 About Author: DEVANG V. PATEL*, Manju Misra
About Author: DEVANG V. PATEL*, Manju Misra
M.S.(PHARM.) PHARMACEUTICS
NATIONAL INSTITUTE OF PHARMACEUTICAL EDUCATION & RESEARCH (NIPER), AHMEDABAD.
Abstract
Drug targeting is the ability to direct a therapeutic agent specifically to desired site of action with little or no interaction with nontarget tissue. Niosomes are one of the best carriers for drug targeting. Niosomes (non-ionic surfactant vesicles) are microscopic lamellar structures formed on admixture of non-ionic surfactant of the alkyl or dialkyl polyglycerol ether class and cholesterol with subsequent hydration in aqueous media. Niosomes are biodegradable, relatively nontoxic, more stable and inexpensive, an alternative to liposomes. Niosomes can be SUV (Small Unilamellar Vesicles), MLV (Multilamellr Vesicles) or LUV (Large Unilamellar Vesicles). The method of preparation of niosome is the based on liposome technology. The basic process of preparation is the same i.e. hydration of the lipid phase by aqueous phase. After preparing niosomal dispersion, unentrapped drug is separated by dialysis, centrifugation or gel filtration. Niosomes are characterized by vesicle size, bilayer formation, number of lamellae, membrane rigidity and entrapment efficiency. A method of in-vitro release rate study includes the use of dialysis tubing. Niosomal drug delivery is potentially applicable to many pharmacological agents for their action against various diseases including cancer and leishmaniasis.
[adsense:336x280:8701650588]
Introduction
The ideal drug delivery system delivers drug at rate decided by the need of the body throughout the period of treatment and it provides the active entity solely to the site of action.
The concept of targeted drug delivery is designed for attempting to concentrate the drug in the tissues of interest while reducing the relative concentration of the medication in the remaining tissues. As a result, drug is localized on the targeted site. Hence, surrounding tissues are not affected by the drug. In addition, loss of drug does not happen due to localization of drug, leading to get maximum efficacy of the medication. Different carriers have been used for targeting of drug, such as immunoglobulin, serum proteins, synthetic polymers, liposome, microspheres, erythrocytes and niosomes.
Niosomes are one of the best among these carriers.[1]
Niosomes (non-ionic surfactant vesicles) are microscopic lamellar structures obtained on admixture of non-ionic surfactant of the alkyl or dialkyl polyglycerol ether class and cholesterol with subsequent hydration in aqueous media.[2]
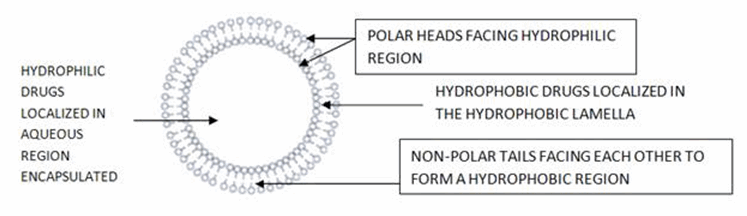
The non-ionic surfactants form a closed bilayer vesicle in aqueous media based on its amphiphilic nature using some energy for instance heat, physical agitation to form this structure. In the bilayer structure, hydrophobic parts are oriented away from the aqueous solvent, whereas the hydrophilic heads remain in contact with the aqueous solvent. The properties of the vesicles can be changed by varying the composition of the vesicles, size, lamellarity, tapped volume, surface charge and concentration. Various forces act inside the vesicle, e.g., van der Waals forces among surfactant molecules, repulsive forces emerging from the electrostatic interactions among charged groups of surfactant molecules, entropic repulsive forces of the head groups of surfactants, short-acting repulsive forces, etc. These forces are responsible for maintaining the vesicular structure of niosomes. But, the stability of niosomes are affected by type of surfactant, nature of encapsulated drug, storage temperature, detergents, use of membrane spanning lipids, the interfacial polymerization of surfactant monomers in situ, inclusion of charged molecule.[3]
Niosomes may act as a depot, releasing the drug in a controlled manner. The therapeutic performance of the drug molecules can also be improved by delayed clearance from the circulation, protecting the drug from biological environment and restricting effects to target cells.[4] It can also be used as vehicle for poorly absorbable drugs to design the novel drug delivery system. It enhances the bioavailability by crossing the anatomical barrier of gastrointestinal tract via transcytosis of M cells of Peyer's patches in the intestinal lymphatic tissues.[5]
The niosomal vesicles are taken up by reticulo-endothelial system. Such localized drug accumulation is used in treatment of diseases, such as leishmaniasis, in which parasites invade cells of liver and spleen.[6,7] Some non-reticulo-endothelial systems like immunoglobulins also recognize lipid surface of this delivery system.[3,4,6-12] Encapsulation of various anti-neoplastic agents in this carrier vesicle has minimized drug-induced toxic side effects while maintaining, or in some instances, increasing the anti-tumour efficacy.[13] Many drugs are administered through niosomes via transdermal route to improve the therapeutic efficacy.
Niosomes provides better drug concentration at the site of action administered by oral, parenteral and topical routes. The evolution of niosomal drug delivery technology is still at the stage of infancy, but this type of drug delivery system has shown promise in cancer chemotherapy and anti-leishmanial therapy.
Advantages of Niosomes[14]
• Since the structure of the niosome offers place to accommodate hydrophilic, lipophilic as well as ampiphilic drug moieties, they can be used for a variety of drugs.
• Niosomes exhibits flexibility in their structural characteristics (composition, fluidity and size) and can be designed according to the desired situation.
• They improve the therapeutic performance of the drug by protecting it from the biological environment and restricting effects to target cells, thereby reducing the clearance of the drug.
• Niosomes can act as a depot to release the drug slowly and offer a controlled release.
• They can increase the oral bioavailability of drugs.
• They are osmotically active and stable.
• They increase the stability of the entrapped drug.
• They can enhance the skin penetration of drugs.
• They can be made to reach the site of action by oral, parenteral as well as topical routes.
• The surfactants are biodegradable, biocompatible, and non-immunogenic
• The niosomal dispersions in an aqueous phase can be emulsified in a non-aqueous phase to control the release rate of the drug and administer normal vesicles in external non-aqueous phase.
• Handling and storage of surfactants do not require any special conditions.
• The vesicle suspension being water based offers greater patient compliance over oily dosage forms.
Comparison of Niosomes vs. Liposomes
(i) Liposomes exhibit certain disadvantages such as: they are expensive, their ingredients like phospholipids are chemically unstable because of their predisposition to oxidative degradation, they require special storage and handling and purity of natural phospholipids is variable. Niosomes do not have any of these problems.
(ii) Differences in characteristics exist between liposomes and niosomes, especially since niosomes are prepared from uncharged single-chain surfactant and cholesterol whereas liposomes are prepared from double-chain phospholipids (neutral or charged).
(iii) Niosomes behave in-vivo like liposomes, prolonging the circulation of entrapped drug and altering its organ distribution and metabolic stability.[10] Such vesicular drug carrier systems alter the plasma clearance kinetics, tissue distribution, metabolism and cellular interaction of the drug.[10,15] They can be expected to target the drug to its desired site of action and/or to control its release.[4]
(iv) As with liposomes, the properties of niosomes depends both on the composition of the bilayer and on method of their production.[11]
(v) The entrapment efficiency increases with increase in the concentration and lipophilicity of surfactant. Chandraprakash et al made Methotrexate loaded non-ionic surfactant vesicles using lipophilic surfactants like Span 40, Span 60 and Span 80 and found that Span 60 (HLB = 4.7) gave highest percent entrapment while Span 85 (HLB = 9.8) gave least entrapment. They also observed that as HLB value of surfactant decreased, the mean size was reduced.[16]
Types of Niosomes Based on the vesicle size, niosomes can be divided into three groups: (i) Small Unilamellar Vesicles (SUV, Size=0.025-0.05 μm), (ii) Multilamellar Vesicles (MLV, Size=>0.05 μm), (iii) Large Unilamellar Vesicles (LUV, Size=>0.10 μm).
Methods of Preparation of Niosomes
Niosomes are prepared by different methods based on the desired sizes of the vesicles and their distribution, number of double layers, entrapment efficiency of the aqueous phase and permeability of vesicle membrane.
(i) Preparation of Small Unilamellar Vesicles
(a) Sonication: The aqueous phase containing drug is added to the mixture of surfactant and cholesterol in a scintillation vial.[7] The mixture is probe sonicated at 60°C for 3 minutes to produce small and uniform in size niosomes.
(b) Micro fluidization: Micro fluidization is a recent technique to prepare unilamellar vesicles of defined size distribution. This method is based on submerged jet principle in which two fluidized streams interact at ultra high velocities, in precisely defined micro channels within the interaction chamber. The impingement of thin liquid sheet along a common front is arranged such that the energy supplied to the system remains within the area of niosomes formation. The result is a greater uniformity, smaller size and better reproducibility of niosomes formed.[17]
(ii) Preparation of Multilamellar Vesicles
(a) Hand shaking method (Thin film hydration technique): In the hand shaking method, surfactant and cholesterol are dissolved in a volatile organic solvent (such as diethyl ether, chloroform or methanol) in a round bottom flask. The organic solvent is removed at room temperature (20°C) using rotary evaporator leaving a thin layer of solid mixture deposited on the wall of the flask. The dried surfactant film is hydrated with aqueous phase containing drug at 50-60°C with gentle agitation. This process forms typical multilamellar niosomes.[7]
(b) Trans-membrane pH gradient (inside acidic) drug uptake process (remote loading): Surfactant and cholesterol are dissolved in chloroform.[18] The solvent is then evaporated under reduced pressure to obtain a thin film on the wall of the round-bottom flask. The film is hydrated with 300 mM citric acid (pH 4.0) by vortex mixing. The multilamellar vesicles are frozen and thawed three times and later sonicated. To this niosomal suspension, aqueous solution containing 10 mg/ml of drug is added and vortexed. The pH of the sample is then raised to 7.0-7.2 with 1M disodium phosphate. This mixture is later heated at 60°C for 10 minutes to produce the desired multilamellar vesicles.
(iii) Preparation of Large Unilamellar Vesicles
(a) Reverse phase evaporation technique (REV): In this method, cholesterol and surfactant are dissolved in a mixture of ether and chloroform.[19] An aqueous phase containing drug is added to this and the resulting two phases are sonicated at 4-5°C. The clear gel formed is further sonicated after the addition of a small amount of phosphate buffered saline. The organic phase is removed at 40°C under low pressure. The resulting viscous niosome suspension is diluted with phosphate-buffered saline and heated in a water bath at 60°C for 10 min to yield niosomes.
(b) Ether injection method: The ether injection method is essentially based on slow injection of niosomal ingredients in diethyl ether through a 14-gauge needle at the rate of approximately 0.25 ml/min into a preheated aqueous phase maintained at 60°C.[7,20] The probable reason behind the formation of larger unilamellar vesicles is that the slow vaporization of solvent results in an ether gradient extending towards the interface of aqueous-nonaqueous interface. The former may be responsible for the formation of the bilayer structure. The disadvantages of this method are that a small amount of ether is frequently present in the vesicles suspension and is difficult to remove.
(iv) Miscellaneous
(a) Multiple membrane extrusion method: A mixture of surfactant, cholesterol, and diacetyl phosphate in chloroform is made into thin film by evaporation. The film is hydrated with aqueous drug solution and the resultant suspension extruded through polycarbonate membranes, which are placed in a series for up to eight passages. This is a good method for controlling niosome size.[17]
(b) Emulsion method: The oil in water (o/w) emulsion is prepared from an organic solution of surfactant, cholesterol, and an aqueous solution of the drug.[21,22] The organic solvent is then evaporated, leaving niosomes dispersed in the aqueous phase.
(c) Lipid injection method: This method does not require expensive organic phase. Here, the mixture of lipids and surfactant is first melted and then injected into a highly agitated heated aqueous phase containing dissolved drug. Here, the drug can be dissolved in molten lipid and the mixture will be injected into agitated, heated aqueous phase containing surfactant.
(d) The “bubble” method: It is novel technique for the one step preparation of liposomes and niosomes without the use of organic solvents. The bubbling unit consists of round-bottomed flask with three necks positioned in water bath to control the temperature. Water-cooled reflux and thermometer is positioned in the first and second neck and nitrogen supply through the third neck. Cholesterol and surfactant are dispersed together in this buffer (pH 7.4) at 70°C, the dispersion mixed for 15 seconds with high shear homogenizer and immediately afterwards “bubbled” at 70°C using nitrogen gas.[23]
(e) Formation of niosomes from proniosomes:

Another method of producing niosomes is to coat a water-soluble carrier such as sorbitol with surfactant. The result of the coating process is a dry formulation. In which each water-soluble particle is covered with a thin film of dry surfactant. This preparation is termed “Proniosomes”. The niosomes are recognized by the addition of aqueous phase at T > Tm and brief agitation.[24]
T = Temperature
Tm = Mean phase transition temperature
Table 1: Drugs Incorporated into Niosomes by Various Methods
|
Method of Preparation |
Drug Incorporated |
|
Ether Injection |
Sodium stibogluconate[7,25] Doxorubicin |
|
Hand Shaking |
Methotrexate[16] Doxorubicin |
|
Sonication |
9-desglycinamide 8-arginine Vasopressin Oestradiol[24] |
Separation of Unentrapped Drug
The removal of unentrapped solute from the vesicles can be accomplished by various techniques, which include:
(i) Dialysis
The aqueous niosomal dispersion is dialyzed in dialysis tubing against phosphate buffer or normal saline or glucose solution.[23]
(ii) Gel Filtration
The unentrapped drug is removed by gel filtration of niosomal dispersion through a Sephadex-G-50 column and elution with phosphate buffered saline or normal saline.[26,27]
(ii) Centrifugation
The niosomal suspension is centrifuged and the supernatant is separated. The pellet is washed and then resuspended to obtain a niosomal suspension free from unentrapped drug.[9,28]
Characterization of Niosomes
(i) Size
Shape of niosomal vesicles is assumed to be spherical, and their mean diameter can be determined by using laser light scattering method.[29] Also, diameter of these vesicles can be determined by using electron microscopy, molecular sieve chromatography, ultracentrifugation, photon correlation microscopy, optical microscopy and freeze fracture electron microscopy.[10,30] Freeze thawing (keeping vesicles suspension at -20°C for 24 hrs and then heating to ambient temperature) of niosomes increases the vesicle diameter, which might be attributed to fusion of vesicles during the cycle.[17]
(ii) Bilayer Formation
Assembly of non-ionic surfactants to form a bilayer vesicle is characterized by an X-cross formation under light polarization microscopy.[31]
(iii) Number of Lamellae
This is determined by using nuclear magnetic resonance (NMR) spectroscopy, small angle X-ray scattering and electron microscopy.[30]
(iv)Membrane Rigidity
Membrane rigidity can be measured by means of mobility of fluorescence probe as a function of temperature.[31]
(v) Entrapment Efficiency
After preparing niosomal dispersion, unentrapped drug is separated by dialysis, centrifugation, or gel filtration as described above and the drug remained entrapped in niosomes is determined by complete vesicle disruption using 50% n-propanol or 0.1% Triton X-100 and analyzing the resultant solution by appropriate assay method for the drug.[32]
Entrapment efficiency = (Amount entrapped / total amount) x 100
In VitroRelease Study
A method of in vitro release rate study was reported with the help of dialysis tubing.[26] A dialysis sac was washed and soaked in distilled water. The vesicle suspension was pipetted into a bag made up of the tubing and sealed. The bag containing the vesicles was then placed in 200 ml buffer solution in a 250 ml beaker with constant shaking at 25°C or 37°C. At various time intervals, the buffer was analyzed for the drug content by an appropriate assay method. In another method, isoniazid-encapsulated niosomes were separated by gel filtration on Sephadex G- 50 powder kept in double distilled water for 48 h for swelling.[33] At first, 1 ml of prepared niosome suspension was placed on the top of the column and elution was carried out using normal saline. Niosomes encapsulated isoniazid elutes out first as a slightly dense, white opalescent suspension followed by free drug. Separated niosomes were filled in a dialysis tube to which a sigma dialysis sac was attached to one end. The dialysis tube was suspended in phosphate buffer of pH (7.4), stirred with a magnetic stirrer, and samples were withdrawn at specific time intervals and analyzed using high-performance liquid chromatography (HPLC) method.
In VivoRelease Study
Albino rats were used for this study. These rats were subdivided with groups. Niosomal suspension used for in vivo study was injected intravenously (through tail vein) using appropriate disposal syringe.
Factors Affecting Physico-chemical Properties of Niosomes
Various factors that affect the physico-chemical properties of niosomes are discussed further.
(i) Nature of Surfactants
A surfactant used for preparation of niosomes must have a hydrophilic head and hydrophobic tail. The hydrophobic tail may consist of one or two alkyl or perfluoroalkyl groups or in some cases a single steroidal group.[22]The ether type surfactants with single chain alkyl as hydrophobic tail is more toxic than corresponding dialkyl ether chain.[13]The ester type surfactants are chemically less stable than ether type surfactants and the former is less toxic than the latter due to ester-linked surfactant degraded by esterases to triglycerides and fatty acid in vivo.[13]The surfactants with alkyl chain length from C12-C18 are suitable for preparation of niosomes.[34,35]Surfactants such as C16EO5 (poly-oxyethylene cetyl ether) or C18EO5 (polyoxyethylene steryl ether) are used for preparation of polyhedral vesicles.[36]Span series surfactants having HLB number of between 4 and 8 can form vesicles.[26]
Table 2: Different Types of Non-Ionic Surfactant
|
Type of Non-ionic surfactant |
Examples |
|
Fatty alcohol |
Cetyl alcohol, Steryl alcohol, Cetosteryl alcohol, oleyl alcohol |
|
Ethers |
Brij, Decyl glucoside, Lauryl glucoside, Octyl glucoside, Triton X-100, Nonoxynol-9 |
|
Esters |
Glyceryl laurate, Polysorbates, Spans |
|
Block copolymers |
Poloxamers |
(ii) Structure of Surfactants
The geometry of vesicle to be formed from surfactants is affected by its structure, which is related to critical packing parameters. On the basis of critical packing parameters of surfactants, we can predicate geometry of vesicle to be formed. Critical packing parameters can be defined using following equation,
CPP (Critical Packing Parameters) = v/lc ×a0
Where v = hydrophobic group volume, lc = the critical hydrophobic group length, a0= the area of hydrophilic head group.
From the critical packing parameter value type of miceller structure formed can be ascertained as given below,
If CPP < ½, then formation of spherical micelles,
If ½ < CPP < 1, then formation of bilayer micelles,
If CPP > 1, then formation inverted micelles.
(iii) Amount and type of surfactant
The mean size of niosomes increases proportionally with increase in the HLB of surfactants like Span 85 (HLB 1.8) to Span 20 (HLB 8.6) because the surface free energy decreases with an increase in hydrophobicity of surfactant.[26]
The bilayers of the vesicles are either in the so-called liquid state or in gel state, depending on the temperature, the type of lipid or surfactant and the presence of other components such as cholesterol. In the gel state, alkyl chains are present in a well-ordered structure, and in the liquid state, the structure of the bilayers is more disordered. The surfactants and lipids are characterized by the gel-liquid phase transition temperature (TC).[49] Phase transition temperature (TC) of surfactant also effects entrapment efficiency i.e. Span 60 having higher TC, provides better entrapment.
(iv) Membrane Composition
The stable niosomes can be prepared with addition of different additives along with surfactants and drugs. Niosomes formed have a number of morphologies and their permeability and stability properties can be altered by manipulating membrane characteristics by different additives. In case of polyhedral niosomes formed from C16G2, the shape of these polyhedral niosome remains unaffected by adding low amount of solulan C24 (cholesteryl poly-24-oxyethylene ether), which prevents aggregation due to development of steric hindrance.[37] The mean size of niosomes is influenced by membrane composition such as Polyhedral niosomes formed by C16G2: solulan C24 in ratio (91:9) having bigger size (8.0 ± 0.03mm) than spherical/tubular niosomes formed by C16G2: cholesterol: solulan C24 in ratio (49:49:2) (6.6±0.2mm).[37] Addition of cholesterol molecule to niosomal system provides rigidity to the membrane and reduces the leakage of drug from niosome.[38]
Inclusion of cholesterol in niosomes increases its hydrodynamic diameter and entrapment efficiency.[26] In general, the action of cholesterol is two folds; on one hand, cholesterol increases the chain order of liquid-state bilayers and on the other, cholesterol decreases the chain order of gel state bilayers. At a high cholesterol concentration, the gel state is transformed to a liquid-ordered phase.[28]
An increase in cholesterol content of the bilayers resulted in a decrease in the release rate of encapsulated material and therefore an increase of the rigidity of the bilayers obtained.[28,46] Presence of charge tends to increase the interlamellar distance between successive bilayers in multilamellar vesicle structure and leads to greater overall entrapped volume.
(v) Nature of Encapsulated Drug
The physico-chemical properties of encapsulated drug influencecharge and rigidity of the niosome bilayer. The drug interacts withsurfactant head groups and develops the charge that creates mutualrepulsion between surfactant bilayers and hence increases vesiclesize.[39]The aggregation of vesicles is prevented due to the chargedevelopment on bilayer. In polyoxyethylene glycol (PEG) coated vesicles, some drug is entrapped in the long PEG chains, thus reducing the tendency to increase the size.[9] The hydrophilic lipophilic balance of the drug affects degree of entrapment.
Table 3: Effect of the nature of drug on the formation of niosomes
|
Nature of the drug |
Leakage from the vesicles |
Stability |
Other properties |
|
Hydrophobic drug |
Decreased |
Increased |
Improved transdermal delivery |
|
Hydrophobic drug |
Increased |
Decreased |
- |
|
Amphiphilic drug |
Decreased |
- |
Increased encapsulation, Altered elecrophoretic mobility |
|
Macromolecules |
Decreased |
Increased |
- |
(vi) Temperature of Hydration
Hydration temperature influences the shape and size of the niosome.For ideal condition it should be above the gel to liquid phase transitiontemperature of system. Temperature change of niosomal systemaffects assembly of surfactants into vesicles and also induces vesicleshape transformation.[22,37] Arunothayanun et al. reported that a polyhedralvesicle formed by C16G2: solulan C24 (91:9) at 25°C which onheating transformed into spherical vesicle at 48°C, but on coolingfrom 55°C, the vesicle produced a cluster of smaller spherical niosomesat 49°C before changing to the polyhedral structures at 35°C. In contrastvesicle formed by C16G2: cholesterol: solulanC24 (49:49:2) showsno shape transformation on heating or cooling[42]. Along with theabove mentioned factors, volume of hydration medium and time ofhydration of niosomes are also critical factors. Improper selection ofthese factors may result in formation of fragile niosomes or creationof drug leakage problems.
(vii) Methods of Preparation
Hand shaking method forms vesicles with greater diameter (0.35-13 nm) compared to the ether injection method (50-1000 nm).[17] Small sized niosomes can be produced by Reverse Phase Evaporation method.[19,40] Micro fluidization method gives greater uniformity and small size vesicles.[17] Niosomes obtained by trans membrane pH gradient (inside acidic) drug uptake process showed greater entrapment efficiency and better retention of drug.[40]
(viii) Resistance To Osmotic Stress
Addition of a hypertonic salt solution to a suspension of niosomes brings about reduction in diameter. In hypotonic salt solution, there is initial slow release with slight swelling of vesicles probably due to inhibition of eluting fluid from vesicles, followed by faster release, which may be due to mechanical loosening of vesicles structure under osmotic stress.[2,41]
Therapeutic Applications of Niosomes
Niosomal drug delivery is potentially applicable to many pharmacological agents for their action against various diseases. Some of their therapeutic applications are discussed below.
(i) Targeting of Bioactive Agents
(a) To reticulo-endothelial system (RES)
The cells of RES preferentially take up the vesicles. The uptake of niosomes by the cells is also by circulating serum factors known as opsonins, which mark them for clearance. Such localized drug accumulation has, however, been exploited in treatment of animal tumors known to metastasize to the liver and spleen and in parasitic infestation of liver.[2]
(b) To organs other than RES
It has been suggested that carrier system can be directed to specific sites in the body by use of antibodies.[12] Immunoglobulins seem to bind quite readily to the lipid surface, thus offering a convenient means for targeting of drug carrier.[42] Many cells possess the intrinsic ability to recognize and bind particular carbohydrate determinants and this can be exploited to direct carriers system to particular cells.
(ii) Neoplasia
Doxorubicin, the anthracyclic antibiotic with broad spectrum anti tumor activity, shows a dose dependant irreversible cardio toxic effect. Niosomal delivery of this drug to mice bearing S-180 tumor increased their life span and decreased the rate of proliferation of sarcoma.[43] Niosomal entrapment increased the half-life of the drug, prolonged its circulation and altered its metabolism. Intravenous administration of methotrexate entrapped in niosomes to S-180 tumor bearing mice resulted in total regression of tumor and also higher plasma level and slower elimination.[44,45]
(iii) Leishmaniasis
Niosomes can be used for targeting of drug in the treatment of diseases in which the infecting organism resides in the organ of reticulo-endothelial system. Leishmaniasis is such a disease in which parasite invades cells of liver and spleen. The commonly prescribed drugs are antimonials, which are related to arsenic, and at high concentration they damage the heart, liver and kidney.
The study of antimony distribution in mice, performed by Hunter et al showed high liver level after intravenous administration of the carriers forms of the drug.[13]
(iv) Delivery of Peptide Drugs
Yoshida et al investigated oral delivery of 9-desglycinamide, 8-arginine vasopressin entrapped in niosomes in an in-vitro intestinal loop model and reported that stability of peptide increased significantly.[46]
(v) Immunological Application of Niosomes
Niosomes have been used for studying the nature of the immune response provoked by antigens. Brewer and Alexander have reported niosomes as potent adjuvant in terms of immunological selectivity, low toxicity and stability.[47]
(vi) Niosomes as a Carrier for Hemoglobin
Niosomes can be used as a carrier for hemoglobin.[48,49]
(vii) Transdermal Delivery of Drugs by Niosomes
Slow penetration of drug through skin is the major drawback of transdermal route of delivery. An increase in the penetration rate has been achieved by transdermal delivery of drug incorporated in niosomes.
(viii) Other Applications
a) Sustained Release
Sustained release action of niosomes can be applied to drugs with low therapeutic index and low water solubility since those could be maintained in the circulation via niosomal encapsulation.
b) Localized Drug Action
Drug delivery through niosomes is one of the approaches to achieve localized drug action, since their size and low penetrability through epithelium and connective tissue keeps the drug localized at the site of administration.
Localized drug action results in enhancement of efficacy of potency of the drug and at the same time reduces its systemic toxic effects e.g. Antimonials encapsulated within niosomes are taken up by mononuclear cells resulting in localization of drug, increase in potency and hence decrease both in dose and toxicity.[13]
Conclusion:
Recent advancements in the field of pharmaceutical and biotechnological research have resulted in the endorsement of proteins and vaccines as a major class of therapeutic agents. These, however, pose numerous drug-associated challenges such as poor bioavailability, suitable route of drug delivery, physical and chemical instability and potentially serious side effects. The usefulness of niosomes in the delivery of proteins and biologicals can be unsubstantiated with a wide scope in encapsulating toxic drugs such as anti-cancer drugs and anti-viral drugs.
Niosomes are considered to be better candidates for drug delivery as compared to liposomes due to various factors like cost, stability etc.
Niosomal drug delivery system is the one of the best targeted drug delivery system. However, the technology utilized in niosomes is still in its infancy. Hence, researches are going on to develop a suitable technology for large production because it is a promising targeted drug delivery system.
References
1. Allen TM. Liposomal drug formulations: Rationale for development and what we can expect for the future. Drugs 1998;56:747-56.
2. Malhotra M, Jain NK. Niosomes as drug carriers. Indian Drugs 1994;31:81-6.
3. Udupa N. Niosomes as drug carriers. In: Jain NK, editor. Controlled and novel drug delivery. 1st ed. New Delhi: CBS Publishers and Distributors; 2002.
4. Baillie AJ, Florence AT, Hume LR, Muirhead GT, Rogerson A. The preparation and properties of niosomes-non-ionic surfactant vesicles. J Pharm Pharmacol 1985;37:863-8.
5. Jadon PS, Gajbhiye V, Jadon RS, Gajbhiye KR, Ganesh N. Enhanced oral bioavailability of griseofulvin via niosomes. AAPS PharmSciTech 2009;10:1186-92.
6. Sheena IP, Singh UV, Kamath R, Uma Devi P, Udupa N. Niosomal withaferin A, with better tumor efficiency. Indian J Pharm Sci 1998;60:45-8.
7. Baillie AJ, Coombs GH, Dolan TF, Laurie J. Non-ionic surfactant vesicles, niosomes, as delivery system for the anti-leishmanial drug, sodium stibogluconate. J Pharm Pharmacol 1986;38:502-5.
8. Kaur IP, Garg A, Singla AK, Aggarwal D. Vesicular systems in ocular drug delivery: An overview. Int J Pharm 2004;269:1-14.
9. Hu C, Rhodes DG. Proniosomes: A novel drug carrier preparation. Int J Pharm 1999;185:23-35.
10. Azmin MN, Florence AT, Handjani-Vila RM, Stuart JF, Vanlerberghe G, Whittaker JS. The effect of non-ionic surfactant vesicle (niosome) entrapment on the absorption and distribution of methoterxate in mice. J Pharm Pharmacol 1985;37:237-42.
11. Szoka Jr F, Papahadjopoulos D. Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu Rev Biophys Bioeng 1980;9:467-508.
12. Gregoriadis G. Targeting of drugs: Implications in medicine. Lancet 1981;2:241-6.
13. Hunter CA, Dolan TF, Coombs GH, Baillie AJ. Vesicular systems (Niosome and Liposomes) for delivery of sodium stibogluconate in experimental murine visceral leishmaniasis. J Pharm Pharmacol 1988;40:161-5.
14. Buckton G, Harwood. Interfacial phenomena in drug delivery and targeting. Switzerland: Academic Publishers; 1995. pp. 154-5.
15. McCormack B, Gregordias G. Drugs-in-cyclodextrins-in-liposomes: An approach to controlling the fate of water insoluble drugs in vivo. Int. J. Pharm. 1998;162:59-69.
16. Chandraprakash KS, Udupa N, Umadevi P, Pillai GK. Pharmacokinetic evaluation of surfactant vesicles containing methotrexate in tumor bearing mice. Int. J. Pharm. 1990;61:R1-R3.
17. Khandare JN, Madhavi G, Tamhankar BM. Niosomes: Novel drug delivery system. The Eastern Pharmacist. 1994;37:61-4.
18. Mayer LD, Bally MB, Hope MJ, Cullis PR. Uptake of antineoplastic agents into large unilamellar vesicles in response to a membrane potential. Biochem Biophys Acta 1985;816:294-302.
19. Naresh RA, Chandrashekhar G, Pillai GK, Udupa N. Anti-inflammatory activity of niosome encapsulated diclofenac sodium with Tween-85 in Arthitic rats. Ind J Pharmacol 1994;26:46-8.
20. Rogerson A, Cummings J, Willmott N, Florence AT. The distribution of doxorubicin in mice following administration in niosomes. J Pharm Pharmacol 1988;40:337-42.
21. Hao Y, Zhao F, Li N, Yang Y, Li K. Studies on a high encapsulation of colchicines by a niosome system. Int J Pharm 2002;244:73-80.
22. Uchegbu IF, Vyas SP. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm 1998;172:33-70.
23. Chauhan S, Luorence MJ. The preparation of polyoxyethylene containing non-ionic surfactant vesicles. J. Pharm. Pharmacol. 1989;4:6.
24. Blazek-Walsh AI, Rhodes DG. SEM imaging predicts quality of niosomes from maltodextrin-based proniosomes. Pharm. Res. 2001;18:656-61.
25. Carter KC, Dolan TF, Baillie AJ, MacColgan C. Visceral leishmaniasis: Drug carrier system characteristics and the ability to clear parasites from the liver, spleen and bone marrow in Leishmania donovani infected BALB/c mice. J.Pharm. Pharmcol. 1989;41:87-91.
26. Yoshioka T, Stermberg B, Florence AT. Preparation and properties of vesicles (niosomes) of sobitan monoesters (Span 20, 40, 60, and 80) and a sorbitan triester (Span 85). Int J Pharm. 1994;105:1-6.
27. Gayatri Devi S, Venkatesh P, Udupa N. Niosomal sumatriptan succinate for nasal administration. Int. J. Pharm. Sci. 2000;62:479-81.
28. Silver BL. The physical chemistry of membranes. New York: Alan/Unwin and Solomon Press; 1985. pp. 209-230.
29. Almira I, Blazek-welsh IA, Rhodes GD. Maltodextrin based proniosomes. AAPS PharmSciTech 2001;3:1-8.
30. Biswal S, Murthy PN, Sahu J, Sahoo P, Amir F. Vesicles of non-ionic surfactants (niosomes) and drug delivery potential. Int J Pharm Sci Nanotech 2008;1:1-8.
31. Manosroi A, Wongtrakul P, Manosroi J, Sakai H, Sugawara F, Yuasa M, et al. Characterization of vesicles prepared with various non-ionic surfactants mixed with cholesterol. Colloids Surf B 2003;30:129-38.
32. Balasubramaniam A, Kumar VA, Pillai KS. Formulation and in-vivo evaluation of niosome encapsulated daunorubicin hydrochloride. Drug Dev Ind Pharm 2002;28:1181-93.
33. Karki R, Mamatha GC, Subramanya G, Udupa N. Preparation, characterization and tissue disposition of niosomes containing isoniazid. Rasayan J Chem 2008;1:224-7.
34. Nasseri B, Florence AT. Some properties of extruded nonionic surfactant micro-tubes. Int. J. Pharm. 2003;254:11.
35. Nasseri B, Florence AT. Microtubules formed by capillary extrusion and fusion of surfactant vesicles. Int. J. of Pharm. 2003;266:91.
36. Ozer AY, Hincal AA, Bouwstra JA. A novel drug delivery system: Non-ionic surfactant vesicle. Eur. J. Pharm. Biopharm. 1991;37:75.
37. Arunothayanun P, Bernard MS, Uchegbu IF, Florence AT. The effect of processing variables on the physical characteristics of nonionic surfactant vesicles (niosomes) formed from hexadecyl diglycerol ether. Int. J. Pharm. 2000;201:7-14.
38. Rogerson A, Cummings J, Florence AT. Adriamycin-loaded niosomes: Drug entrapment, stability and release. J. Microencap. 1987;4:321.
39. Stafford S, Ballie AJ, Florence AT. Drug effect on the size of chemically defined nonionic surfactant vesicles. J. Pharm. Pharmacol. 1988;40:26.
40. Parthasarathi G, Udupa N, Umadevi P, Pillai GK. Niosome encapsulated of vincristine sulfate: Improved anticancer activity with reduced toxicity in mice. J. Drug Target. 1994;2:173-82.
41. Kiwada H, Nimura H, Kato Y. Chem. Pharm. Bull. 1985;33:2475-82.
42. Weissman G, Bloomgarden D, Kaplan R, Cohen C, Hoffstein S, Collins T, et al. A general method for the introduction of enzymes, by means of immunoglobulin-coated liposomes, into lysosomes of deficient cells. Proc. Natl. Acad. Sci.1975;72:88-92.
43. Cummings J, Staurt JF, Calman KC. Determination of adriamycin, adriamycinol and their 7-deoxyaglycones in human serum by high-performance liquid chromatography. J. Chromatogr. 1984;311:125-33.
44. Chandraprakash KS, Udupa N, Umadevi P, Pillai GK. Formulation and evaluation of methotrexate niosomes. Ind. J. Pharm. Sci. 1992;54:197.
45. Suzuki K, Sokan K. The application of liposomes to cosmetics. Cosmetic and Toiletries. 1990;105:65-78.
46. Yoshida H, Lehr CM, Kok W, Junginger HE, Verhoef JC, Bouwistra JA. Niosomes for oral delivery of peptide drugs. J.Control Rel. 1992;21:145-53.
47. Brewer JM, Alexander J. The adjuvant activity of non-ionic surfactant vesicles (niosomes) on the BALB/c humoral response to bovine serum albumin. Immunology 1992;75:570-5.
48. Moser P, Marchand-Arvier M, Labrude P, Handjani-Vila RM, Vignerson C. Hemoglobin niosomes. I. Preparation, functional and physico-chemical properties, and stability. Pharm Acta Helv. 1989;64:192-202.
49. Moser P, Arvier MM, Labrude P, Vignerson C. Hemoglobin niosomes. II. In Vitro interactions with plasma proteins and phagocytes. Pharm Acta Helv. 1990;65:82-92.
Reference ID: PHARMATUTOR-ART-1030
FIND OUT MORE ARTICLES AT OUR DATABASE









