 About Authors:
About Authors:
Rahul kumar Singh
M.Pharm (Quality Assurance)
Sr. Officer Quality Assurance at Shreya Life Sciences Pvt. Ltd.,
Roorkee, Uttarakhand
rahulsingh.qa@gmail.com
Abstract
Miglitol, an oral alpha-glucosidase inhibitor, is a desoxynojirimycin derivative that delays the digestion of ingested carbohydrates, thereby resulting in a smaller rise in blood glucose concentration following meals. As a consequence of plasma glucose reduction, miglitol reduce levels of glycosylated hemoglobin in patients with Type II (non-insulin-dependent) diabetes mellitus. Systemic nonenzymatic protein glycosylation, as reflected by levels of glycosylated hemoglobin, is a function of average blood glucose concentration over time. Because its mechanism of action is different, the effect of miglitol to enhance glycemic control is additive to that of sulfonylureas when used in combination. In addition, miglitol diminishes the insulinotropic and weight-increasing effects of sulfonylureas. Miglitol has minor inhibitory activity against lactase and consequently, at the recommended doses, would not be expected to induce lactose intolerance.
Miglitol inhibits glycoside hydrolase enzymes called alpha-glucosidases. Since miglitol works by preventing digestion of carbohydrates, it lowers the degree of postprandial hyperglycemia. It must be taken at the start of main meals to have maximal effect. Its effect will depend on the amount of non-monosaccharide carbohydrates in a person's diet.
In contrast to acarbose (another alpha-glucosidase inhibitor), miglitol is systemically absorbed; however, it is not metabolized and is excreted by the kidneys.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1586
Introduction
Anti-diabetic drugs treat diabetes mellitusby lowering glucose levels in the blood. With the exceptions of insulin, exenatide, and pramlintide, all are administered orally and are thus also called oral hypoglycemic agents or oral antihyperglycemic agents. There are different classes of anti-diabetic drugs, and their selection depends on the nature of the diabetes, age and situation of the person, as well as other factors.
Diabetes mellitus type 1 is a disease caused by the lack of insulin. Insulin must be used in Type I, which must be injected or inhaled.
Diabetes mellitus type 2 is a disease of insulin resistance by cells. Treatments include (1) agents which increase the amount of insulin secreted by the pancreas, (2) agents which increase the sensitivity of target organs to insulin, and (3) agents which decrease the rate at which glucose is absorbed from the gastrointestinal tract.
Classification –
1-Insulin
2- Secretagogues
· 2.1 Sulfonylureas
· 2.2 Meglitinides
3- Sensitizers
· 3.1 Biguanides
· 3.2 Thiazolidinediones
4- Alpha-glucosidase inhibitors
5- Peptide analogs
· 5.1 Incretin mimetics
· 5.1.1 Glucagon-like peptide (GLP) analogs and agonists
· 5.1.2 Gastric inhibitory peptide (GIP) analogs
· 5.2 DPP-4 inhibitors
· 5.3 Amylin analogues
6- Experimental agents
7- Herbal extracts
Alpha-glucosidase inhibitorsare oral anti-diabetic drugs used for diabetes mellitus type 2that work by preventing the digestion of carbohydrates (such as starchand table sugar). Carbohydrates are normally converted into simple sugars (monosaccharides) which can be absorbed through the intestine. Hence, alpha-glucosidase inhibitors reduce the impact of carbohydrates on blood sugar.
Drugs used in alpha-glucosidase inhibitors:
- Acarbose- Precose
- Miglitol- Glyset
- Voglibose
[adsense:468x15:2204050025]
Even though the drugs have a similar mechanism of action, there are subtle differences between acarbose and miglitol. Acarbose is an oligosaccharide, whereas miglitol resembles a monosaccharide. Miglitol is fairly well-absorbed by the body, as opposed to acarbose. Moreover, acarbose inhibits pancreatic alpha-amylase in addition to alpha-glucosidase.
Clinical use
Alpha-glucosidase inhibitors are used to establish greater glycemic controlover hyperglycemia in diabetes mellitus type 2, particularly with regard to postprandial hyperglycemia. They may be used as monotherapy in conjunction with an appropriate diabetic diet and exercise, or they may be used in conjunction with other anti-diabetic drugs.
Alpha-glucosidase inhibitors may also be useful in patients with diabetes mellitus type 1; however, this use has not been officially approved by the Food and Drug Administration.
*Combination of miglitol, an anti-diabetic drug, and nicorandil markedly reduces myocardial infarct size through opening the mitochondrial KATP channels in rabbits.1
*Antidiabetic drug miglitol inhibits myocardial apoptosis involving decreased hydroxyl radical production and Bax expression in an ischaemia/reperfusion rabbit heart.2
*The α-glucosidase inhibitor miglitol suppresses postprandial hyperglycaemia and interleukin-1β and tumour necrosis factor-α gene expression in rat peripheral leucocytes induced by intermittent sucrose loading.
Mechanism of action
Alpha-glucosidase inhibitors are saccharides that act as competitive inhibitors of enzymes needed to digest carbohydrates: specifically alpha-glucosidase enzymes in the brush border of the small intestines. The membrane-bound intestinal alpha-glucosidaseshydrolyze oligosaccharides, trisaccharides, and disaccharides to glucose and other monosaccharides in the small intestine.
Acarbose also blocks pancreatic alpha-amylase in addition to inhibiting membrane-bound alpha-glucosidases. Pancreatic alpha-amylase hydrolyzes complex starches to oligosaccharides in the lumen of the small intestine.
Inhibition of these enzyme systems reduces the rate of digestion of carbohydrates. Less glucose is absorbed because the carbohydrates are not broken down into glucose molecules. In diabetic patients, the short-term effect of these drugs therapies is to decrease current blood glucose levels: the long term effect is a small reduction in haemoglobin.
In contrast to sulfonylureas, miglitol does not enhance insulin secretion. The antihyperglycemic action of miglitol results from a reversible inhibition of membrane-bound intestinal α-glucoside hydrolase enzymes. Membrane-bound intestinal α-glucosidases hydrolyze oligosaccharides and disaccharides to glucose and other monosaccharides in the brush border of the small intestine. In diabetic patients, this enzyme inhibition results in delayed glucose absorption and lowering of postprandial hyperglycemia.
Because its mechanism of action is different, the effect of miglitol to enhance glycemic control is additive to that of sulfonylureas when used in combination. In addition, miglitol diminishes the insulinotropic and weight-increasing effects of sulfonylureas.
Miglitol has minor inhibitory activity against lactase and consequently, at the recommended doses, would not be expected to induce lactose intolerance.
Dosing
There is no fixed dosage regimen for the management of diabetes mellitus with miglitol any other pharmacologic agent. Dosage of miglitolmust be individualized on the basis of both effectiveness and tolerance while not exceeding the maximum recommended dosage of 100 mg 3 times daily. Miglitol should be taken three times daily at the start (with the first bite) of each main meal. miglitolshould be started at 25 mg, and the dosage gradually increased as described below, both to reduce gastrointestinal adverse effects and to permit identification of the minimum dose required for adequate glycemic control of the patient.
During treatment initiation and dose titration , one-hour postprandial plasma glucose may be used to determine the therapeutic response to miglitoland identify the minimum effective dose for the patient. Thereafter, glycosylated hemoglobin should be measured at intervals of approximately three months. The therapeutic goal should be to decrease both postprandial plasma glucose and glycosylated hemoglobin levels to normal or near normal by using the lowest effective dose of miglitoleither as monotherapy or in combination with a sulfonylurea.
Since alpha-glucosidase inhibitors are competitive inhibitors of the digestive enzymes, they must be taken at the start of main meals to have maximal effect. Their effects on blood sugar levels following meals will depend on the amount of complex carbohydrates in the meal.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Initial Dosage
The recommended starting dosage of miglitol is 25 mg, given orally three times daily at the start (with the first bite) of each main meal. However, some patients may benefit by starting at 25 mg once daily to minimize gastrointestinal adverse effects, and gradually increasing the frequency of administration to 3 times daily.
Maintenance Dosage
The usual maintenance dose of miglitol is 50 mg 3 times daily, although some patients may benefit from increasing the dose to 100 mg 3 times daily. In order to allow adaptation to potential gastrointestinal adverse effects, it is recommended that miglitol therapy be initiated at a dosage of 25 mg 3 times daily, the lowest effective dosage, and then gradually titrated upward to allow adaptation. After 4 - 8 weeks of the 25 mg 3 times daily regimen, the dosage should be increased to 50 mg 3 times daily for approximately three months, following which a glycosylated hemoglobin level should be measured to assess therapeutic response. If, at that time, the glycosylated hemoglobin level is not satisfactory, the dosage may be further increased to 100 mg 3 times daily, the maximum recommended dosage.
Maximum Dosage
The maximum recommended dosage of miglitol is 100 mg 3 times daily. In one clinical trial, 200 mg 3 times daily gave additional improved glycemic control but increased the incidence of the gastrointestinal symptoms described above.
Side effects & precautions
Since alpha-glucosidase inhibitors prevent the degradation of complex carbohydrates into glucose, the carbohydrates will remain in the intestine. In the colon, bacteria will digest the complex carbohydrates, thereby causing gastrointestinal side effects such as flatulence and diarrhea. Since these effects are dose-related, it is generally advised to start with a low dose and gradually increase the dose to the desired amount. Voglibose, in contrast to acarbose, has less of these side effects, and is hence preferred lately[citation needed]. It is also more economical compared to acarbose[citation needed].
If a patient using an alpha-glucosidase inhibitor suffers from an episode of hypoglycemia, the patient should eat something containing monosaccharides, such as glucose tablets. Since the drug will prevent the digestion of polysaccharides (or non-monosaccharides), non-monosaccharide foods may not effectively reverse a hypoglycemic episode in a patient taking an alpha-glucosidase inhibitor.
Pharmacokinetics
Miglitol is a desoxynojirimycin derivative that delays the digestion of ingested carbohydrates, thereby resulting in a smaller rise in blood glucose concentration following meals. As a consequence of plasma glucose reduction, GLYSET Tablets reduce levels of glycosylated hemoglobin in patients with Type II (non-insulin-dependent) diabetes mellitus. Systemic nonenzymatic protein glycosylation, as reflected by levels of glycosylated hemoglobin, is a function of average blood glucose concentration over time.Some pharmacokinitic parameters are as follows -
Absorption
Absorption of miglitol is saturable at high doses: a dose of 25 mg is completely absorbed, whereas a dose of 100 mg is only 50% - 70% absorbed. For all doses, peak concentrations are reached in 2-3 hours. There is no evidence that systemic absorption of miglitol contributes to its therapeutic effect.
Distribution
The protein binding of miglitol is negligible (<4.0%). Miglitol has a volume of distribution of 0.18 L/kg, consistent with distribution primarily into the extracellular fluid.
Metabolism
Miglitol is not metabolized in man or in any animal species studied. No metabolites have been detected in plasma, urine, or feces, indicating a lack of either systemic or pre-systemic metabolism.
Excretion
Miglitol is eliminated by renal excretion as unchanged drug. Thus, following a 25-mg dose, over 95% of the dose is recovered in the urine within 24 hours. At higher doses, the cumulative recovery of drug from urine is somewhat lower due to the incomplete bioavailability. The elimination half-life of miglitol from plasma is approximately 2 hours.
Half life
The elimination half life of meglitol from plasma is approximately 2 hours .
Protein binding
Protein binding of meglitol is negligible (< 4%)
Food Interactions
Take with food, at beginning of each meal. Iron needs increased.
Side effects-
Gastrointestinal
Gastrointestinal symptoms are the most common reactions to GLYSET Tablets. In U.S. placebo-controlled trials, the incidences of abdominal pain, diarrhea, and flatulence were 11.7%, 28.7%, and 41.5% respectively in 962 patients treated with GLYSET 25-100 mg 3 times daily, whereas the corresponding incidences were 4.7%, 10.0%, and 12.0% in 603 placebo-treated patients. The incidence of diarrhea and abdominal pain tended to diminish considerably with continued treatment.
Dermatologic
Skin rash was reported in 4.3% of patients treated with GLYSET compared to 2.4% of placebo-treated patients. Rashes were generally transient and most were assessed as unrelated to miglitol by physician-investigators.
Abnormal Laboratory Findings
Low serum iron occurred more often in patients treated with GLYSET (9.2%) than in placebo-treated patients (4.2%) but did not persist in the majority of cases and was not associated with reductions in hemoglobin or changes in other hematologic indices.
druginteraction
Several studies investigated the possible interaction between miglitol and glyburide. In six healthy volunteers given a single dose of 5-mg glyburide on a background of 6 days treatment with miglitol (50 mg 3 times daily for 4 days followed by 100 mg 3 times daily for 2 days) or placebo, the mean Cmax and AUC values for glyburide were 17% and 25% lower, respectively, when glyburide was given with miglitol. In a study in diabetic patients in which the effects of adding miglitol 100 mg 3 times daily x 7 days or placebo to a background regimen of 3.5 mg glyburide daily were investigated, the mean AUC value for glyburide was 18% lower in the group treated with miglitol, although this difference was not statistically significant. Further information on a potential interaction with glyburide was obtained from one of the large U.S. clinical trials (Study 7) in which patients were dosed with either miglitol or placebo on a background of glyburide 10 mg twice daily. At the 6-month and 1-year clinic visits, patients taking concomitant miglitol 100 mg 3 times daily exhibited mean Cmax values for glyburide that were 16% and 8% lower, respectively, compared to patients taking glyburide alone. However, these differences were not statistically significant. Thus, although there was a trend toward lower AUC and Cmax values for glyburide when co-administered with miglitol (GLYSET), no definitive statement regarding a potential interaction can be made based on the foregoing three studies.
The effect of miglitol (100 mg 3 times daily x 7 days) on the pharmacokinetics of a single 1000-mg dose of metformin was investigated in healthy volunteers. Mean AUC and Cmax values for metformin were 12% to 13% lower when the volunteers were given miglitol as compared with placebo, but this difference was not statistically significant.
In a healthy volunteer study, co-administration of either 50 mg or 100 mg miglitol 3 times daily together with digoxin reduced the average plasma concentrations of digoxin by 19% and 28%, respectively. However, in diabetic patients under treatment with digoxin, plasma digoxin concentrations were not altered by co-administration of miglitol 100 mg 3 times daily x 14 days.
Other healthy volunteer studies have demonstrated that miglitol may significantly reduce the bioavailability of ranitidine and propranolol by 60% and 40%, respectively. No effect of miglitol was observed on the pharmacokinetics or pharmacodynamics of either warfarin or nifedipine.
Intestinal adsorbents (e.g., charcoal) and digestive enzyme preparations containing carbohydrate-splitting enzymes (e.g., amylase, pancreatin) may reduce the effect of miglitol and should not be taken concomitantly.
In 12 healthy males, concomitantly administered antacid did not influence the pharmacokinetics of miglitol.
Precautions
Hypoglycemia
Because of its mechanism of action, miglitol when administered alone should not cause hypoglycemia in the fasted or postprandial state. Sulfonylurea agents may cause hypoglycemia. Because miglitol Tablets given in combination with a sulfonylurea will cause a further lowering of blood glucose, it may increase the hypoglycemic potential of the sulfonylurea, although this was not observed in clinical trials. Oral glucose (dextrose), whose absorption is not delayed by miglitol, should be used instead of sucrose (cane sugar) in the treatment of mild-to-moderate hypoglycemia. Sucrose, whose hydrolysis to glucose and fructose is inhibited by miglitol, is unsuitable for the rapid correction of hypoglycemia. Severe hypoglycemia may require the use of either intravenous glucose infusion or glucagon injection.
Loss of Control of Blood Glucose
When diabetic patients are exposed to stress such as fever, trauma, infection, or surgery, a temporary loss of control of blood glucose may occur. At such times, temporary insulin therapy may be necessary.
Renal Impairment
Plasma concentrations of miglitol in renally impaired volunteers were proportionally increased relative to the degree of renal dysfunction. Long-term clinical trials in diabetic patients with significant renal dysfunction (serum creatinine >2.0 mg/dL) have not been conducted. Therefore, treatment of these patients with miglitol is not recommended.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Miglitol was administered to mice by the dietary route at doses as high as approximately 500 mg/kg body weight (corresponding to greater than 5 times the exposure in humans based on AUC) for 21 months. In a two-year rat study, miglitol was administered in the diet at exposures comparable to the maximum human exposures based on AUC. There was no evidence of carcinogenicity resulting from dietary treatment with miglitol.
In vitro, miglitol was found to be nonmutagenic in the bacterial mutagenesis (Ames) assay and the eukaryotic forward mutation assay (CHO/HGPRT). Miglitol did not have any clastogenic effects in vivo in the mouse micronucleus test. There were no heritable mutations detected in dominant lethal assay.
A combined male and female fertility study conducted in Wistar rats treated orally with miglitol at dose levels of 300 mg/kg body weight (approximately 8 times the maximum human exposure based on body surface area) produced no untoward effect on reproductive performance or capability to reproduce. In addition, survival, growth, development, and fertility of the offspring were not compromised.
Pregnancy
The safety of miglitol (GLYSET) in pregnant women has not been established. Developmental toxicology studies have been performed in rats at doses of 50, 150 and 450 mg/kg, corresponding to levels of approximately 1.5, 4, and 12 times the maximum recommended human exposure based on body surface area. In rabbits, doses of 10, 45, and 200 mg/kg corresponding to levels of approximately 0.5, 3, and 10 times the human exposure were examined. These studies revealed no evidence of fetal malformations attributable to miglitol. Doses of miglitol up to 4 and 3 times the human dose (based on body surface area), for rats and rabbits, respectively, did not reveal evidence of impaired fertility or harm to the fetus. The highest doses tested in these studies, 450 mg/kg in the rat and 200 mg/kg in the rabbit promoted maternal and/or fetal toxicity. Fetotoxicity was indicated by a slight but significant reduction in fetal weight in the rat study and slight reduction in fetal weight, delayed ossification of the fetal skeleton and increase in the percentage of non-viable fetuses in the rabbit study. In the peri-postnatal study in rats, the NOAEL (No Observed Adverse Effect Level) was 100 mg/kg (corresponding to approximately four times the exposure to humans, based on body surface area). An increase in stillborn progeny was noted at the high dose (300 mg/kg) in the rat peri-postnatal study, but not at the high dose (450 mg/kg) in the delivery segment of the rat developmental toxicity study. Otherwise, there was no adverse effect on survival, growth, development, behavior, or fertility in either the rat developmental toxicity or peri-postnatal studies. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Nursing Mothers
Miglitol has been shown to be excreted in human milk to a very small degree. Total excretion into milk accounted for 0.02% of a 100-mg maternal dose. The estimated exposure to a nursing infant is approximately 0.4% of the maternal dose. Although the levels of miglitol reached in human milk are exceedingly low, it is recommended that miglitol not be administered to a nursing woman.
Pediatric Use
Safety and effectiveness of miglitol in pediatric patients have not been established.
Geriatric Use
Of the total number of subjects in clinical studies of miglitol in the United States, patients valid for safety analyses included 24% over 65, and 3% over 75. No overall differences in safety and effectiveness were observed between these subjects and younger subjects. The pharmacokinetics of miglitol were studied in elderly and young males (n=8 per group). At the dosage of 100 mg 3 times daily for 3 days, no differences between the two groups were found.
overdose
Unlike sulfonylureas or insulin, an overdose of GLYSET Tablets will not result in hypoglycemia. An overdose may result in transient increases in flatulence, diarrhea, and abdominal discomfort. Because of the lack of extra-intestinal effects seen with GLYSET, no serious systemic reactions are expected in the event of an overdose.
Missed dose:If you miss a dose, use it as soon as you remember. If it is near the time of the next dose, skip the missed dose and resume your usual dosing schedule. Do not double the dose to catch up.
Storage:Store at room temperature at 77 degrees F (25 degrees C) away from light and moisture. Brief storage between 59-86 degrees F (15-30 degrees C) is permitted. Do not store in the bathroom. Keep all medicines away from children and pets.
Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company for more details about how to safely discard your product.
Special Populations
Renal Impairment
Because miglitol is excreted primarily by the kidneys, accumulation of miglitol is expected in patients with renal impairment. Patients with creatinine clearance <25 mL/min taking 25 mg 3 times daily exhibited a greater than two-fold increase in miglitol plasma levels as compared to ubjects with creatinine clearance >60 mL/min. Dosage adjustment to correct the increased plasma concentrations is not feasible because miglitol acts locally. Little information is available on the safety of miglitol in patients with creatinine clearance <25 mL/min.
Hepatic impairment
Miglitol pharmacokinetics were not altered in cirrhotic patients relative to healthy control subjects. Since miglitol is not metabolized, no influence of hepatic function on the kinetics of miglitol is expected.
Gender
No significant difference in the pharmacokinetics of miglitol was observed between elderly men and women when body weight was taken into account.
Race
Several pharmacokinetic studies were conducted in Japanese volunteers, with results similar to those observed in Caucasians. A study comparing the pharmacodynamic response to a single 50-mg dose in Black and Caucasian healthy volunteers indicated similar glucose and insulin responses in both populations.
Miglitol (GLYSET Tablets)
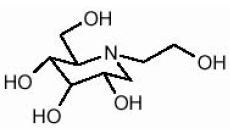
GLYSET Tablets contain miglitol, an oral alpha-glucosidase inhibitor for use in the management of non-insulin-dependent diabetes mellitus (NIDDM). Miglitol is a desoxynojirimycin derivative, and is chemically known as 3,4,5-piperidinetriol, 1-(2-hydroxyethyl)-2-(hydroxymethyl)-, [2R-(2α,3β,4α, 5β)]-. It is a white to pale-yellow powder with a molecular weight of 207.2. Miglitol is soluble in water and has a pKa of 5.9. Its empirical formula is C8H17NO5 and its chemical structure is as follows:
GLYSET is available as 25 mg, 50 mg and 100 mg tablets for oral use. The inactive ingredients are starch, microcrystalline cellulose, magnesium stearate, hypromellose, polyethylene glycol, titanium dioxide, and polysorbate 80.
|
Strength |
NDC |
Tablet Identification |
|
|
Front |
Back |
||
|
25 mg |
0009-5012-01 |
GLYSET |
25 |
|
50 mg |
0009-5013-01 |
GLYSET |
50 |
|
100 mg |
0009-5014-01 |
GLYSET |
100 |
Table 1: Results of Monotherapy Study with GLYSET
|
Study |
Treatment |
HbA1c (%) |
1-hour Postprandial Glucose (mg/dL) |
||
|
Mean Change from Baseline* |
Treatment Effect** |
Mean Change from Baseline |
Treatment Effect** |
||
|
1 (U.S.) |
Placebo |
+0.71 |
--- |
+24 |
--- |
|
GLYSET 50 mg t.i.d.*** |
+0.13 |
-0.58† |
-39 |
-63† |
|
|
2 (U.S.) |
Placebo |
+0.47 |
--- |
+15 |
--- |
|
GLYSET 50 mg t.i.d. |
-0.22 |
-0.69† |
-52 |
-67† |
|
|
GLYSET 100 mg t.i.d. |
-0.28 |
-0.75† |
-59 |
-74† |
|
|
3 (non-U.S.) |
Placebo |
+0.18 |
--- |
+2 |
--- |
|
GLYSET 25 mg t.i.d. |
-0.08 |
-0.26 |
-33 |
-35† |
|
|
GLYSET 50 mg t.i.d. |
-0.22 |
-0.40 |
-45 |
-47† |
|
|
GLYSET 100 mg t.i.d. |
-0.63 |
-0.81† |
-62 |
-64† |
|
|
GLYSET 200 mg t.i.d.‡ |
-0.84 |
-1.02† |
-85 |
-87† |
|
|
4 (non-U.S.) |
Placebo |
+0.01 |
--- |
+8 |
--- |
|
GLYSET 50 mg t.i.d. |
-0.35 |
-0.36† |
-20 |
-28† |
|
|
GLYSET 100 mg t.i.d. |
-0.57 |
-0.58† |
-25 |
-33† |
|
|
5 (non-U.S.) |
Placebo |
+0.32 |
--- |
+17 |
--- |
|
GLYSET 100 mg t.i.d. |
-0.43 |
-0.75† |
-38 |
-55† |
|
|
*Mean baseline ranged from 7.54 to 8.72% in these studies. |
|||||
Table 2: Results of Combination Therapy with GLYSET Plus Sulfonylurea (SFU)
|
Study |
Treatment |
HbA 1c (%) |
1-hour Postprandial Glucose (mg/dL) |
||
|
Mean Change from Baseline* |
Treatment Effect** |
Mean Change from Baseline |
Treatment Effect** |
||
|
6 (U.S.) |
Placebo + SFU |
+0.33 |
--- |
-1 |
--- |
|
GLYSET 50 mg t.i.d.*** + SFU |
-0.49 |
-0.82† |
-69 |
-68† |
|
|
GLYSET 100 mg t.i.d. + SFU |
-0.41 |
-0.74† |
-73 |
-72† |
|
|
7 (U.S.) |
Placebo + SFU |
+1.01 |
--- |
48 |
--- |
|
GLYSET 25 mg t.i.d. + SFU |
+0.71 |
-0.30 |
-2 |
-50† |
|
|
GLYSET 50 mg t.i.d. + SFU |
+0.39 |
-0.62† |
-13 |
-61† |
|
|
GLYSET 100 mg t.i.d. + SFU |
+0.28 |
-0.73† |
-33 |
-81† |
|
|
8 (non-U.S.) |
Placebo + SFU |
+0.16 |
--- |
+10 |
--- |
|
GLYSET 100 mg t.i.d. + SFU |
-0.50 |
-0.66† |
-36 |
-46† |
|
|
|
|||||
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Clinical Studies
Clinical Experience in Non-Insulin-Dependent Diabetes Mellitus (NIDDM) Patients on Dietary Treatment Only
GLYSET Tablets were evaluated in two U.S. and three non-U.S. controlled, fixed-dose, monotherapy studies, in which 735 patients treated with GLYSET were evaluated for efficacy analyses (see Table 1).
In Study 1, a one-year study in which GLYSET was evaluated as monotherapy and also as combination therapy, there was a statistically significantly smaller increase in mean glycosylated hemoglobin (HbA1c) over time in the miglitol 50 mg 3 times daily monotherapy arm compared to placebo. Significant reductions in mean fasting and postprandial plasma glucose levels and in mean postprandial insulin levels were observed in patients treated with GLYSET compared with the placebo group.
In Study 2, a 14-week study, there was a significant decrease in HbA1c in patients receiving GLYSET 50 mg 3 times daily or 100 mg 3 times daily compared to placebo. In addition, there were significant reductions in postprandial plasma glucose and postprandial serum insulin levels compared to placebo.
Study 3 was a 6-month dose-ranging trial evaluating GLYSET at doses from 25 mg 3 times daily to 200 mg 3 times daily. GLYSET produced a greater reduction in HbA1c than placebo at all doses, although the effect was statistically significant only at the 100 mg 3 times daily and 200 mg 3 times daily doses. In addition, all doses of GLYSET









