About Authors:
Snehal V.Chopade, Dr.V.R.Patil - Principle
TVES College of pharmacy,
Faizpur accredited by North Maharashtra University,
Jalgaon
Abstract:
UPLC can be regarded as new invention for liquid chromatography. UPLC refers to Ultra Performance Liquid Chromatography. UPLC brings dramatic improvements in sensitivity, resolution and speed of analysis can be calculated. It has instrumentation that operates at high pressure than that used in HPLC & in this system uses fine particles(less than 2.5µm) & mobile phases at high linear velocities decreases the length of column, reduces solvent consumtion & saves time.
This review introduces the theory of UPLC, and it can summarizes some of the most recent work in the field.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1206
Introduction:
Chromatography is a non-destructive procedure for resolving a multi-component mixture of traces, minor or constituents in to individual fractions.It is a method of separating a mixture of components in to individual components through a porous medium under the influence of solvent [3]
For many years, researchers have looked at “fast LC” as a way to speed up analyses.[4,5] The need for speed , the availability of affordable and easy to use mass spectrometers. Smaller columns and faster flow rates (amongst other parameters) have been used. Elevated temperature, having the dual advantages of lowering viscosity, and increasing mass transfer by increasing the diffusivity of the analytes, has also been investigated. [6] However, using conventional particle sizes and pressures, limitations are soon reached and compromises must be made, sacrificing resolution.
High performance liquid chromatography (HPLC) is approved technique as it has been used in laboratories worldwide over the past 30-plus years. HPLC technology simply doesn’t have the capability to take full advantages of sub-2µm particles.[7].
UPLC can be regarded as new invention for liquid chromatography. UPLC refers to Ultra Performance Liquid Chromatography. UPLC brings dramatic improvements in sensitivity, resolution and speed of analysis can be calculated. It has instrumentation that operates at high pressure than that used in HPLC & in this system uses fine particles(less than 2.5µm) & mobile phases at high linear velocities decreases the length of column, reduces solvent consumption & saves time. This review introduces the theory of UPLC, and it can summarizes some of the most recent work in the field.
According to the van Deemter equation, as the particle size decreases to less than2.5 µm, there is a significant gain in efficiency, while the efficiency does not diminish at increased flow rates or linear velocities[8].
Therefore by using smaller particles, speed and peak capacity (number of peaks resolved per unit time in gradient separations) can be extended to new limits, termed Ultra PerformanceLiquid Chromatography, or UPLC [8].
The technology takes full advantage of chromatographic principles to run separations
Using columns packed with smaller particles(less than2.5µm) and/or higher flow rates for increased speed, this gives superior resolution and sensitivity [7,8].Now a days in industrial area UPLC refers for some of the most recent work field.
Principle:
The UPLC is based on the principal of use of stationary phase consisting of particles less than 2.5 μm (while HPLC columns are typically filled with particles of 3 to 5 μm). The underlying principles of this evolution are governed by the Van Deemter equation, which is an empirical formula that describes the relationship between linear velocity (flow rate) and plate height (HETP or column efficiency) [8,11].
H=A+B/v+Cv
Where;
A, B and C are constants
v is the linear velocity, the carrier gas flow rate.
*The A term is independent of velocity and represents "eddy" mixing. It is smallest when the packed column particles are small and uniform.
* The B term represents axial diffusion or the natural diffusion tendency of molecules.This effect is diminished at high flow rates and so this term is divided by v.
* The C term is due to kinetic resistance to equilibrium in the separation process.The kinetic resistance is the time lag involved in moving from the gas phase to the packing stationary phase and back again. The greater the flow of gas, the more a molecule on the packing tends to lag behind molecules in the mobile phase. Thus term is proportional to v.
Therefore it is possible to increase throughput, and thus the speed of analysis without affecting the chromatographic performance. The advent of UPLC has demanded the development of a new instrumental system for liquid chromatography, which can take advantage of the separation performance (by reducing dead volumes) and consistent with the pressures (about 8000 to 15,000 PSI, compared with 2500 to 5000 PSI in HPLC). Efficiency is proportional to column length and inversely proportional to the particle size.[18]
As shown in Figure 1, smaller particles provide increased efficiency as well as the ability to work at increased linear velocity without a loss of efficiency, providing both resolution and speed. Efficiency is the primary separation parameter behind UPLC since it relies on the same selectivity and retentivity as HPLC. In the fundamental
resolution (Rs) equation [8]:
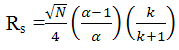
Resolution is proportional to the square root of N. But since N is inversely proportional
to particle size (dp):
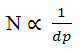
as the particle size is lowered by a factor of three, from, for example, 5 µm (HPLC-scale)
to 1.7 µm (UPLC-scale), N is increased by three and resolution by the square root of three or 1.7N is also inversely proportional to the square of the peak width
eg: (Fig 1)UPLC stability indicating assay.
UPLC conditions:
1. Column: 2.1 x 30 mm 1.7μm ACQUITY BEH C18
2. Temp: 30 °C. A 45 s, 5–85%B linear gradient,
3. flow rate: 0.8 mL/min was used.
4. Mobile phase: A was 10 mm ammonium formate, pH 4.0,
B was acetonitrile. UV detection at 273 nm and 40 pts/s
5. Peaks in order: 5-nitroso-2,4,6-triaminopyrimidine, 4-amino-6-chloro-1,3-benzenesulfanamide, hydrochlorthiazide, triamterine, and methylbenzenesulfanamide; 5 µL injection, 0.1 mg/mL each.
So as the particle size decreases to increase N and subsequently Rs, an increase in sensitivity is obtained.Also, peak height is inversely proportional to the peak width:
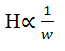
an increase in sensitivity is obtained, since narrower peaks are taller peaks. Narrower peaks also mean more peak capacity per unit time in gradient separations, desirable for many applications. eg. peptide maping.
As earlier said efficiency is proportional to column length and inversely proportional to the particle size [8]:
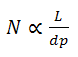
Therefore, the column can be shortened by the same factor as the particle size without loss of resolution. Using a flow rate three times higher due to the smaller particles and shortening the column by one third (again due to the smaller particles), the separation is completed in 1/9 the time while maintaining resolution. The application of UPLC resulted in the detection of additional drug metabolites, superior separation and improved spectral quality [13,14,8].
Chemistry of small particles:
The design and developmentof sub-2 µm particles is a significant challenge, and researchers have been active in this area for some time to capitalize on t their advantages.[9,14]
A commercially available non-porous, high iffiest small particle has poor loading capacity and retention due to low surface area. To maintain retention and capacity must use novel porous particles that can withstand high pressures. In 2000,hybride of silica & polymeric column was introduced which consist of classical sol-gel synthesis that incorporates carbon in the form of methyl groups, these columns are mechanically strong. They are highly efficient & can be operate at wide range of pH.[20]
But, in order to provide the kind of enhanced mechanical stability required for UPLC, a second generation bridged ethane hybrid (BEH) technology was developed.[10] These 1.7 µm particles derive their enhanced mechanical stability by bridging the methyl groups in the silica matrix.[20].
Capitalizing on smaller particles:
Only small particles are not responsible for fast resolution & sensitivity, some special instrumentation system should be design. So some special kind of system capable of delivering the pressures required to realize the potential of UPLC have been reported in the literature and elsewhere.[20]
In early 2004, the first commercially available UPLC system that embodied these requirements was described for the separation of various pharmaceutical related small organic molecules, proteins, and peptides; it is called the ACQUITY UPLCTM System.[20,8] The ACQUITY UPLC System consists of a binary solvent manager, sample manager(including the column heater), detector, and optional sample organizer. The binary solvent manager uses two individual serial flow pumps to deliver a parallel binary gradient. mixed under high pressure.[8,20]
Instrumentation:
The UPLC System has been holistically designed to match the performance needs of innovative column chemistries with robust hardware, easy-to-use software and specialized support services. It consists of:
· Small, pressure-tolerant particles
· High-pressure fluidic modules
· Minimized system volume
· Negligible carryover
· Reduced cycle times
· Last response detectors
· Integrated system software and diagnostics
The ACQUITY UPLC System’s high-pressure fluidics optimizes flow rates to make the most of small particle technology. The ACQUITY UPLC System’s sample-handling design is designed to ensure exceptionally low carryover and reduced cycle time. And when interfaced with the Sample Organizer, it increases unattended sample capacity by ten times. High-speed detectors, both optical and mass, contribute to increased sensitivity and help manage the heightened speed and resolution requirements of UPLC.
[adsense:468x15:2204050025]
ACQUITY UPLC Systems are easily controlled, diagnosed, and monitored via a graphical system console interface. The console offers:
· Quick and easy access to critical instrument parameters
· Simple system start-up, elegant system status monitoring and predictive performance indicators to ensure maximum productivity
· Data management capabilities that are supported by both MassLynx™ and Empower™ software
The ACQUITY UPLC System is also supported by Intelligent Device Management technology with our Connections INSIGHT™ service, providing instrument diagnostics.
1. Sample injection
2. Uplc columns
3. Column maneger & heater or cooler
4. Detectors
5. Softwares
6. Accesories
7. Connection insight service (if provided by mfg.company.water provided it)
1.Sample inject:
Lee et al. described the design of injection valves and separation reproducibility [15] and the use of a carbon dioxide enhanced slurry packing method onthe capillary scale for the separation of some benzodiazepines, herbicides, and various pharmaceutical compounds [17].Jorgenson et al. modified a commerciallyavailable HPLC system to operate at 17,500 psi and used 22 cm longcapillaries packed with 1.5 mm C18-modified particles for the analysis of proteins [16].
The calculated pressure drop at the optimum flow rate for maximum efficiency across a 15-cm long column packed with 1.7µm particles is approximately 15,000 psi. Therefore, a pump capable of delivering solvent smoothly and reproducibly at these pressures and that can compensate for solvent compressibility, while operating in both the gradient and isocratic separation modes is required [7].
With 1.7µm particles, half-height peak In UPLC, sample introduction is critical. Conventional injection valves, either automated or manual, are not designed and hardened to work at extreme pressure. To protect the column from extreme pressure fluctuations, the injection process must be relatively pulse-free and the swept volume of the device also needs to be minimal to reduce potential band spreading. A fast injection cycle time is needed to fully capitalise on the speed afforded by UPLC, which in turn requires a high sample capacity. Low volume injections with minimal carryover are also required to increase sensitivity [8]. There are also direct injection approaches for biological samples [18,19].
2. UPLC Columns:
The design and development of sub-2 µm particles is a significant challenge, and researchers have been active in this area for some time, trying to capitalize on their advantages 2–4. Although high efficiency, nonporous 1.5µm particles are commercially available, they suffer from poor loading capacity and retention due to low surface area. Silica-based particles have good mechanical strength but can suffer from a number of disadvantages, which include a limited pH range and tailing of basic analytes. Polymeric columns can overcome pH limitations, but they have their own issues including low efficiencies, limited loading capacities, and poor mechanical strength. In 2000, Waters introduced XTerra®,a first generation hybrid chemistry that took advantage of the best of both the silica and Polymeric column worlds. XTerra columns are mechanically strong, with high efficiency and operate over an extended pH range. They are produced using a classical sol-gel synthesis that incorporates carbon in the form of methyl groups. But in order to provide the necessary mechanical stability for UPLC, a second generation bridged ethyl hybrid (BEH) technology was developed called ACQUITY BEH, these 1.7µm particles derive their enhanced mechanical stability by bridging the methyl groups in the silica matrix. Packing 1.7µm particles into reproducible and rugged columns was also a challenge that needed to be overcome. Requirements include a smoother interior surface of the column hardware and re-designing the end frits to retain the small particles and resist clogging. Packed bed uniformity is also critical, especially if shorter columns are to maintain resolution while accomplishing the goal of faster separations. All ACQUITY UPLC BEH columns also include eCord™ microchip technology that captures the manufacturing information for each column including the quality control tests and certificates of analysis [7,8,20].
Fig. 1: ACQUITY UPLC BEH Column chemistries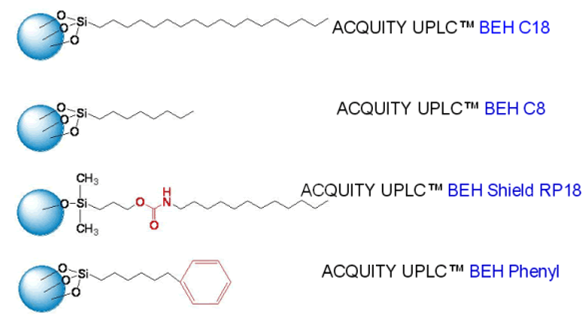
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
3. Column manager & heater cooler:
The ACQUITY UPLC Column Manager, with automatic column switching, is for high productivity UPLC sample processing, and its Column Heater/Cooler enables labs to use temperature as a method parameter. The ACQUITY UPLC Column Manager allows users to take full advantage of the performance, range of stationary phases, and mechanical strength offered by ACQUITY UPLC BEH Columns. The Column Manager provides temperature regulation from 10 °C to 90 °C, automated switching for up to four columns with dimensions to 2.1 mm in internal diameter (I.D.) and 150 mm in length, as well as a bypass channel for flow injections.
The ACQUITY UPLC System consists of a binary solvent manager, sample manager including the column heater, detector, and optional sample organiser. The binary solvent manager uses two individual serial flow pumps to deliver a parallel binary gradient. There are built-in solvent select valves to choose from up to four solvents. There is a 15,000-psi pressure limit (about 1000 bar) to take full advantage of the sub-2μm particles. The sample manager also incorporates several technology advancements. Using pressure assisted sample introduction, low dispersion is maintained through the injection process, and a series of pressures transducers facilitate self-monitoring and diagnostics. It uses needle-in-needle sampling for improved ruggedness and a needle calibration sensor increases accuracy. Injection cycle time is 25 seconds without a wash and 60 sec with a dual wash used to further decrease carry over. A variety of microtiter plate formats (deep well, mid height, or vials) can also be accommodated in a thermostatically controlled environment. Using the optional sample organiser, the sample manager can inject from up to 22 microtiter plates. The sample manager also controls the column heater. Column temperatures up to 65°C can be attained.
4. Detectors:
Half-height peak widths of less than one second are obtained with 1.7μm particles, which gives significant challenges for the detector. In order to integrate an analyte peak accurately and reproducibly, the detector sampling rate must be high enough to capture enough data points across the peak. The detector cell must have minimal dispersion (volume) to preserve separation efficiency. Conceptually, the sensitivity increase for UPLC detection should be 2-3 times higher than HPLC separations, depending on the detection technique. MS detection is significantly enhanced by UPLC; an increased peak concentration with reduced chromatographic dispersion at lower flow rates promotes increased source ionization efficiencies.
Waters detectors enhance ability to analyze a variety of compounds. Its ACQUITY UPLC detectors—
· The Photodiode Array (PDA),
· Tunable UV (TUV),
· Evaporative Light Scattering (ELS)
-- are optimized for UPLC system technology by virtue of their low dispersion characteristics, high data acquisition rates, and reliable performance with cost-effective maintenance support and parts.
Tunable UV (TUV):
The ACQUITY Tunable UV/Visible detector cell consists of a light guided flow cell equivalent to an optical fibre. Light is efficiently transferred down the flow cell in an internal reflectance mode that still maintains a 10mm flow cell path length with a volume of only 500mL. Tubing and connections in the system are efficiently routed to maintain low dispersion and to take advantage of leak detectors that interact with the software to alert the user to potentialproblems [26].
UPLC is an ideal inlet for the sensitivity and specificity offered by mass spectrometry. The low dispersion, high-speed detection performance of Waters MS Technologies, in combination with the performance characteristics of UPLC,can dramatically extend detection capabilities.
5. Softwares:
ACQUITY UPLC Systems can be easily controlled, diagnosed and monitored via a graphical system console interface with Empower™ and MassLynx™ software. Both Empower and MassLynx provide the dynamic data processing and information management tools to convert the results generated by the ACQUITY UPLC System into valuable knowledge.
6. ACCESORIES:
Waters is continually extending the functionality of the ACQUITY UPLC System:
· eCord™ technology, available on all ACQUITY UPLC columns, records column history
· Sample Organizer that increases system capacity by more than 10 times
· FlexCart system platform improves usability, accessibility and convenience
7.connection INSIGHTTM service:
( If provided by mfg.company WATER’S provided it)
Connections INSIGHT™ uses Intelligent Device Management technology to provide diagnostic information for the ACQUITY UPLC Systems. Connections INSIGHT creates a virtual technical support presence in your lab, enabling Waters to provide you with proactive and timely service, with the highest level of support and satisfaction.
|
Table 1: Comparison between UPLC and HPLC |
||
|
Characteristics |
HPLC |
UPLC |
|
Particle size |
3 to 5m |
Less than 2m
|
|
Maximum backpressure |
35-40 MPa |
103.5 MPa |
|
Analytical column |
Alltima C18 |
Acquity UPLC BEH C18 |
|
Column dimensions |
150 X 3.2 mm |
150 X 2.1 mm |
|
Column temperature |
30 °C |
65 °C |
|
Injection volume |
5μL(Std.In100% MeOH) |
2μL(Std.In100% MeOH) |
Advantages:
• Decreases run time and increases sensitivity
• Provides the selectivity, sensitivity, and dynamic range of LC analysis
• Maintaining resolution performance.
• Expands scope of Multiresidue Methods
• UPLC’s fast resolving power quickly quantifies related and unrelated compounds
• Faster analysis through the use of a novel separation material of very fine particle size
• Operation cost is reduced
• Less solvent consumption
• Reduces process cycle times, so that more product can be produced with existing
resources
• Increases sample throughput and enables manufacturers to produce more material that
consistently meet or exceeds the product specifications, potentially eliminating
variability, failed batches, or the need to re-work material [22,23]
• Delivers real-time analysis in step with manufacturing processes
• Assures end-product quality, including final release testing
Disadvantages:
Due to increased pressure requires more maintenance and reduces the life of the columns of this type. So far performance similar or even higher has been demonstrated by using stationary phases of size around 2 μm without the adverse effects of high pressure.
In addition, the phases of less than 2 μm are generally non-regenerable and thus have limited use [24, 25].
Applications Of UPLC:
1.Identification of Metabolite:
Biotransformation of new chemical entities (NCE) is necessary for drug discovery. When a compound reaches the development stage, metabolite identification becomes a regulated process.Discovery studies are generally carried out in vitro to identify major metabolites so that metabolic weak spots on the drug candidate molecule can be recognized and protected by changing the compound structure. Key for analysts in metabolite identification is maintaining high sample throughput and providing results to medicinal chemists as soon as they are available. UPLC/MS/MS addresses the complex analytical requirements of biomarker discovery by offering unmatched sensitivity, resolution, dynamic range, and mass accuracy[21].(fig.4)
2.Study of Metabonomics / Metabolomics
Metabonomics studies are carried out in labs to accelerate the development of new medicines. The ability to compare and contrast large sample groups provides insight into the biochemical changes that occur when a biological system is exposed to a new chemical entity (NCE). Metabonomics provides a rapid and robust method for detecting these changes, improves understanding of potential toxicity, and allows monitoring the efficacy. The correct implementation of metabonomic and metabolomic information helps similar discovery, development, and manufacturing processes in the biotechnology and chemical industry companies. With these studies, scientists are better able to visualize and identify fundamental differences in sample sets. The UPLC/MS System combines the benefits of UPLC analyses, high resolution exact mass MS, and specialized application managers to rapidly generate and interpret information-rich data, allowing rapid and informed decisions to be made[21]
Eg :Figure 4: Extracted ion chromatograms for major N and O dealkylated and double de-alkylation metabolites of dextromethorphan by HPLC/Tof MS.
3.Analysis of Natural Products and Traditional Herbal Medicine
UPLC is widely used for analysis of natural products and herbal medicines. For traditional herbal medicines, also known as natural products or traditional Chinese medicines, analytical laboratories need to expand their understanding of their pharmacology to provide evidence-based validation of their effectiveness as medicines and to establish safety parameters for their production. The main purpose of this is to analyze drug samples arise from the complexity of the matrix and variability from sample to sample.UPLC provides high-quality separations and detection capabilities to identify active compounds in highly complex samples that results from natural products and traditional herbal medicines.
Metabonomics-based analysis, using UPLC, exact mass MS, and MarkerLynx Software data processing for multivariate statistical analysis,can help quickly and accurately characterize these medicines and also their effect on human metabolism.
Preparative-scale fractionation and purification is used along with classic quantitative bioanalytical tools used in drug development.[21]
4.ADME (Absorption, Distribution, Metabolism, Excreation) Screening:
Pharmacokinetics studies include studies of ADME (Absorption, Distribution, Metabolism and Excreation). ADME studies measure physical and biochemical properties – absorption, distribution, metabolism, elimination, and toxicity of drugs where such compounds exhibit activity against the target disease. A significant number of candidate medicines fall out of the development process due to toxicity. If toxic reactions or any side effect occurs in the discovery/development process, then it becomes more costly. It is difficult to evaluate candidate drugs for possible toxicity, drug-drug interactions, inhibition, and/or induction of metabolizing enzymes in the body. Failure to properly identify these potential toxic events can cause a compound to be withdrawn from the market.
The high resolution of UPLC enables accurate detection and integration of peaks in complex matrices and extra sensitivity allows peak detection for samples generated by lower concentration incubations and sample pooling. These are important for automated generic methods as they reduce failed sample analyses and save time. UPLC/MS/MS provides following advantages:-
UPLC can more than double throughput with no loss in method robustness.
UPLC is also simpler and more robust than the staggered separations sometimes applied with HPLC methods.
Tandem quadrupole MS provides sensitivity and selectivity for samples in matrix using multiple reaction monitoring (MRM) for detection and automated compound optimization.
UPLC/MS/MS operating with rapid, generic gradients has been shown to increase analytical throughput and sensitivity in high throughput pharmacokinetics or bioanalysis studies, including the rapid measurement of potential p450 inhibition, induction, and drug-drug interactions. As well, since this UPLC-based approach can help labs pre- emptively determine candidate toxicity and drug-drug interactions, it enables organizations to be more confident in the viability of candidate medicines that do progress to late-stage clinical trials.
Tandem quadrupole MS combines with UPLC in ADME screening for sensitivity and selectivity with fast analyses of samples in matrix to be achieved with minimal cleanup, using MRM (multiple reaction monitoring) for detection and automated compound optimization.[21]
5.Bioanalysis / Bioequivalence Studies
For pharmacokinetic, toxicity, and bioequivalence studies, quantitation of a drug in biological samples is an important part of development programs. The drugs are generally of low molecular weight and are tested during both preclinical and clinical studies. Several biological matrices are used for quantitative bioanalysis, the most common being blood, plasma, and urine [27]
The primary technique for quantitative bioanalysis is LC/MS/MS. The sensitivity and selectivity of UPLC/MS/MS at low detection levels generates accurate and reliable data that can be used for a variety of different purposes, including statistical pharmacokinetics (PK) analysis.
UPLC/MS/MS helps in the processes of method development for bioanalysis into logical steps for MS, LC, and sample preparation.Quantitative bioanalysis is also an integral part of bioequivalence studies, which are used to determine if new formulations of existing drugs allow the compound to reach the bloodstream at a similar rate and exposure level as the original formulation.UPLC/MS/MS solutions are proven to increase efficiency, productivity, and profitability for bioequivalence laboratories. Applications of UPLC/MS/MS in bioequivalence and bioanalysis are:-
In UPLC/MS/MS, LC and MS instruments and software combine in a sophisticated and integrated system for bioanalysis and bioequivalence studies, providing unprecedented performance and compliance support
Eg. Fig5-Comparison HPLC and. UPLC for the separation of a ginger root extract.& 6- UPLC Separation of Seven Coumarins illustrating fast method development.
UPLC/MS/MS delivers excellent chromatographic resolution and sensitivity
MS delivers simultaneous full-scan MS and multiple reaction monitoring (MRM) MS data with high sensitivity to address matrix monitoring
UPLC Sample Organizer maximizes efficiency by accommodating large numbers of samples in a temperature-controlled environment, ensuring maximum throughput
Increase the sensitivity of analyses, quality of data including lower limits of quantitation (LLOQ), and productivity. efficient separations with fast acquisition rates of tandem quadrupole MS systems Easily acquire, quantify and report full system data in a compliant environment using a security-based data collection software Ensure the highest quality results and reliable system operation in regulated environment[21]
Eg Fig5- Comparison HPLC and. UPLC for the separation of a ginger roots extract.
6.Dissolution Testing :
For quality control and release in drug manufacturing,dissolution testing is essential in sustained-release dosage formulations, testing higher potency drugs is particularly important where dissolution can be the rate-limiting step in medicine delivery. The dissolution profile is used to demonstrate reliability and batch-to-batch uniformity of the active ingredient. Additionally, newer and more potent formulations require increased analytical sensitivity. UPLC provides precise and reliable automated online sample acquisition. It automates dissolution testing, from pill drop to test start, through data acquisition and analysis of sample aliquots, to the management of test result publication and distribution[21].
7.Forced Degradation Studies:
The FDA and ICH require stability testing data to understand how the quality of an API (active pharmaceutical ingredient) or a drug product changes with time under the influence of environmental factors such as heat, light, pressure and moisture or humidity. Knowledge of these stability characteristics defines storage conditions and shelf life, the selection of proper formulations and protective packaging, and is required for regulatory documentation. Forced degradation, or stress testing, is carried out under even harsher conditions than those used for accelerated stability testing. Generally performed early in the drug development process, laboratories cause the potential drug to degrade under a variety of conditions like peroxide oxidation, acid and base hydrolysis, photostability, and temperature to understand resulting by-products and pathways that are necessary to develop stability indicating methods.
PDA/MS (photodiode array and MS), allows for faster and higher peak capacity separations, for complex degradation product profiles also. Combining the chromatographic speed, resolution, and sensitivity of UPLC separations with the high-speed scan rates of UPLC-specific photodiode array and MS detection will give confidence for identifying degradation products and thus shortening the time required to develop stability-indicating methods[21].
8.Manufacturing / QA / QC :
Identity, purity, quality, safety and efficacy are the important factors to be considered while manufacturing a drug product .The successful production of quality pharmaceutical products requires that raw materials meet purity specifications. That manufacturing processes proceed as designed. Those final pharmaceutical products meet, and hopefully exceed, defined release specifications. Continued monitoring of material stability is also a component of quality assurance and control. UPLC is used for the highly regulated, quantitative analyses performed in QA/QC laboratories.
The supply of consistent, high quality consumable products plays an important role in a registered analytical method. The need for consistency over the lifetime of a drug product which could be in excess of 30 years is essential in order to avoid method revalidation and associated production delays.
9.Iodinated disinfection byproduct:
Iodinated disinfection by products (DBPs) are generally more toxic than their chlorinated and brominated analogues. Up to date, only a few iodinated DBPs in drinking water have been identified by gas chromatography/mass spectrometry. In this work, a method for fast selective detection of polar iodinated DBPs was developed using an electrospray ionization-triple quadrupole mass spectrometer (ESI-tqMS) by conducting precursor ion scan of iodide at m/z 126.9. With such a method, pictures of polar iodinated DBPs in chlorinated, chloraminated, and chlorine-ammonia treated water samples were achieved. By coupling state-of-the-art ultra performance liquid chromatography (UPLC) to the ESI-tqMS, structures of 17 iodinated DBPs were tentatively proposed. The results fully demonstrate that, with respect to the DBP number/levels among the three disinfection processes, chloramination generally generated the most/highest iodinated DBPs, chlorination generally produced the fewest/lowest iodinated DBPs, and chlorine-ammonia sequential treatment formed iodinated DBPs lying in between; the numbers of iodinated DBPs in chloraminated Suwannee River Fulvic Acid (SRFA) and Humic Acid (SRHA) were nearly the same, but the levels of aliphatic iodinated DBPs were higher in the chloraminated SRFA while the levels of aromatic iodinated DBPs were higher in the chloraminated SRHA; a couple of nitrogenous iodinated DBPs were found in chloramination and chlorine-ammonia treatment. The ratio of total organic iodine levels in chlorine-ammonia sequential treatment and chloramination could be expressed as a function of the lag time of ammonia addition.[1]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Chromatographs:
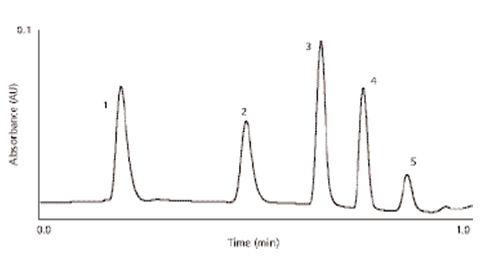
Fig1: UPLC stability indicating assay8.
UPLC conditions:
|
column: |
2.1 ×30 mm 1.7μm ACQUITY BEH C18 at 30 °C. A 45 s, 5–85%B linear gradient, |
|
Injaction |
5 µL injection, 0.1 mg/mL each. |
|
Flow rate |
0.8 mL/min |
|
Mobile phase |
A was 10 mm ammonium formate, pH 4.0, B was acetonitrile. |
|
Peaks in order |
(1)5-nitroso-2,4,6-triaminopyrimidine, 4-amino-6-chloro-1, (2)3-benzenesulfanamide, (3)hydrochlorthiazide, (4) triamterine, (5) methylbenzenesulfanamide; |
|
UV detection |
at 273 nm and 40 pts/s |
|
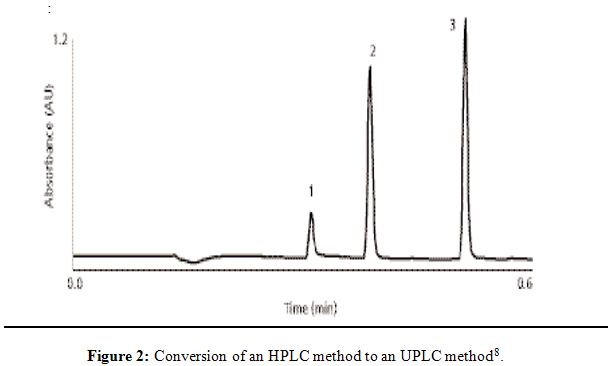
Column |
2.1 _ 50 mm, 1.7 _m ACQUITY BEH C18 at 40 °C. A 10 mM ammonium phosphate pH 9.2) acetonitrile linear gradient from 10–90% acetonitrile |
|
Injection |
3 µL of a 5 mg/mL mixture of each of the compounds |
|
flow rate |
at 1.0 mL/min |
|
UV detection |
at 210 nm |
|
Peaks are in order |
(1) pseudoephedrine, (2) ibuprofen, and (3) butylparaben internal Standard. |
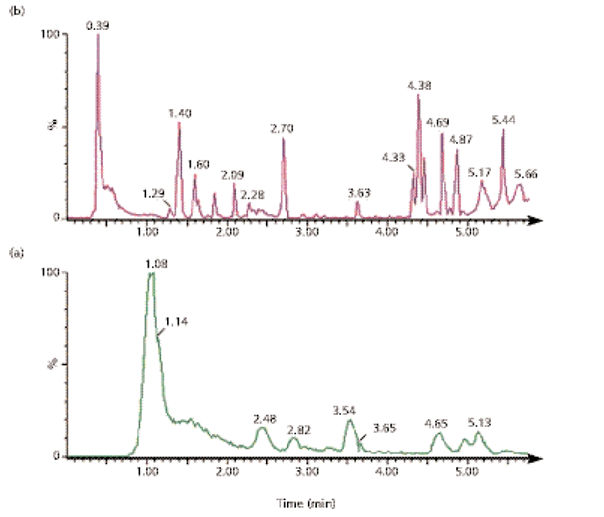
Figure 3: Full scan T of MS for HPLC (Figure 3a) and UPLC analyses (Figure 3b) of the metabolites of dextromethorphan8.
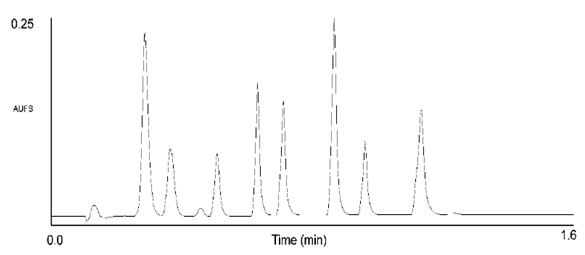
Figure 4.UPLC separation of eight diuretics7.
|
Column: |
2.1 by 30mm 1.7 mm ACQUITY UPLC BEH C18 @ 358C. A 9–45%B linear gradient over 0.8 minutes
|
|
flow rate |
0.86mL/min
|
|
Mobile phase |
A was 0.1% formic acid, B was acetonitrile
|
|
UV detection |
273 nm
|
|
Peaks are in order |
(1) Acetazolamide, |
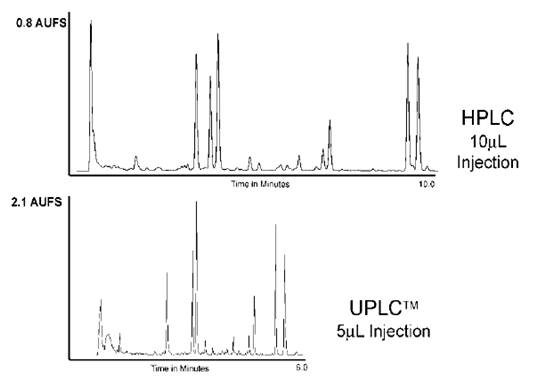
Figure 5. Comparison HPLC and. UPLC for the separation of a ginger root extract7.
|
|
HPLC conditions |
UPLC condition |
|
Column: |
2.1 by 100mm 5.0 mm prototype BEH C18 at 288C. A25–96%B linear gradient over 10 minutes. |
2.1 by 100mm 1.7 mm ACQUITY BEH C18 at 288C. A 50–100%B linear gradient from 1.4 to 3.7 minutes, followed by a hold until 6.0 minutes |
|
flow rate |
1.0mL/min |
0.3mL/min |
|
Mobile phase |
A was water, B was acetonitrile |
A was water, B was acetonitrile |
|
UV detection |
230 nm |
230 nm |
|
Injection. |
10 mL |
5mL |
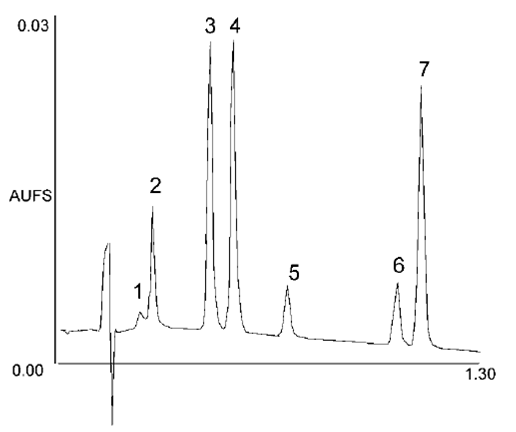
Figure 6:UPLC Separation of Seven Coumarins illustrating fast method development7.
|
Column |
2.1 by 30mm 1.7 mm ACQUITY UPLC BEH C18 @ 358C. A20–40%B linear gradient over 1.0 minute |
|
flow rate |
0.86mL/min |
|
Mobile phase |
A was 0.1% formic acid, B was acetonitrile |
|
UV detection |
254 nm and 40 pts/sec |
|
Peaks are in order |
1: 7-hydroxycoumarin-glucuronide, 7-hydroxycoumarin, 4-hydroxycoumarin, coumarin, 7-methoxycoumarin, 7-ethoxycoumarin, and 4-ethoxycoumarin.
|
Conclusion:
UPLC gives increased resolution, speed & sensitivity for liquid chromatography therefore due to UPLC new chemistry & instrumentation technology can provide more information per unit of work.UPLC has main advantage over others is reduction of analysis time which also helps to reduce solvent consumption.A negative aspect of UPLC could be the higher backpressure than in conventional HPLC. This backpressure can be reduced by increasing the column temperature.Butit seems that UPLC can offer significant improvements in speed, sensitivity and resolution compared with conventional HPLC.
References:
1. Guoyu D and Xiangru Z, A Picture of Polar Iodinated Disinfection Byproducts in Drinking Water by (UPLC/)ESI-tqMS , SAR, Environ. Sci. Technol 2009, 43 (24), pp 9287–9293
2. Lucie Nováková et al, Department of Analytical Chemistry, Faculty of Pharmacy, Charles University, Heyrovského , Advantages of application of UPLC in pharmaceutical analysis, may 2005
3. Gurdeep R. Chatwal, Sham K.Anand,Instrumental methods of chemical analysis,1979,reprint 2005.pg no. 2.556-2.558
4. Cheng, Y.-F.; Lu, Z.; Neue, U. Rapid Commun. Mass Spectrom. 2001, 15, 141.
5. Tiller, P.R.; Romanyshyn, L.A.; Neue, U.D. Anal. Bioanal. Chem. 2003, 377, 788.
6. Neue, U.D.; Mazzeo, J.R. J. Sep. Sci. 2001, pg no.24, 1.
7. Michael E. Swartz, UPLCTM: An Introduction and Review Waters Corporation, Milford, Massachusetts, USA, Journal of Liquid Chromatography & Related Technologiesw, 2005; 28: pg no.1253–1263
8. Swartz M. E. , Ultra Performance Liquid Chromatography (UPLC): An Introduction, Separation Science Re-Defined, LCGC Supplement, p. 8-13(MAY 2005)
9. Jerkovich A.D., Mellors J.S., and Jorgenson J.W., LCGC 21(7), 600–610 (2003).
10.MacNair J.E., Lewis K.C. , and Jorgenson J.W., Anal. Chem. 69, 983–989 (1997).
11.van Deemter, J.J.; Zuiderweg, F.J.; Klinkenberg, A. Chem. Eng. Sci. 1956, 5, 271.
12.Jerkovich A.D., Mellors J.S. and Jorgenson J.W., LCGC 21, 600–610 (2003).
13. MacNair J.E., Patel K.D. aqnd Jorgenson J.W., Anal. Chem. 71, 700–708 (1999)
14. Wu N., Lippert J.A. and Lee M.L., J. Chromatogr., A 911, 1–12 (2001).
15. Wu, N.; Lippert, J.A.; Lee, M.L. J. Chromatogr. A 2001, 911, 1.
16. Tolley, L.; Jorgenson, J.W.; Mosely, M.A. Anal. Chem. 2001, 73, 2985
17. Lippert, J.A.; Xin, B.; Wu, N.; Lee, M.L. J. Microcol. Sepn. 1997, 11, 631.
18. Lars Y. and Honore H.S., J. Chromatogr., A 1020, 59–67 (2003).
19 McLoughlin D.A., Olah T.V., and Gilbert J.D., J. Pharm. Biomed. Anal. 15,
1893– 1901 (1997).
20.Swartz M. E. , Ultra Performance Liquid Chromatography (UPLC): An Introduction, & review,Journal of Liquid Chromatography & Related Technologiesw, 28: 1253–1263, 2005
21. B. Srivastava , B. K. Sharma , Uttam Singh Baghel , Yashwant , Neha Sethi, Ultra performance liquid chromatography (uplc) : a chromatography technique,International Journal of Pharmaceutical Quality Assurance 2010; 2(1): 19-25
22 Jerkovitch A.D., Mellors J.S., and Jorgenson J.W., LCGC 21(7), 2003.
23. Wu N., Lippert J.A., and Lee M.L., J. Chromotogr., 911(1), 2001.
24. Swartz M., LCGC 23(1), 46–53 (2005).
25. Broske A.D., et al., Agilent Technologies application note 5988-9251EN (2004).
26.Goodwin L, White SA, Spooner N. Evaluation of ultra-performance liquid chromatography in the bioanalysis of small molecule drug candidates in plasma.J. Chromatogr. Sci.; 45(6): 298–304, (2007).
Annexure
UPLC : Ultra performance liquid chromatography
HPLC : High performance liquid chromatography
PSI : Pressure square index
BEH : Brigdeg ethane hybrid
I.D : Internal diameter
PDA : Photodiode Array
TUV : Tunable UV
ELS : Evaporative light scattering
MeOH : methanol
NCE : New chemical entity
MS : Mas spectroscopy
MRM : Multiple reaction monitoring
LLOQ: Lower limits of quantitation
API : Active pharmaceutical ingredient
DBPs : Disinfection by products
SRFA : Suwannee river fulvic acid
SRHA: Suwanee river humic acid
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









