 About Authors:
About Authors:
Swathi sree Karumuri*, Venkata Naveen Kasagana
Department of Pharmaceutics,
Sankaralingam Bhuvaneswari College of Pharmacy, Anaikuttam,
Sivakasi - 626 130, Tamilnadu, India
*swathi.karumuri2011@gmail.com
Abstract:
New drugs pursuing novel delivery technologies with the objective of improving drug safety, efficacy and site Targeting in a cost effective way has been a challenging area of work for researchers. Their eminent work in the field of Novel technology has introduced many drug delivery methods to fulfil the requirements of pharmaceutical manufacturers and customers. Multiunit particulate systems (MUPS) are novel MDDS techniques that have gained importance, not only because of their ability to control drug release, but also for the modified drug-release profiles they facilitate. Similarly Acoustic targeted drug delivery (ATDD) is a method that uses ultrasound energy to enhance the transport of molecules into and/or across specific tissues. P.L.E.A.S.E. (Painless Laser Epidermal System) is a transdermal delivery device that uses a special laser source to create micro pores in the skin through which high molecular weight drugs are delivered painlessly in a highly controlled and accurate fashion. Oral delivery of insulin with increased lipophilicity across the gastrointestinal epithelium by the application of Eligen technology. Development of nanorobots with biosensors for medical target identification and drug delivery plays a prominent role in the diagnosis and treatment of Diseases. Gene delivery technology that turn off or on gene responsible for causing diseases like cancer and diabetes. The present article describes how Novel Drug delivery fulfils the Patient Needs on a Global Level and forms a key technology in the field of pharmaceuticals.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1447
INTRODUCTION
During the past decade a new era of science and technology has emerged in pharmaceutical research aimed at the development of novel or advanced drug delivery systems. The researchers have advanced in many new drug delivery systems that have solved the problems of many conventional dosage forms and fulfilled the needs of many patients. The advancement of targeted novel drug delivery was given much importance for treating the diseases like cancer, brain targeted drug delivery, diabetes, cardiac disorders and GI targeted drug delivery systems where the treatment by the conventional dosing methods have failed to deliver the drug in required concentration and required target site. In the present article we are going through a brief presentation of some of the novel drug delivery systems that has been show much interest by the pharmaceutical researchers.
[adsense:468x15:2204050025]
MULTIUNIT PARTICULATE SYSTEMS (MUPS)
Oral controlled release (CR) multiple unit dosage forms such as micro particles, hydrogel micro beads and pellets are gaining considerable importance in recent years in view of their advantages over the conventional single unit formulations. Polymeric hydrogels are three-dimensional cross-linked networks that have the ability to absorb water and swell without losing their shape. Their most remarkable macroscopic property is their high swelling ability, which depends largely on the external conditions (i.e. pH, temperature) and the parameters of the gel (i.e. mesh size). Hydrogels have been widely used in medicine and pharmacy as Targeted controlled delivery devices of various active materials.1
Hydrogel Beads in GI drug release
The multiple unit dosage forms spread uniformly throughout the gastrointestinal tract (GIT), this will avoid the release of drug at one particular site, thus avoiding the risk of toxicity. Uniform distribution of multiple units in GIT results in more reproducible absorption and will reduces the risk of local irritations when compared to single unit systems. Simvastatin is a powerful lipid-lowering drug that is used in the treatment of hypercholesterolemia is a Multiunit oral controlled release drug.
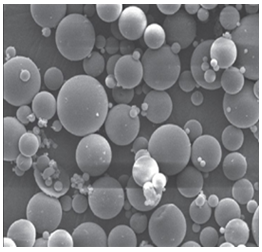
Preparation of carboxymethylcellulose-aluminum based hydrogels beads by ionotropic gelation method for the controlled release of simvastatin
Simvastatin was dispersed in an aqueous solution of carboxymethylcellulose sodium (NaCMC) and mixed homogeneously using magnetic stirrer. Twenty milliliters of dispersion was extruded in the form of droplets into 100 ml aqueous solution of AlCl3 solution using 25 ml hypodermic syringe through a needle (number 23). When a dispersion of drug and NaCMC was extruded though the needle into a solution containing Al3+ cations, the beads were formed instantaneously. As soon as the trivalent cations (Al3+) are bought in contact with NaCMC, they form ionic cross-links between two polymer molecules and different parts of the same polymer chain. The exchange of Na+ ions occurs with Al3+. These ions are ionically substituted at the carboxylate site and a second strand of NaCMC can also be connected with Al3+ forming a link in which the cations are attached to three NaCMC strands together. As the concentration of AlCl3 was increased, smaller beads were produced. This suggests that during crosslinking, the hydrogel might have undergone rapid shrinking leading to the formation of smaller and rigid matrix at higher crosslink densities. Also by increasing the polymer concentration in the beads, an increase in size of the beads was observed, which could be attributed to the formation of bigger droplets due to increase in the viscosity of the solution with increasing concentration of polymer during extruding through a needle. On the other hand, increase in amount of simvastatin increased the bead size because simvastatin might have occupied the interstitial spaces between polymer segments (Agnihotri and Aminabhavi 2004). The beads were removed after the gelation period of 15 min and washed with distilled water repeatedly to make free from un-reacted ions and dried at room temperature for 24 h and then at 40OC for 10 h.
Similarly a multiparticulate system with the potential for site-specific delivery to the colon has been investigated. Two drugs, indomethacin and sulphamethoxazole were successfully incorporated into the beads. Drug release from the beads was a function of media pH and drug loading.2
Calcium-carboxymethyl chitosan hydrogel beads for protein drug delivery system prepared by crosslinking with Ca2+. The beads were loaded with a protein (bovine serum albumin, BSA).3
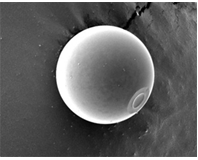
The multiunit hydrogel beads can act as targeted drug delivery systems based on different mechanism like pH sensitive, temperature sensitive or may be glucose sensitive that act as GI specific, colon specific, topical drug delivery, tissue engineering, cosmetology ocular drug delivery and protein drug delivery.4
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Acoustic targeted drug delivery(ATDD)
Acoustic targeted drug delivery(ATDD) is a method that uses ultrasound energy to enhance the transport of molecules into and/or across specific tissues. Generally this class of ultrasound energy falls under the class of therapeutic ultrasound, and ranges in ultrasonic frequencies of 1-20MHz and Sound intensities of 0-30 watts/cm2 [1] The use of ATDD in conjunction with local drug delivery by injection, topical application and convection enhanced deliveryshows promise to significantly enhance the treatment of various diseases in the human body by specifically targeting the drug into the tissue.
The success of treating brain cancer such as neuroblastomas and neurofibromatosis has not been very effective. Convection-enhanced drug delivery has been used to bypasses the blood-brain barrier by infusing drugs directly into the brain through a needle or microcatheter. Small molecules can be delivered over relatively large distances in the brain, but larger molecules, including certain proteins with proven efficacy against malignant cells, are hindered in their transport. Drugs can be packaged inside nanoparticles such as polymeric spheres or liposomes, which protects the drug from elimination, but transport of these nanoparticles in the interstitium is even more hindered than that of proteins. The transport of proteins and nanoparticles can be improved by increasing the porosity or the effective “pore size” of the brain interstitium. Transport is enhanced by the use of hyperosmolar solution that induces tissue swelling locally by drawing fluid into the interstitium from surrounding blood vessels and cells. Similarly Infusing an enzyme that temporarily degrades the extracellular matrix, which increases the permeability of the interstitium to proteins and nanoparticles, also enhances transport. Although these methods work to some extent,additional transport enhancement is required to realize the potential of convection enhanced drug delivery.
A recent study shows that, 1.58 MHz ultrasound is used to increase the pore size where there is an increase the perfusion of locally delivered Evans blue dye, into an agar gel that acts as a mimic of brain tissue for drug delivery studies, equine brain and avian muscle tissue. The ultimate goal is to increase the rate of transport of pharmaceutical agents relative to their elimination rate, and thereby extend the distance that drugs penetrate and maintain therapeutically useful concentrations.5
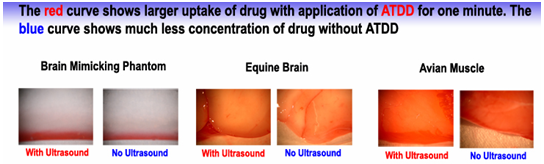
Chemotherapy drug delivery using ultrasonic stimulation as a release trigger has also been developed to improve the release rate of the drugs.6 Similarly use of Surface Acoustic Wave (SAW) device based on MEMS technology for drug delivery in the treatment of ocular diseases.7
P.L.E.A.S.E. = Painless Laser Epidermal System
Peptides/proteins play important roles in the drug therapy of various diseases such as cancers, autoimmune diseases, memory impairment, mental disorders, hypertension, and metabolic disorders.8 Peptide drugs are also currently approved for preventive applications such as vaccines.9,10 The poor oral bioavailability of peptides and vaccines, and problems associated with their parenteral delivery have led to continued interest in non-invasive delivery techniques.11 The transdermal delivery of peptides/vaccines is an attractive possibility.12 The main advantages of the transdermal route are the possibility of continuously delivering drugs with short half-lives, thus prolonging their effect.13 Although transdermal drug delivery has many potential benefits, the permeability of skin to macromolecules is extremely low because of the formidable barrier function of the stratum corneum (SC). A variety of physical methods and penetration enhancers have been tested to deliver a large amount of vaccines in an effort to generate a desirable immune response.14 So far, most physical methods have had limited success. Penetration enhancers are not always desirable due to their toxicity. Thus there is a need to develop new enhancing methods.15

P.L.E.A.S.E is a novel transdermal delivery method based on laser ablation of skin, needle free and painless intra epidermal drug delivery technology. It is designed to enhance delivery of large molecular weight drugs and vaccines via specialized laser micro-poration technology. A handheld laser device creates controlled aqueous micro pores in the epidermis. Due to the special features of the device the micro pores do not reach the dermis, where nerves and blood vessels reside. An intelligent graphical user interface guarantees simple and safe use by the medical personnel or the patient, who can use the device without supervision. A special laser source ablates outer skin tissue painless in a highly controlled and accurate fashion.16
It has an added advantage over other conventional drug delivery device for transdermal route.
1. Very short pulses practically eliminate thermal damage
2. No carbonization, only fast ablation of skin tissue
3. Very accurate skin ablation in 5-10 µm steps
4. Scanner optics allows flexible formation of pore arrays
5. No need for installation through a qualified technician
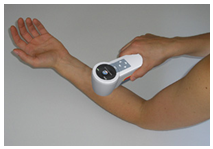
laser can effectively enhance and precisely control drug delivery via the skin,including nalbuphine17, 5-fluorouracil18, vitamin C19, 5-aminolevulinic acid20, and genes21.
Eligen Technology
This technology makes it possible to deliver a therapeutic molecule orally without altering its chemical form or biological integrity.
Barriers to Oral Drug Delivery22
Degradation
The high acid content and enzymes of the digestive tract can degrade some drugs, particularly protein and peptide drugs, before they are absorbed into the bloodstream.
Poor Absorption
Many macromolecules and charged compounds are poorly absorbed through biological membranes due to poor partitioning properties.
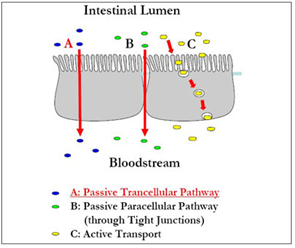
The Eligen Technology can inhibit acid and peptidase mediated degradation allowing sufficient time for the drug to be absorbed via the GI epithelium. Carrier’s interact with the drug molecule to transiently alter the poor absorption properties and facilitate drug molecules to cross cell membranes.
The Eligen Technology uses passive transcellular transport to enable drug molecules of all sizes to cross cell membranes. The carrier used is promptly eliminated by normal excretion pathways. This process will not affect the chemical nature of the drug molecule and the integrity of gastrointestinal absorptive cells remains unaffected.
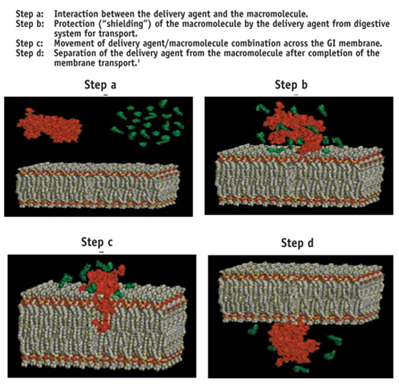
Eligen Technology has many advantages
a. Broad Applicability
The Eligen Technology is applicable to a diverse group of drug molecules (e.g., proteins, peptides and other poorly absorbed compounds). This technology can orally delivered a wide range of drugs successfully; from small molecules to drugs with molecular weight of more than 20,000 Daltons.
b. Stand-Alone Delivery Approach
The Eligen system does not rely upon the addition of other agents that can have adverse effects on the intestinal membranes or other physiologic process (e.g., penetration enhancers or enzyme inhibitors).
c. Versatility of Formulation
Various types of oral formulations have been introduced using the Eligen Technology, including solutions, tablets, and capsules. This system is also applicable to controlled release dosage forms.
d. Ease of Manufacture
The manufacturing set up required to produce Eligen delivery agents at commercial scale is easy.
e. Ease of manufacture of Drug/carrier Mixture
A simple blending step with drug and carrier in the appropriate proportions is all that is required to produce an Eligen formulation.
Oral delivery of insulin with the Eligen technology
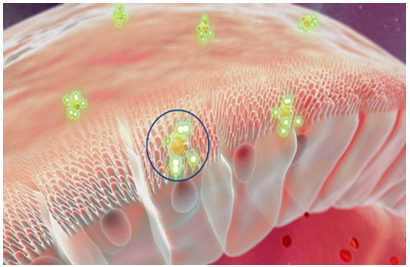
The development of oral insulin using the Eligen technology represents a significant advance in insulin administration which is expected to improve the quality of life of diabetic patients. As clinical studies progress, a great deal of interest has focused on the process by which this technology enables insulin absorption from the intestinal lumen into the bloodstream. The eligen technology employs low molecular weight compounds (termed drug delivery agents or carriers) which interact weakly and non-covalently with insulin, increasing its lipophilicity and thereby its ability to cross the gastrointestinal epithelium. By the use of this technology we can overcome many problems related to oral drug delivery formulations and can provide easy way of delivering the drug by the oral route.23
Eligen Technology in action. As depicted inside the blue circle, delivery agents (small white dots) chaperone a therapeutic molecule (the green entity) entering into the outer membrane of an epithelial gastrointestinal cell. The gastrointestinal tract is on the top and the blood stream is on the bottom right
Also the oral administration of Vit.B 12 using Eligen technology has been successfully studied. These studies shows that in future more oral drug delivery formulations can be available in the market based on this technology.24
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Nano Robotics
Nanorobots would constitute any smart structure capable of actuation, sensing, signalling, information processing, intelligence, manipulation and swarm behaviour at nano scale (10-9 m). Bionanorobits designed by harnessing properties of biological materials (peptides, DNA’s), their designs and functionalities. These are inspired not only by nature but machines too. Nanorobots could propose solutions at most of the nanomedicine problems.
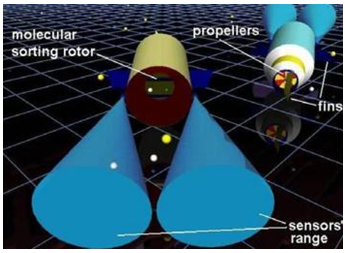
You can see above the “nanorobots’ design-sensors, molecular sorting rotors, fins and propellers. The depicted blue cones shows the sensors ‘touching’ areas.” They also have specific sensory capabilities to detect the target regions, obstacles and chemicals relevant for their medical application.”

Nanorobotics in Cancer therapy
Human knowledge, with all its growth and development, is still in its initial stages of finding efficient ways to treat cancer. The elevated number of cancer patients puts cancer treatment amongst the top priority of scientific research facilities. An important aspect in cancer therapy is the development of a targeted drug delivery system that decreases the toxic side effects of chemotherapy. The current conventional method in treating cancer involves inserting catheters to allow for chemotherapy, to reduce the amount of cancer present, and then to surgically remove the tumors, followed by more chemotherapy and radiation sessions. However, the delivery of the drug is not localized, so even the healthy cells that normally divide rapidly will be targeted by the treatment. The main manufacturing technique in early nanorobot sensor design takes advantage of the high precision technology of CMOS (Complementary Metal Oxide Semiconductor) VLSI (Very Large Scale Integration) system design.25 CMOS-based biosensors use nanowires as material for their circuit assembly. They can detect minimal chemical changes,26 such as E-cadherin and beta-catenin gradients, which can serve as chemical targets for detection of early metastatic phases.27
To help propel the nanorobot inside the body, an actuator needs to be implemented in the design. An actuator is a device that serves as an engine and helps the nanorobot move.
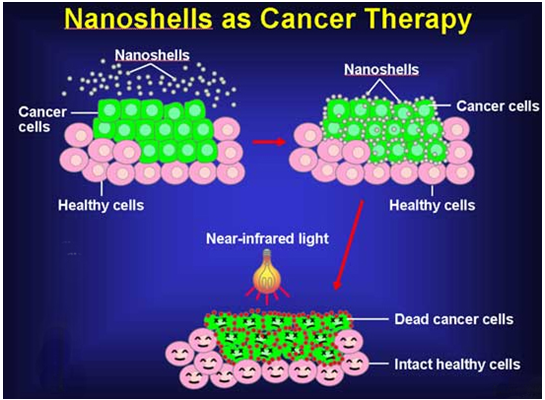
Nanorobots could be used to tag the cancerous cells, so that the surgeon could efficiently and precisely remove the tumor. Two methods have been used to target the nanoparticles to tumor sites, which are commonly known as active and passive targeting. In active targeting, the nanoparticle is linked to tumor-specific ligands,28 whereas passive targeting relies on the mere similarity in size of the nanoparticle with the unique properties of the tumor’s vasculature.29 Thus nanorobotics plays an important role in cancer therapy. It has its step on other diseases like arteriosclerosis, brain targeted diseases and even in heart transplantation.
Gene delivery Technology
The objective of gene therapy is to rescue mutant phenotypes by providing deficient cells with a normally functioning copy of the gene associated with the disease. In such rescues the mutant cells are complemented by the rescuing gene and reverted to wildtype function. This paradigm for gene therapy is only be useful in treating diseases caused by recessive mutations such as cystic fibrosis in which in a single functional copy of the gene is sufficient for the viability of the individual. Diseases caused by dominant alleles such as Huntington’s disease would most likely not be cured under this scheme because the presence of the mutant gene product from a single allele is lethal.
Gene therapy in Cancer
The gene delivery technology in cancer involves identifying the genes whose mutation causes cancer to spread and kill. The next is to develop more powerful gene delivery and expression technologies, so that we can correct these cancer-causing abnormal genes directly in patients.
The new gene therapy technologies are currently being tested in human patients with lung cancer and melanoma.
References:
1.Rashmi Boppana, Raghavendra V. Kulkarni, Chitrali Mallikarjun Setty and Navanath V. Kalyane., Carboxymethylcellulose ? aluminum hydrogel microbeads for prolonged release of simvastatin, Acta Pharmaceutica Scienci,52: 137-143,2010.
2.O Munjeri, J.H Collett, J.T Fell., Hydrogel beads based on amidated pectins for colon-specific drug delivery: the role of chitosan in modifying drug release, journal of controlled release, vol 46(3),1997.
3.Liu, Z., Jiao, Y. and Zhang, Z. (2007), Calcium-carboxymethyl chitosan hydrogel beads for protein drug delivery system. J. Appl. Polym. Sci., 103: 3164–3168. doi: 10.1002/app.24867
4.Saima Amin*, Saeid Rajabnezhad and Kanchan Kohli, Hydrogels as potential drug delivery systems, Scientific Research and Essay Vol. 3 (11), pp. 1175-1183, November,2009.
5.George K. Lewis Jr., Willam L. Olbricht, George K. Lewis., Acoustic enhanced evans blue dye perfusion in neurological tissues, Acoustical society of America, vol.2(1),2007.
6.Ghaleb A. Husseini, Mario A. Diaz de la Rosa, Eric S. Richardson, Douglas A. Christensen, and William G. Pitt, The Role of Cavitation in Acoustically Activated Drug Delivery, J Control Release, vol. 107(2), 2006.
7.Nabili, M. ;Mahesh, S. ; Ji Liu ; Belyea, D. ; Geist, C. ; Zderic, V. ; Zaghloul, M., Surface Acoustic Wave devices for ocular drug delivery,ultrasonic symposium (IUS),2010 IEEE.
8.O. Pillai, V. Nair, R. Poduri, R. Panchagnula, Transdermal iontophoresis. Part II: peptide and protein delivery, Meth. Find. Exp. Clin. Pharmacol. 21 (1999) 229–240.
9.A. Beignon, J. Briand, S. Muller, C.D. Partidos, Immunization onto bare skin with synthetic peptides: immunomodulation with a CpG-containing oligodeoxynucleotide and effective priming of influenza virus-specific CD4+T cells, Immunology 105 (2002) 204–212.
10.Y.L. Zhao, S.N. Murthy, M.H. Manjli, L.J. Guan, A. Sen, S.W. Hui, Introduction of cytotoxic T-lymphocytes by electroporation-enhanced needle-free skin immunization, Vaccine 24 (2006) 1282–1290.
11.N. Abla, A. Naik, R.H. Guy, Y.N. Kalia, Effect of charge and molecular weight on transdermal peptide delivery by iontophoresis, Pharm. Res. 22 (2005) 2069–2078.
12.G.M. Glenn, D.N. Taylor, X. Li, S. Frankel, A. Montemarano, C.R. Alving, Transcutaneous immunization: a human vaccine delivery strategy using a patch, Nat. Med. 6 (2000) 1403–1406.
13.T. Ogiso, M. Iwaki, T. Tanino, A. Yono, A. Ito, In vitro skin penetration and degradation of peptides and their analysis using a kinetic model, Biol. Pharm. Bull.23 (2000) 1346–1351.
14.S.Mitragotri, Immunization without needles, Nat. Rev. Immunol.12 (2005) 905–916.
15.P. Upadhyay, Enhanced transdermal-immunization with diphtheria-toxoid using local hyperthermia, Vaccine 24 (2006) 5593–5598.
16.medgadget.com/2008/02/pleasepainless_laser_epidermal_system.html
17.W.R. Lee, S.C. Shen, H.H. Lai, C.H. Hu, J.Y. Fang, Transdermal drug delivery enhanced and controlled by erbium:YAG laser: a comparative study of lipophilic and hydrophilic drugs, J. Control Release 75 (2001) 155–166.
18.W.R. Lee, S.C. Shen, K.H. Wang, C.H. Hu, J.Y. Fang, The effect of laser treatment on skin to enhance and control transdermal delivery of 5-fluorouracil, J. Pharm. Sci. 91 (2002) 1613–1626.
19.W.R. Lee, S.C. Shen, K.H. Wang, C.H. Hu, J.Y. Fang, Lasers and microdermabrasion enhance and control topical delivery of vitamin C, J. Invest. Dermatol. 121 (2003) 1118–1125.
20.S.C. Shen, W.R. Lee, Y.P. Fang, C.H. Hu, J.Y. Fang, In vitro percutaneous absorption and in vivo protoporphyrin IX accumulation in skin and tumors after topical 5-aminolevulinic acid application with enhancement using an erbium:YAG laser, J. Pharm. Sci. 95 (2006) 929–938.
21.W.R. Lee, S.C. Shen, C.J. Liu, C.L. Fang, C.H. Hu, J.Y. Fang, Erbium:YAG laser-ediated oligonucleotide and DNA delivery via the skin: an animal study, J. Control. Release 115 (2006) 344–353.
22.emisphere.com/technology_advantages.html
23.Malkov D, Angelo R, Wang HZ, Flanders E, Tang H, Gomez-Orellana I., oral delivery of insulin with the eligen technology, current drug delivery, Vol.2(2), 2005.
24.Michael V. Novinski, The “New” Emisphere: Impacting the Future of Drug Delivery, Drug Delivery Technology, Vol 8(5),2008.
25.Lambert B and Weitekamp D P. “Mechanical sensors of electromagnetic fields”. US Patent Specification 6835926, 2004.
26.A. S. G. Curtis, M. Dalby, N. Gadegaard. “Cell signaling arising from nanotopography: implications for nanomedical devices”. Nanomedicine J., Future Medicine. 2006; 1(1): 67-72.
27.Janda E, Nevolo M. Lehmann K, Downward J, Beug H and Grieco M. “Raf plus TGF beta-dependent EMT is initiated by endocytosis and lysosomal degradation of E-cadherin”. Nat. Oncogene. 2006; 25: 117-30.
28.Akerman ME, Chan WC, Laakkonen P, Bhatia SN, Ruoslahti, E. “Nanocrystal targeting in vivo”. Proc Natl Acad Sci USA. 2002; 99: 12617–12621.
29.Vasir JK, Labhasetwar V. “Targeted drug delivery in cancer therapy”. Technol Cancer Res Treat. 2005; 4: 363–374.
30.invitrogen.com/site/us/en/home/References/Molecular-Probes-The- and book/ Technical-Notes-and-Product-Highlights/BacMam-Gene-Delivery-Technology.html
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









