 About Authors:
About Authors:
YASWANTH ALLAMNENI*, P DAYANANDA CHARY, S CHAITANYA KUMAR, VENKATA BALAKRISHNA RAO N
Research and Development Department,
Natco Pharma Limited,
Kothur, Mahaboobnagar,
Andhra Pradesh – 509228.
Abstract:
The main objective of this study is to provide the clear procedure for technology transfer process in pharmaceutical industry. Technology transfer is a process to transfer information and technologies necessary to manufacture quality drug product consistently or technology transfer is the process of taking an invention from its inception in a laboratory to a commercialized product. Investment in R&D is a necessary but not a sufficient condition for economic growth. Productivity gains only result from the natural diffusion of innovation to the marketplace (technology transfer). Responsible departments for successful technology transfer of a product in pharmaceutical industry are R&D, Production, Engineering, QC and QA.
[adsense:336x280:8701650588]
Reference ID: PHARMATUTOR-ART-1252
Introduction:
In the pharmaceutical industry, “technology transfer” refers to the processes that are needed for successful progress from drug discovery to product development to clinical trials to full-scale commercialization or it is the process by which a developer of technology makes its technology available to commercial partner that will exploit the technology.
The importances of technology transfer are:
* To elucidate necessary information to transfer technology from R&D to actual manufacturing by sorting out various information obtained during R&D.
* To elucidate necessary information to transfer technology of existing products between various manufacturing places.
* To exemplify specific procedures and points of concern for the two types of technology transfer in the above to contribute to smooth technology transfer. This is applicable to the technology transfer through R&D and production of drug (chemically synthesized drug substances and drug products) and the technology transfer related to post-marketing changes in manufacturing places.
Various stages of formulation development were as follows
- Preformulation studies.
- Bench scale - (1/1000th of X)
- Lab scale – (1/100th of X)
- Scale up- (1/10th of X or 0.1M whichever is maximum)
- Commercial (X)
Where X is the final commercial scale batch size.
Technology Transfer Process:
Technology transfer is both integral and critical to the drug discovery and development process for new medicinal products. The decision to transfer products between manufacturing sites is frequently driven by economics. Key stages of the process include data collection, data review, regulatory impact with particular emphasis on any change approvals, analytical validation, pilot or full-scale process batch, stability set down (if required). Typical technology transfer process was described in Fig. 1.
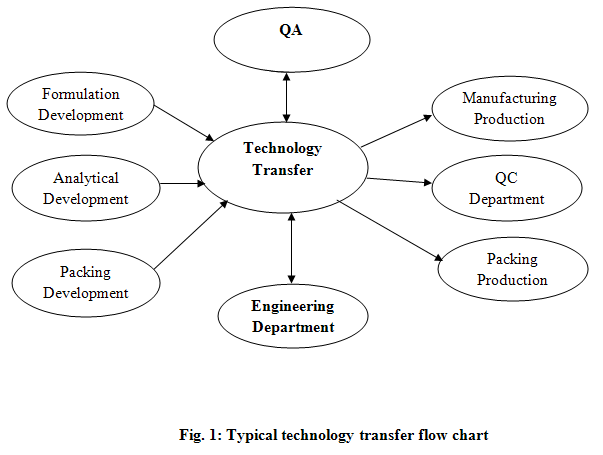
For a typical research-based pharmaceutical company, drug discovery and development can be broken down into distinct stages which were clearly described in Fig. 2.
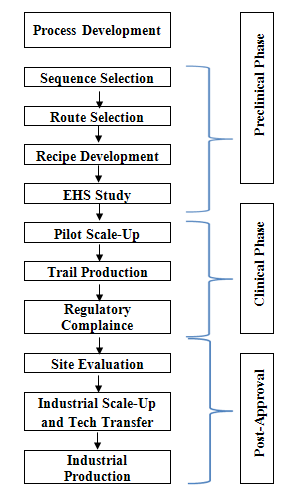
Fig. 2: Typical process development work flow in pharmaceutical industry.
1. Research phase.
a. Quality Design.
2. Development phase.
a. Research for Factory Production.
b.Consistency between Quality and Specification.
c.Assurance of consistency through development and manufacturing.
d.Technology Transfer from R&D to Production.
3. Production phase.
a.Validation & Production.
b.Feedback from Production and Technology Transfer of Marketed Products.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Technology Transfer Team:
The technology transfer team consists of the following members and their responsibilities are given below:
Table 1: constitution of technology transfer team and their responsibilities
|
Technology Transfer Team Member |
Responsibilities |
|
Process Technologist |
a. Central focus for transfer activities. b. Collates documentation from donor site c. Performs initial assessment of transferred project for Feasibility, Compatibility with site capabilities and Establishes resource requirements. |
|
Technology Transfer Team Member |
Responsibilities |
|
QA Representative |
a. Reviews documentation to determine compliance with marketing authorization (MA). b. Reviews analytical methods with QC to determine capability, equipment training requirements. c. Initiates conversion of donor site documentation into local systems or format. d. Initiates or confirms regulatory requirements, e.g., change to manufacturing license; variations to MA if process changes needed, etc. |
|
Production Representative |
a. Reviews process instructions (with process technologist) to confirm capacity and capability. b. Considers any safety implications, e.g., solvents; toxic; sanitizing materials. c. Considers impact on local standard operating procedures (SOPs). d. Considers training requirements of supervisors or operators. |
|
Engineering Representative |
a. Reviews (with production representative) equipment requirement. b. Initiates required engineering modifications, change or part purchase. c. Reviews preventative maintenance and calibration impact, e.g., use of more aggressive ingredients; more temperature sensitive process, and modifies accordingly. |
|
QC Representative |
a. Reviews analytical requirement. b. Availability with instruments. c. Responsible for analytical method transfer for drug substance and drug product. |
Technology Transfer Checklist:
A checklist that summarizes the details that should be collated during the process, the majority of which have already been referenced.
Copy of Part 2 of marketing authorization, Production master formula, Manufacturing instructions, Dispensing instructions, Analytical methods, Previous process validation, Previous analytical validation, Cleaning instructions and previous cleaning validation, Stability reports, Excipient specifications and source, Active specifications and source, Primary packaging material specifications and source, Packaging instructions, Customer complaints, Process deviations file, Analytical deviations file, Reject and rework file, Specimen manufacturing batch record, Specimen cartons, labels, leaflets.
Factors affecting the Success of Technology Transfer:
* Unlike the greenfield R & D project, the technology transfer is the importation of a developed R & D product from outside the company. Therefore, a complete background of technology with it’s possible strengths & weaknesses need to be understood in the beginning.
* Issues related to costs, royalty, royalty payment, milestone payments need to be considered before the technology transfer is to be opted. If this due diligence is not done correctly, the project may loose it’s priority within the company.
* Regulatory issues including ‘difficult to ressolve’ issues need to be correctly addressed while the project is on.
* Accurate calculations of time-lines with enough margins for development/ scale-up related issues, country of licencing, regulatory guidelines existing in the country of origin, need to be taken into account.
* Lastly, the major factor is a team which accepts the technology & the team that offers the technology should have complete understanding of the entire project & needs to develop a personal raport besides official project structure.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Technology Transfer Procedure:
1. Procedure for Manufacturing and Packaging:
* After the completion of three validation/commercial batches, R&D shall prepare the technology transfer dossier (TTD), which shall be reviewed by Head- Production, Head-QC, Head- Engineering and approved by Head-QA.
* The dossier shall contain the details of unitary formula, process flow chart, raw material and packing material specifications, in-process and finished product specifications, master formula card, safety parameters, critical process steps, critical process parameters and their specifications and measured response, process validation protocol, process validation report, stability data, deviation and change controls, product development report.
* After successful transfer of technology (manufacturing process), manufacturing of the respective product is the responsibility of production department. If any problem arises, QA shall investigate and refer to R&D through investigation report in the form of Inter Office Communication (IOC).
* Any deviation in the process shall be supported by deviation/change control form as applicable.
* For third party and loan license products, respective organization will provide the TTD to QA and manufacturing process shall be demonstrated to production personnel on minimum of two or three batches.
2. Procedure for Analytical Method Transfer:
a. For Drug Product:
* Analytical method transfer shall be initiated by analytical department for all the validated methods.
* Analytical department analyst along with quality control analyst has to perform analysis as per the analytical method transfer protocol.
* The transfer activity shall be established on the optimization batch or process batch with the final formula.
* Analytical method transfer activity shall be initiated with analytical method transfer protocol prepared by AR&D, reviewed by DQA/QC and finally approved by QA.
* The analytical method transfer protocol shall explain the transfer activities of the drug product and also the parameters that shall be transferred are Assay, Dissolution, Related substances, Content uniformity and Residual solvents.
* In case of multiple strengths, analytical method transfer shall be performed for lower strength.
* All chromatograms, record of results and other information have to be interlinked and records have to be maintained.
* A report has to made with summarized results and conclusions and the same shall be reviewed by Head-DQA and Head-QC, approved by Head-QA.
* The analytical method transfer process is considered to be completed upon the certification by analytical department, quality control & quality assurance that the method under consideration meets the acceptance criteria.
* Finally analytical method transfer report shall be prepared.
b. For Drug Substances:
· Non Pharmacopoeial Methods:
The vendor has to transfer the analytical methods to QC. In case, if the analytical method transfer activity was not performed by the vendor the same shall be prepared by AR&D as per the STP.
· Pharmacopoeial Methods:
The vendor has to transfer the analytical methods to QC. If the methods are same as per respective pharmacopoeia, the method suitability shall be performed by AR&D. Suitability of the analytical method shall verified by performing the specificity (RRT verification) and precision study (Triplicate) and the same shall be reviewed by DQA and communicated to QC.
* Analytical method transfer protocol and report shall be prepared.
* The analytical method transfer protocol shall explain the transfer activities of the drug substance and also the parameters that shall transferred are assay, residual solvents, related substances,.
* All chromatograms, record of results and other information have to be interlinked and records have to be maintained.
* A drug substance analytical method transfer report has to be made with summarized results with conclusions as per drug product method transfer report.
* The limits and parameters described for drug product/drug substance are indicative, but shall altered based on customer’s requirements and nature of drug product/drug substances to be followed as per respective analytical method transfer protocol.
* Documentation for analytical method transfer activity shall be done by analytical R&D department.
The Technology Transfer Report:
Regulatory inspectors and sometimes assessors will ask for evidence of successful transfer. This is more likely when the technology being transferred, be it the process or analytical method, is new to the site or poses particular challenges e.g. the introduction of bioassays. The report should also serve a similar function to the original development pharmaceutics report in that it provides a "ready reckoner" of key aspects of the product and a reference point in the future if problems are encountered.
Discussion:
The process of technology transfer is considered to be completed after successful completion of three validation batches by demonstration of the manufacturing process by R&D personnel to production personnel. Ensure that the development team reliably transfer all relevant quality, technical information to the receiving site such that the process must operate consistently and the critical process parameters are well defined and understood by the production personnel.
Conclusion:
Appropriate technology transfer is important to upgrade the quality of design to be the quality of product, and ensure stable and high quality of the product. The technology transfer does not mean one-time actions taken by the transferring party toward the transferred party, but means continuous information exchange between the both parties to maintain the product manufacturing.
Acknowledgement:
Authors wish to give thanks to Natco Pharma Ltd., Hyderabad for constant support and given literature to carry out this review.
References:
1. Analytical Procedures Technology Transfer. ISPE Draft Guidelines. International Society of Pharmaceutical Engineers, ISPE European Office, 7 Avenue des Gaulois, 1040 Brussels, Belgium.
2. Draft Guidelines for Validation of Analytical Procedures. International Committee for Harmonization (ICH).
3. Guideline for Technology Transfer (Draft), Cited 2009 December 12. Available from:
URL: nihs.go.jp/drug/GMP/04BDH0149-1post.pdf
4. Technology Transfer from R & D to production, Cited 2010 February 6.
Available from URL: bioqc.org/workshopdata/1Technology.pdf
5. Singh Amanjeet, Aggarwal Geeta. Technology Transfer In Pharmaceutical Industry: A Discussion. International Journal of Pharma and Bio Sciences. July-September 2009; 1, Issue-3.
6. YUE Feifei and YUE Yingming. Research on Technology Transfer in the Pharmaceutical Industry. Available from: 2010cygchy01a3.pdf
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









