In 2023, FDA approved 55 new drugs never before approved or marketed in the U.S., known as “novel” drugs. We also made other important approval decisions, such as expanding the use or patient population of previously approved drugs.
The 2023 actions, both novel and other important drug approvals, focus on prevention, diagnosis, and treatment of diseases and conditions such as:
• Infectious diseases, including COVID-19, respiratory syncytial virus (RSV), hospital acquired and ventilator associated bacterial pneumonia, and HIV-1.
• Neurological conditions, such as amyotrophic lateral sclerosis (ALS), Alzheimer’s disease, and migraine.
• Opioid use, misuse, and abuse.
• Heart, blood, kidney, and endocrine diseases, including type 2 diabetes in pediatrics, types of anemia, pediatric hormone deficiency, and chronic weight management.
• Lung diseases, such as asthma and cystic fibrosis.
• Gastrointestinal conditions, including inflammatory bowel disease and pediatric functional constipation.
• Different types of cancers, such as colorectal, prostate, lung, and low-grade gliomas (tumors that start in the brain).
• Women’s health, such as postpartum depression, hot flashes due to menopause, and nonprescription oral contraception
Reference Id: PHARMATUTOR-ART-3011
New Drugs for Patients with Rare Diseases
Patients with rare diseases are often in critical need of new therapies, as these individuals generally have few or no existing treatment options. In 2023, 28 of 55, or 51% of our novel drug approvals received orphan drug designation because they target rare diseases, including:
• Friedreich’s ataxia, an inherited, degenerative disease that damages the nervous system.
• Candidemia and invasive candidiasis, which are serious and life-threatening fungal infections
• Rett syndrome, a genetic, neurological disorder that affects brain development.
• CD55-deficient protein-losing enteropathy (CHAPLE disease), a genetic disease that affects the immune system.
• Paroxysmal nocturnal hemoglobinuria, a disease that causes red blood cells to break apart.
• Activated phosphoinositide 3-kinase delta, a genetic disorder that impairs the immune system.
In 2023,
CDER also approved many therapies for rare cancers or tumors, including:
• Mantle cell lymphoma, an aggressive form of non-Hodgkin’s lymphoma.
• Nasopharyngeal carcinoma, a rare head and neck cancer.
• Large B-cell lymphoma.
• Desmoid tumors, noncancerous growths in the connective tissue.
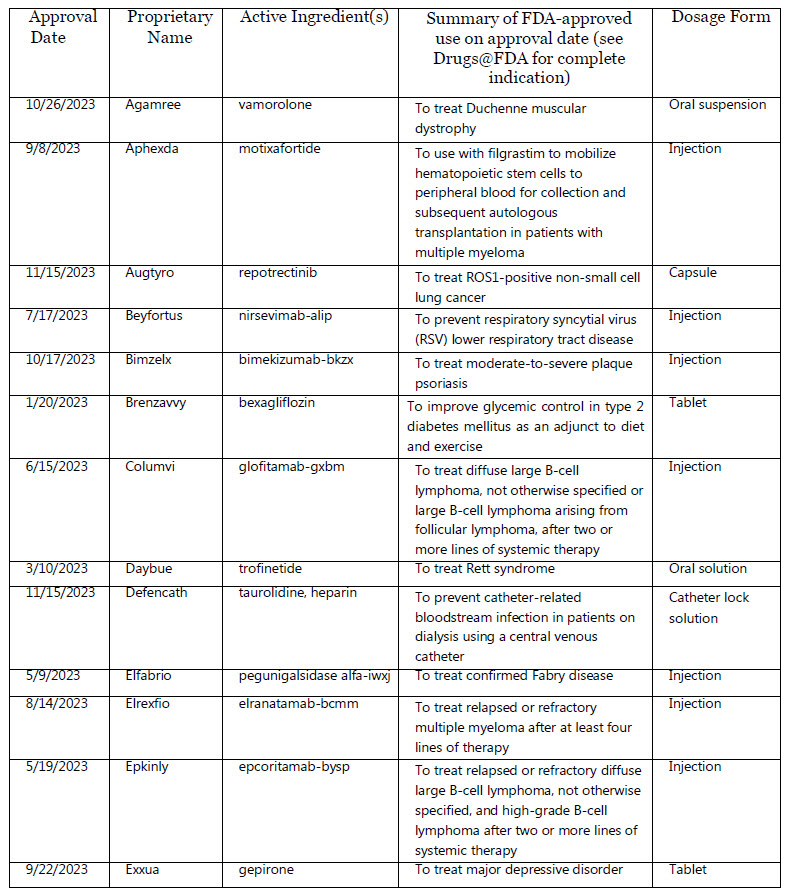
CDER’s Novel Drug Approvals of 2023
2023, CDER approved 55 novel drugs, either as new molecular entities (NMEs) under New Drug Applications (NDAs), or as new therapeutic biologics under Biologics License Applica- tions (BLAs). The active ingredient(s) in a novel drug have not been approved in the U.S.
Trade Name & Active Ingredient(s)
Agamree : vamorolone
Aphexda : motixafortide
Augtyro : repotrectinib
Beyfortus : nirsevimab-alip
Bimzelx : bimekizumab-bkzx
Brenzavvy bexagliflozin
Columvi : glofitamab-gxbm
Daybue : trofinetide
Defencath : taurolidine, heparin
Elfabrio : pegunigalsidase alfa-iwxj
Elrexfio : elranatamab-bcmm
Epkinly : epcoritamab-bysp
Exxua : gepirone
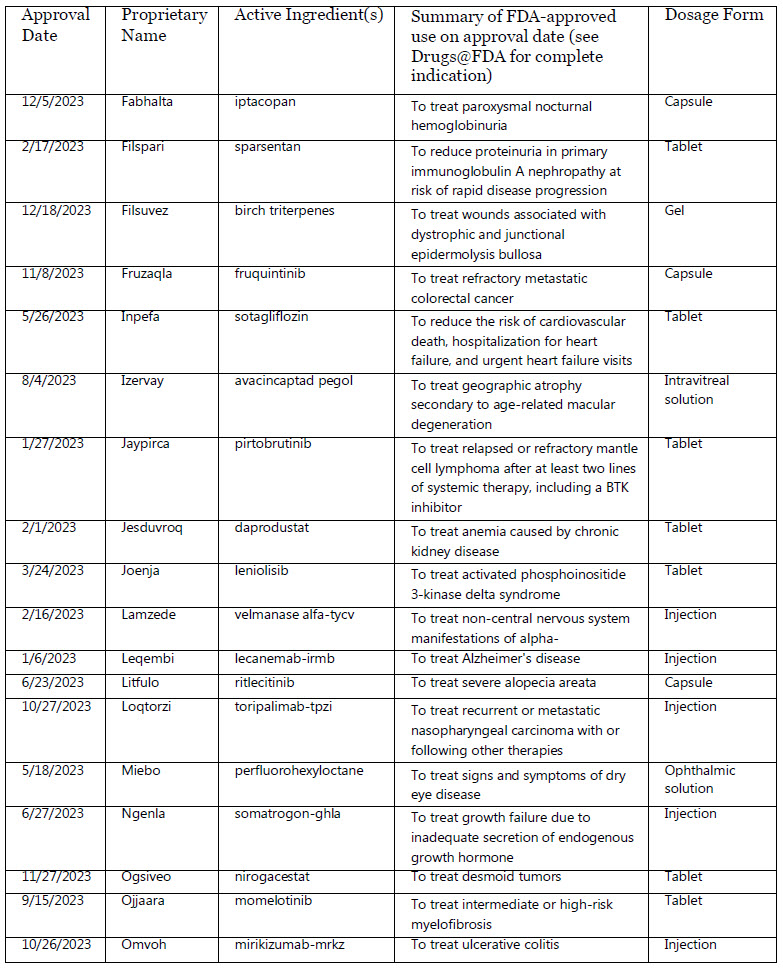
Fabhalta : iptacopan
Filspari : sparsentan
Filsuvez : birch triterpenes
Fruzaqla : fruquintinib
Inpefa : sotagliflozin
Izervay : avacincaptad pegol
Jaypirca : pirtobrutinib
Jesduvroq : daprodustat
Joenja : leniolisib
Lamzede : velmanase alfa-tycv
Leqembi : lecanemab-irmb
Litfulo : ritlecitinib
Loqtorzi : toripalimab-tpzi
Miebo : perfluorohexyloctane
Ngenla : somatrogon-ghla
Ogsiveo : nirogacestat
Ojjaara : momelotinib
Trade Name & Active Ingredient(s)
Omvoh : mirikizumab-mrkz
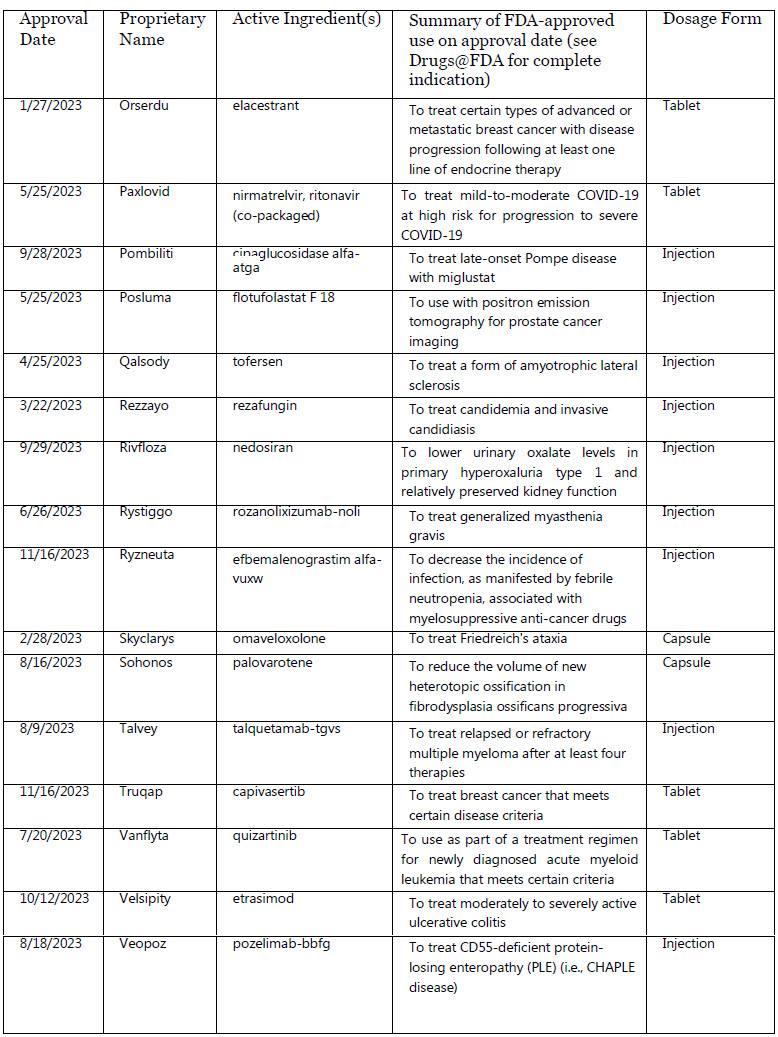
Orserdu : elacestrant
Paxlovid : nirmatrelvir, ritonavir (co-packaged)
Pombiliti : cipaglucosidase alfa-atga
Posluma : flotufolastat F 18
Qalsody : tofersen
Rezzayo : rezafungin
Rivfloza : nedosiran
Rystiggo : rozanolixizumab-noli
Ryzneuta : efbemalenograstim alfa-vuxw
Skyclarys : omaveloxolone
Sohonos : palovarotene
Talvey : talquetamab-tgvs
Truqap : capivasertib
Vanflyta : quizartinib
Velsipity : etrasimod
Veopoz : pozelimab-bbfg
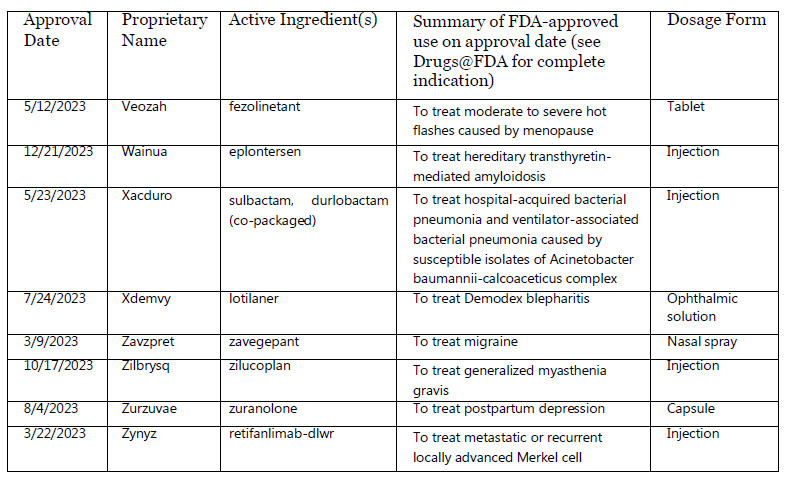
Veozah : fezolinetant
Wainua : eplontersen
Xacduro : sulbactam, durlobactam (co-packaged)
Xdemvy : lotilaner
Zavzpret : zavegepant
Zilbrysq : zilucoplan
Zurzuvae : zuranolone
Zynyz : retifanlimab-dlwr
First-in-Class Drugs
CDER identified 20 of the 55 novel drugs approved (36%) in 2023 as first-in-class. These drugs have mechanisms of action different from those of existing therapies.
Novel drugs approved in 2023 that CDER identified as first-in-class were:
Daybue, Defencath, Fabhalta, Filspari, Filsuvez, Jesduvroq, Joenja, Lamzede, Miebo, Ogsiveo, Paxlovid, Qalsody, Rivfloza, Skyclarys, Sohonos, Talvey, Truqap, Veopoz, Veozah, Xdemvy
Notable examples of novel first-in-class approvals include:
• Daybue (trofinetide) oral solution as the first treatment for patients aged two years and older with Rett syndrome, a rare, genetic neurological disorder that affects brain development.
• Jesduvroq (daprodustat) tablets as the first oral treatment for anemia (decreased number of red blood cells) caused by chronic kidney disease for adults receiving dialy¬sis for at least four months.
• Miebo (perfluorohexyloctane) ophthalmic solution to treat signs and symptoms of dry eye disease, which occurs when a patient’s tears do not provide sufficient eye lubrica¬tion, leading to potential ocular (eye-related) inflammation and damage.
• Paxlovid (nirmatrelvir and ritonavir, co-packaged for oral use) tablets to treat mild-to-moderate COVID-19 in adults who are at high risk for progression to severe COVID-19, including hospitalization or death. Paxlovid is the first oral antiviral pill approved to treat COVID-19 in adults.
• Skyclarys (omaveloxolone) capsules as the first treatment for Friedreich’s ataxia, a rare, inherited, degenerative disease that damages the nervous system, characterized by impaired coordination and difficulty walking.
• Talvey (talquetamab-tgvs) injection to treat adults with refractory (treatment-resistant) or relapsed (recurring) multiple myeloma who have received other therapies. It was approved through the Accelerated Approval pathway.
• Veozah (fezolinetant) tablets to treat moderate to severe vasomotor symptoms, or hot flashes, due to menopause.
• Xdemvy (lotilaner) ophthalmic solution as the first drug to treat Demodex blepharitis, chronic eyelid inflammation caused by Demodex folliculorum, a microscopic mite.
Drugs for Rare Diseases
In 2023, 28 of CDER’s 55 novel drug approvals (51%) received orphan drug designation because they target rare diseases (diseases that affect fewer than 200,000 people in the U.S.). Patients with rare diseases often have few or no drugs available to treat their conditions.
Novel drugs approved in 2023 with the orphan drug designation were:
Agamree, Aphexda, Augtyro, Daybue, Elrexfio, Fabhalta, Filspari, Filsuvez, Jaypirca, Joenja, Lamzede, Loqtorzi, Ngenla, Ogsiveo, Ojjaara, Pombiliti, Qalsody, Rezzayo,
Rivfloza, Rystiggo, Skyclarys, Sohonos, Talvey, Vanflyta, Veopoz, Wainua, Zilbrysq, Zynyz
Examples of novel approvals of 2023 for rare diseases include:
• Fabhalta (iptacopan) tablets as the first oral treatment for paroxysmal nocturnal hemoglobinuria, a disease that causes red blood cells to break apart.
• Filspari (sparsentan) tablets to reduce proteinuria (elevated protein in the urine) in adults with primary immunoglobulin A (IgA) nephropathy at risk of rapid disease pro¬gression. IgA nephropathy is a chronic kidney disease that can lead to kidney failure. Filspari was approved through the Accelerated Approval pathway.
• Jaypirca (pirtobrutinib) tablets to treat relapsed or refractory mantle cell lymphoma, an aggressive form of non-Hodgkin lymphoma, after receiving other therapies. Later in 2023, CDER approved Jaypirca to treat adults with chronic lymphocytic leukemia or small lymphocytic lymphoma after receiving other therapies. Both indications were approved through the Accelerated Approval pathway.
• Joenja (leniolisib) tablets as the first treatment for activated phosphoinositide 3-kinase delta syndrome (APDS) in patients 12 years and older. APDS is a genetic dis¬order that impairs the immune system
• Ogsiveo (nirogacestat) tablets to treat desmoid tumors, which are noncancerous growths in the connective tissue, such as the abdomen, arms, and legs.
• Ojjaara (momelotinib) tablets to treat myelofibrosis, a type of bone marrow cancer that causes scar tissue buildup in the bone marrow, disrupting the body’s normal pro¬duction of blood cells.
• Rezzayo (rezafungin) injection to treat candidemia and invasive candidiasis, which are serious and life-threatening fungal infections, in patients 18 years or older with lim¬ited or no alternative treatments.
• Rystiggo (rozanolixizumab-noli) injection for adults with the two most common subtypes of generalized myasthenia gravis, a chronic autoimmune neuromuscular con¬dition that causes muscle weakness and severe fatigue.
• Sohonos (palovarotene) capsules to reduce the volume of new heterotopic ossifica¬tion (bone formation outside the skeleton) in females eight years and older and males 10 years and older with fibrodysplasia ossificans progressiva, a disorder in which bones gradually form in muscle tissue and connective tissue.
• Veopoz (pozelimab) injection as the first drug to treat CHAPLE disease, a very rare genetic disease. CHAPLE disease affects the immune system and can be life-threatening.
• Wainua (eplontersen) injection to treat hereditary transthyretin-mediated amyloidosis, a genetic disorder that leads to organ and tissue dysfunction
Other Novel Drug Approvals
In addition to the first-in-class and drugs for rare diseases, CDER approved these notable approvals in 2023 :
• Beyfortus (nirsevimab-alip) injection as the first approved drug to prevent RSV lower respiratory tract disease in all (i.e., not only high-risk) babies born during or entering their first RSV season and certain high-risk children up to 24 months. While most patients experience mild cold-like symptoms, some infants, especially with their first RSV infection, develop lower respiratory tract disease such as pneumonia and bron¬chiolitis (swelling of the lungs’ small airway passages).
• Columvi (glofitamab-gxbm) injection to treat types of relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and high-grade B-cell lymphoma. DLBCL is marked by rapid tumor growth in the lymph nodes, spleen, liver, bone marrow, or other tissues and organs. Columvi was approved through the Accelerated Approval pathway.
• Defencath (taurolidine and heparin) catheter lock solution to reduce catheter-related bloodstream infections in adults with kidney failure who are receiving chronic hemodi¬alysis (a procedure to purify the blood) through a central venous catheter
• Epkinly (epcoritamab-bysp) injection to treat types of relapsed or refractory DLBCL and high-grade B-cell lymphoma. Epkinly was approved through the Accelerated Approval pathway.
• Fruzaqla (fruquintinib) capsules to treat refractory, metastatic colorectal cancer.
• Izervay (avacincaptad pegol) injection to treat geographic atrophy secondary to age-re¬lated macular degeneration (AMD). Geographic atrophy is an advanced form of AMD that leads to progressive and irreversible retinal cell loss and permanent vision loss.
• Leqembi (lecanemab-irmb) injection to treat Alzheimer’s disease, a progressive neu¬rologic disorder that is the most common cause of dementia. Leqembi was approved through the Accelerated Approval pathway and later converted to traditional approval after clinical benefit was confirmed, both in 2023.
• Orserdu (elacestrant) tablets to treat breast cancer with certain genetic features and disease progression after at least one line of endocrine therapy.
• Xacduro (sulbactam and durlobactam) injection to treat hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia caused by Acinetobacter baumannii-calcoaceticus complex.
• Zavzpret (zavegepant) nasal spray to treat acute migraine with or without aura (a migraine warning sign).
• Zurzuvae (zuranolone) tablets as the first oral medication to treat postpartum depres¬sion, a complex mix of physical, emotional, and behavioral changes that can occur around childbirth
Innovation : Use of Expedited Development and Review Pathways
CDER used diverse regulatory approaches to enhance and expedite drug review in 2023. These approaches enable increased flexibility, efficiency, and interactions between CDER staff and drug developers. They often also allow shorter review times to speed the avail- ability of new therapies to patients with serious conditions, especially in cases where there are no satisfactory alternatives, while preserving FDA’s rigorous standards for safety and effectiveness
Fast Track
CDER granted Fast Track status to 25 of the 55 novel drugs (45%) in 2023. Fast Track speeds development and review of new drugs and biologics by increasing the level of communication between FDA and drug developers and by enabling CDER to review portions of a drug applica- tion on a rolling basis.
Drugs granted Fast Track status were: Agamree, Beyfortus, Columvi, Daybue, Defencath, Elfabrio, Elrexfio, Filsuvez, Fruzaqla, Izervay, Jaypirca, Lamzede, Leqembi, Ogsiveo, Orserdu, Paxlovid, Qalsody, Rezzayo, Skyclarys, Truqap, Vanflyta, Veopoz, Xacduro, Zurzuvae, Zynyz
Breakthrough Therapy
CDER designated 9 of the 55 novel drugs (16%) in 2023 as Breakthrough Therapies. A Break- through Therapy designation includes all the Fast Track program features and offers intensive FDA guidance during drug development, including involvement from senior managers.
Drugs designated with Breakthrough Therapy status were: Elrexfio, Fabhalta, Izervay, Leqembi, Loqtorzi, Ogsiveo, Pombiliti, Rivfloza, Talvey
Priority Review
In 2023, 31 of the 55 novel drugs approved (56%) were designated Priority Review. A drug receives Priority Review if CDER determines that the drug treats a serious condition and, if approved, would provide a significant improvement in safety or effectiveness of the treatment, diagnosis, or prevention of the serious condition. Generally speaking, a Priority Review appli- cation is one on which CDER aims to take action within six months of filing (compared to a target date of 10 months under standard review).
Drugs designated Priority Review were: Augtyro, Columvi, Daybue, Defencath, Elfabrio, Elrexfio, Epkinly, Filspari, Filsuvez, Fruzaqla, Izervay, Jaypirca, Joenja, Lamzede, Leqembi, Loqtorzi, Ogsiveo, Orserdu, Paxlovid, Qalsody, Rezzayo, Rystiggo, Skyclarys, Sohonos, Talvey, Truqap, Vanflyta, Veopoz, Xacduro, Zurzuvae, Zynyz
New Uses of Approved Drugs
After CDER approves a new treatment, a drug sponsor may generate new data about the product that suggests an additional use. The drug sponsor may then submit an application to modify or expand the use of an approved drug based on this new data.
The products below are some 2023 CDER approvals for new uses or indications of an approved drug :
• Ayvakit (avapritinib) tablets were first approved in 2020 to treat types of gastrointesti¬nal stromal tumors. Ayvakit was approved in 2023 for indolent systemic mastocytosis, a rare disorder that results in the buildup of too many mast cells (a type of white blood cell).
• Eylea (aflibercept) injection was originally approved in 2011 for neovascular (wet) age-related macular degeneration. In 2023, CDER approved Eylea to treat retinopathy of prematurity (ROP), an eye disease that can occur in babies born prematurely. In ROP, abnormal blood vessels grow in the retina and can lead to vision loss.
• Ilaris (canakinumab) injection was originally approved in 2009. In 2023, CDER approved Ilaris to treat gout flares in adults in whom certain other therapies are not advised. Gout is a type of arthritis characterized by sudden attacks of pain, swelling, and redness in one or more joints.
• Jemperli (dostarlimab-gxly) injection was initially approved in 2021. In 2023, CDER approved Jemperli for two endometrial cancer uses, as a single agent and in combina¬tion with other therapies. Endometrial cancer occurs in the tissues of the endometrium, which is the lining of the uterus.
• Kevzara (sarilumab) injection was first approved in 2017 for moderately to severely active rheumatoid arthritis. In 2023, CDER approved it for adults with polymyalgia rheumatica (PMR) who did not adequately respond to corticosteroids or who cannot tolerate corticosteroid taper (gradual dose reduction). PMR is an inflammatory disorder that causes muscle pain and stiffness around the shoulders and hips.
• Keytruda (pembrolizumab) injection was first approved in 2014. In 2023, CDER approved Keytruda for several new uses, including in combination with Padcev for patients with locally advanced or metastatic urothelial carcinoma who are not eligible for a type of chemotherapy; for types of non-small cell lung cancer as an adjuvant (after other therapies) therapy; and for early-stage non-small cell lung cancer as a neo¬adjuvant (before other therapies) and adjuvant treatment.
• Linzess (linaclotide) capsules were first approved in 2012. In 2023, CDER approved it as the first treatment for pediatric functional constipation in patients six to 17 years. Pediatric functional constipation occurs when patients have infrequent bowel move¬ments with hard stools that can be difficult or painful to pass.
• Lonsurf (tipiracil hydrochloride and trifluridine) tablets were first approved in 2015. In 2023, CDER approved Lonsurf, alone or in combination with another therapy (beva- cizumab), for patients with previously treated metastatic colorectal cancer.
• Lynparza (olaparib) tablets were first approved in 2014. In 2023, Lynparza was approved in combination with another therapy for adults with a type of prostate cancer that is metastatic and castration-resistant (i.e., keeps growing when the amount of tes¬tosterone is reduced to very low levels).
• Padcev (enfortumabvedotin-ejfv) injection was first approved in 2019 as a single agent. CDER approved Padcev in combination with Keytruda in 2023 to treat adults with locally advanced or metastatic urothelial cancer.
• Polivy (polatuzumabvedotin-piiq) injection was first approved in 2019 to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), not otherwise spec¬ified, after at least two therapies. In 2023, CDER expanded the indication for adults with previously untreated DLBCL, not otherwise specified, or high-grade B-cell lym-phoma that is considered low-intermediate risk or higher.
• Rexulti (brexpiprazole) tablets were initially approved in 2015 as an add-on therapy for major depressive disorder and as a treatment for schizophrenia. In 2023, CDER approved Rexulti as the first drug to treat agitation associated with dementia due to Alzheimer’s disease.
• Rinvoq (upadacitinib) tablets were originally approved in 2019 for adults with moder¬ately to severely active rheumatoid arthritis. In 2023, CDER approved Rinvoq for adults with moderately to severely active Crohn’s disease with an inadequate response or intolerance to other therapies (specifically TNF blockers). Rinvoq is the first approved oral product for moderately to severely active Crohn’s disease, a chronic disease that causes inflammation in the digestive tract.
• Talzenna (talazoparib) capsules were first approved in 2018 to treat types of breast cancer. In 2023, Talzenna was approved in combination with another therapy to treat a type of metastatic castration-resistant prostate cancer.
• Tukysa (tucatinib) tablets were first approved in 2020. In 2023, CDER approved Tukysa for a type of colorectal cancer that has progressed following certain chemotherapies.
• Verzenio (abemaciclib) tablets were initially approved in 2017. In 2023, CDER approved Verzenio with endocrine therapy (tamoxifen or an aromatase inhibitor) for a type of early breast cancer with a high risk of recurrence.
Biosimilar Approvals
The biosimilar pathway is an abbreviated approval pathway for biologics that are highly similar to and have no clinically meaningful differences from an FDA-approved biologi¬cal reference product. This pathway was established to provide more treatment options, increase patient access, and potentially reduce the cost of therapies through competition.
In 2023, CDER approved five new biosimilars for five reference products, including three reference products that did not have a corresponding biosimilar. Several drugs were approved as interchangeable biosimilars, which are biosimilars that may be substituted for the reference product at the pharmacy similar to how generics are substituted, subject to state law.
Avzivi (bevacizumab-tnjn) injection was approved to treat types of metastatic col¬orectal cancer and hepatocellular carcinoma (reference product: Avastin).
Tofidence (tocilizumab-bavi) injection was approved to treat types of arthritis, includ¬ing rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, and systemic juvenile idiopathic arthritis (reference product: Actemra).
Tyruko (natalizumab-sztn) injection was approved as a monotherapy (a single ther¬apy) to treat relapsing forms of multiple sclerosis and to induce and maintain a clinical response in adults with moderately to severely active Crohn’s disease whose condition meets certain criteria (reference product: Tysabri).
Wezlana (ustekinumab-auub) injection was approved to treat plaque psoriasis and psoriatic arthritis in patients six years and older, and Crohn’s disease and ulcerative colitis in adults (reference product: Stelara).Wezlana was approved as an interchange¬able biosimilar.
Yuflyma (adalimumab-aaty) injection was approved to treat a variety of inflammatory conditions (reference product: Humira).
In 2023, CDER also approved notable changes to the following biosimilars:
• Abrilada (adalimumab-afzb) injection was originally approved in 2019 for several inflammatory conditions. In 2023, CDER approved it as an interchangeable biosimilar (reference product: Humira).
• Byoovis (ranibizumab-nuna) injection was initially approved in 2021 to treat certain eye conditions. In 2023, CDER approved it as an interchangeable biosimilar (reference product: Lucentis).
CDER has approved a total of 45 biosimilars for 14 different reference products since 2015. This includes at least one biosimilar for each of these top selling biologics in the U.S.: nine biosimilars to Humira; six biosimilars to Neulasta; five biosimilars to Herceptin and Avastin; four biosimilars to Remicade; three biosimilars to Rituxan and Neupogen; two biosimilars to Lantus, Enbrel, and Lucentis; and one biosimilar to Epogen/Procit, Actemra, Stelara, and Tysabri. Multiple biosimilars for an approved reference product can enhance competition, which may lead to reduced costs for both patients and our health care system.
Other Important Approvals
New formulations of approved drugs can offer significant therapeutic advances. Simi¬larly, new dosage forms (such as from a capsule to a chewable tablet for those unable to swallow pills) can help increase adherence, make sure patients take the proper dose, and improve quality of life for patients who must use the medication on a prolonged basis. Making a drug available as an over-the-counter product can also increase patient access to therapies. Below are examples of new formulations, new dosage forms, over-the-counter actions, and other notable approval actions of 2023:
• Airsupra (albuterol and budesonide) inhalation aerosol for as-needed treatment or prevention of bronchoconstriction (tightening of lung airways) and to reduce the risk of asthma attacks in adults. Airsupra is a combination product of two approved active ingredients, albuterol and budesonide.
• Akeega (niraparib and abiraterone acetate) tablets to treat a type of prostate cancer that meets certain disease criteria, together with prednisone (a steroid). Akeega is a combination product of two approved active ingredients, niraparib and abiraterone.
• Brixadi (buprenorphine) extended-release injection to treat moderate-to-severe opi¬oid use disorder. Brixadi is now approved in both weekly and monthly under-the-skin injectable formulations at varying doses, including lower doses that may be appropriate for those who do not tolerate higher doses of extended-release buprenorphine.
• Entyvio (vedolizumab) injection was approved in 2014 for intravenous (into the vein) administration to treat moderately to severely active ulcerative colitis and Crohn’s dis¬ease in adults. In 2023, CDER approved Entyvio as an under-the-skin injection to treat moderately to severely active ulcerative colitis in certain adults. This will allow patients to self-administer the medication after training, avoiding needing to go to an infusion center.
• Hepzato (melphalan hydrochloride) injection. Melphalan, the active ingredient, has been approved for different oncology indications. In 2023, CDER approved Hepzato with the same active ingredient to treat liver metastases (metastatic growths) in certain patients with uveal melanoma, a rare cancer that develops in a part of the eye.
• Lampit (nifurtimox) tablets were converted from Accelerated Approval to full approval for use in pediatric patients from birth to younger than 18 years to treat Chagas disease (American Trypanosomiasis) caused by the Trypanosoma cruzi parasite. Chagas disease can cause heart, digestive, and neurological problems and may be life-threatening.
• Lodoco (colchicine) tablets. Lodoco, with the previously approved active ingredient colchicine, was approved in 2023 to reduce the risk of certain cardiovascular events in adults with established atherosclerotic disease (thickening of the arteries) or with multi¬ple risk factors for cardiovascular disease.
• Mydcombi (tropicamide and phenylephrine hydrochloride) ophthalmic spray, a combination of two approved active ingredients, was approved in 2023 to induce mydriasis (pupil dilation) for diagnostic procedures and in other conditions.
• Narcan (naloxone hydrochloride) nasal spray had been approved as a prescription drug to reverse the effects of opioid overdose. In 2023, CDER approved Narcan as a nonprescription drug.
• Opill (norgestrel) tablets had been approved as a prescription drug to prevent pregnancy. In 2023, CDER approved Opill as the first nonprescription daily oral contraceptive.
• Opvee (nalmefene hydrochloride) nasal spray was approved in a new dosage form in 2023 to reverse the effects of opioid overdose. Nalmefene hydrochloride (the active ingredient) had been approved as an injection drug.
• Prevymis (letermovir) tablets and injection were first approved in 2017 to prevent cytomegalovirus (CMV) infection and disease in adults at high risk for CMV who received an allogeneic (from a donor) hematopoietic stem cell transplant. In 2023, CDER approved Prevymis for the same indication in adults at high risk for CMV who received a kidney transplant.
• RiVive (naloxone hydrochloride) nasal spray was approved in 2023 as a nonpre¬scription drug to reverse the effects of opioid overdose. Its active ingredient, naloxone hydrochloride, had been approved for prescription use.
• Ryzumi (phentolamine) ophthalmic solution was approved in 2023 to treat pharma¬cologically induced mydriasis (eye dilation). CDER had previously approved its active ingredient, phentolamine.
• Syfovre (pegcetacoplan) injection. Pegcetacoplan, the active ingredient, was first approved as an under-the-skin injection in 2021. In 2023, CDER approved Syfovre for intravitreal use (a shot directly into the eye) to treat geographic atrophy resulting from AMD.
• Technegas (kit for preparation of technetium Tc 99m labeled carbon) inhalation aerosol was approved as a radioactive diagnostic agent for visualization of lung ven¬tilation and evaluation of pulmonary embolism (a blockage in the pulmonary arteries), when paired with perfusion imaging. Other dosage forms of technetium Tc 99m have been previously approved.
• Zepbound (tirzepatide) injection. In 2023, CDER approved Zepbound, with the previously approved active ingredient tirzepatide, for chronic weight management, in addition to a reduced-calorie diet and increased physical activity, in adults with obesity or overweight and at least one weight-related comorbid condition.
CDER’s Novel Approvals of 2023
(in alphabetical order)