 (2004), pp. 103–119.
12.. K. Nishinari and R. Takahashi, Interaction in polysaccharide solutions and gels, Curr. Opin. Colloid Interface Sci. 8 (2003), pp. 396–400.
13. M.G. Cascone, N. Barbani, C. Cristallini, P. Giusti, G. Ciardelli and L. Lazzeri, Bioartificial polymeric materials based on polysaccharides, J. Biomater. Sci., Polym. Ed. 12 (2001), pp. 267–281.
14. J. Venugopal and S. Ramakrishna, Applications of polymer nanofibers in biomedicine and biotechnology, Appl. Biochem. Biotechnol. 125 (2)
15. K.I. Kivirikko, Collagen biosynthesis: a mini-review cluster, Matrix Biol. 16 (1998), pp. 35-37.
16. R. Berisio, L. Vitagliano, L. Mazzarella and A. Zagari, Recent progress on collagen triple helix structure, stability and assembly, Prot. Pept. Lett. 9 (2002), pp. 107–116.
17. B. Brodsky and J.A. Ramshaw, The collagen triple-helix structure, Matrix Biol. 15 (1997), pp. 545–554.
18. Y. Tabata and Y. Ikada, Protein release from gelatin matrices, Adv. Drug Deliv. Rev. 31 (1998), pp. 287–301.
19. S. Young, M. Wong, Y. Tabata and A.G. Mikos, Gelatin as a delivery vehicle for the controlled release of bioactive molecules, J. Control. Release 109 (2005), pp. 256–274.
20. K.B. Djagny, Z. Wang and S. Xu, Gelatin: a valuable protein for food and pharmaceutical
industries: review, Crit. Rev. Food Sci. Nutr. 41 (2001), pp. 481–492.
21. G.H. Altman, F. Diaz, C. Jakuba, T. Calabro, R.L. Horan, J. Chen, H. Lu, J. Richmond and D.L. Kaplan, Silk-based biomaterials, Biomaterials 24 (2003), pp. 401–416.
22. M.B. Hinman, J.A. Jones and R.V. Lewis, Synthetic spider silk: a modular fiber, Trends Biotech. 18 (2000), pp. 374–379.
23. Y. Tamada, New process to form a silk fibroin porous3-D structure, Biomacromolecules 6 (2005), pp. 3100–3106.
24. E. Khor and L.Y. Lim Implantabl applications of chitin and chitosan, Biomaterials 24 (2003), pp. 2339–2349.
25. W.R. Morrison and J. Karkalas, Starch. In: P.M. Dey, Editor, Methods in Plant Biochemistry: Carbohydrates vol. 2, Academic Press Limited, London (1990), pp. 323–352
26. K. Poutanen and P. Forssell, Modification of starch properties with plasticizers, Trends Polym. Sci. 4 (1996), pp. 128–132.
27. P.A. Dell and W.G. Kohlman, Effects of water-content on the properties of starch poly(ethylene vinyl alcohol) blends, J. Appl. Polym. Sci. 52 (1994), pp. 353–363.
28. C. Bastioli, A. Cerutti, I. Guanella, G.C. Romano and M. Tosin, Physical state and biodegradation behavior of starch–polycaprolactone systems, J. Environ. Polym. Degrad. 3 (1995), pp. 81–95.
29. M. George and T.E. Abraham, Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan — a review, J. Control. Release 114 (2006), pp. 1–14.
30. M.M. Stevens, H.F. Qanadilo, R. Langer and V. Prasad Shastri, A rapid-curing alginate gel system: utility in periosteum-derived cartilage tissue engineering, Biomaterials 25 (2004), pp. 887–894.
31. I.L. Jung, K.H. Phyo, K.C. Kim, H.K. Park and I.G. Kim, Spontaneous liberation of intracellular polyhydroxybutyrate granules in Escherichia coli, Res. Microbiol. 156 (2005), pp. 865-870
32. Sujja-areevath J.Munday D L ,Cox P J,KhanKa,Release Characterstics of Diclofenac Sodium for Encapsulated
33. Shangraw, R. F: Compressed Tablets by Direct Compression Granulation Pharmaceutical Dosage Forms: Tablets, Vol-1, 2nd ed. Marcel Dekker, USA, 1989. p.195-246.
34.Shangraw , R. F: Direct Compression Tableting: Encyclopedia of Pharmaceutical Technology, Vo-4, 2nd ed. Marcel Dekker, USA, 1988. p.85-160.
35. Reimerdes, D: The Near Future of Tablet Excipients, Manuf. Chem., 1993; 64:14-15.
36. Abraham B ,Alpsien M , Bake B Lawson A, Sjogren J.In vitro & in vivo erosion of 2 different Hydrophilic Gel matrix tablet, Eur J pharma Biopharm 1998,46,69-75
37. Malviya Rishabh Pranati ,Bbansal Mayank, Sharma P.K preparation &Evaluation of Disintegrating properties of cucurbita Pulp powder, Integration Journal of Pharmaceutical Science.(accepted manuscript (JP-09,119)2010.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









