 About Authors:
About Authors:
Dharmendra Kumar*1, Sumedha Bansal1
1Department of Pharmaceutical Technology, Meerut Institute of Engineering and Technology,
Meerut, Uttar Pardesh, India, 250005
*rvnimiet@gmail.com
Abstract:
In present era, nasal drug delivery system has been considered as potential and favourable route of drug delivery because it provides patient compliance, easy to administration, bypass first pass metabolism, excellent penetration, low dose required, rapid absorption and gives desirable effects. So many times nasal drug delivery has been considered as alternative of parenteral route. The purpose of this review, to provide a complete information about nasal drug delivery system such as advantage, limitations, anatomy of nose, mechanism of drug absorption, factors affecting of nasal drug delivery, absorption improvement aspects, novel drug formulations, types of nasal drug delivery system with uses of nasal drug delivery in various disease. So we have expectation, that researches and others cited person get benefitted from this review.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1593
Intoroduction:
Generally nose is the important part of body for inhalation process. But when it is used as the route of drug delivery attained the great attraction for various drugs. Because nose provides faster and higher level of drug absorption with possibility of self-administration [1].
In recentmany of drugs are used via nasal route but many have various disadvantage such as poor contact of formulation with nasal mucosa, fast clearance and solid dosage formulations also be difficult via nasal route [2].
Researchers have also attempted delivery of drugs to the CNS via nasal route cross the blood brain barrier allowing the direct drug delivery in the bio phase of central nervous system active compound. NDDS also be administration route for vaccines[3-6].
Hydrophobic and low molecular drugs can easily penetrate through nasal mucosa with less degradation. Fast absorption can be achieved due to large absorption surface area and high vascularisation. In emergency nasal route can be used as alternative route of parenteral[7-8].
Advantage of nasal drug delivery system
|
S. NO. |
ADVANTAGES |
FACTORS |
|
1. |
Improving patient compliance[9] |
Needle free (painless) Trained person not required |
|
2. |
Good penetration[10] |
in case of lipophilic drugs in case of low molecular weight
|
|
3. |
rapid absorption and onset of action |
Due to relative large surface area High vascularisation |
|
4. |
Avoidance of the harsh environment |
less chemical and enzymatic degradation |
|
5. |
low dose required |
Free from first pass metabolism |
|
6. |
Direct delivery of drug to central nervous system[11] |
via olfactory region, thus bypass the blood brain barrier |
[adsense:468x15:2204050025]
Limitations of nasal drug delivery system.
|
S.ON. |
LIMITATIONS |
FACTORS |
|
1. |
Risk of local side effect and irreversible damage of cilia on nasal mucosa [12] |
due to constituents added to dosage forms |
|
2. |
Disrupt and even dissolve the nasal membrane[12] |
Due to high concentration of absorption enhancers |
|
3. |
Reduce the capacity of nasal absorption[12] |
Due to nasal atrophic rhinitis and severe vasomotor rhinitis |
|
4. |
Low bioavailability[12] |
due to enzymatic degradation and metabolism at mucosal surface. |
Anatomy of nose:
Breathing and olfaction are the major function of human nose[13]. But it also functioned as filtration and humidify of inhaled air before reaching in lowest airways. Nasal cavity has mucus layer and hairs, those helpful in filtration of particles trapped in inhaled air. Additionally metabolism of endogenous substances, mucociliary clearance also a function of nose[14-17].
An nasal cavity has about a 20 ml capacity with a large surface area (about 180 cm2) for the drug absorption afforded by the microvillus present along the pseudo-stratified columnar epithelia cells of the nasal mucosa. Nasal cavity is divided by septum into two equal parts[18].
PH of mucosal secretions:-
In children – 5.0 to 6.7[19-20]
In adult - 5.5 to 6.5[19-20]
Human nasal cavity have three functional regions called vestibular, olfactory ,and respiratoryareas.the vestibular area provides as a baffle system, and functioned as filter of airborne particle, it has Keratinized and squamous epithelial cells with nasal hairs, it has poor permeability of drug particles. Olfactory epithelium is helpful to metabolizing drugs,it has sustentacular cells, olfactory receptor and basal cells. Drugs can be direct access to CNS from olfactory region. Respiratory region also an important in nasal drug delivery system and provided optimum drug absorption. Respiratory region contains columnar ciliated cells, columnar non ciliated cells, goblet cells and basal cells[21-24].
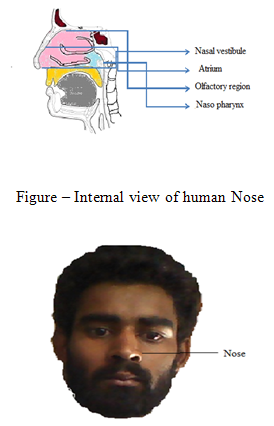
Figure:- External view of Human Nose
(A).Nasal vestibule:-
- It is the anterior part of nasal cavity.
-Surface area is 0.6cm2[24].
-Nasal portion is covered by a stratified squamous keratinized epithelial with sebaceous gland.
-Drug absorption is very difficult in this region but it afforded high resistance against toxic environment [25].
(B). Atrium-
- The area between nasal vestibule and respiratory region is called Atrium.
- The anterior part consists – stratified squamous
- The posterior part consists- pseudostratified columnar cells.
(C). Respiratory region.
- It is the largest part of nasal cavity and also known as conchae.
- Humidification and temperature is the function of it
- Drug delivery is very good in this region
-It consist pseudo stratified columnar epithelial, globet cells, basal cells, mucous and serous glands.
- Microvilli are important to enhance the respiratory surface area.
(D). Olfactory region.
- It located in the roof of the nasal cavity.
-It has neuroepithelium
-Neuroepithelium is the cavity part of the CNS is directly exposed the external environment [26].
-It has pseudostratified.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Mechanism of drug absorption[27-28]:-
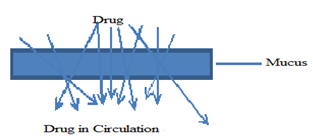
Figure -: Mechanism of Drug Absorption
Types of drug mechanism:
1. Paracellular mechanism – in which process drug transported between cell and transcytosis by vesicle carrier. It is slow process. In which passive diffuse take place. It is suitable mechanism for hydrophilic drugs[28].
2. Transcellular mechanism – in which process drug diffuse through membrane. It is an active transport process. It is more suitable for lipophilic drugs[28-29].
Factors affecting nasal drug delivery system:
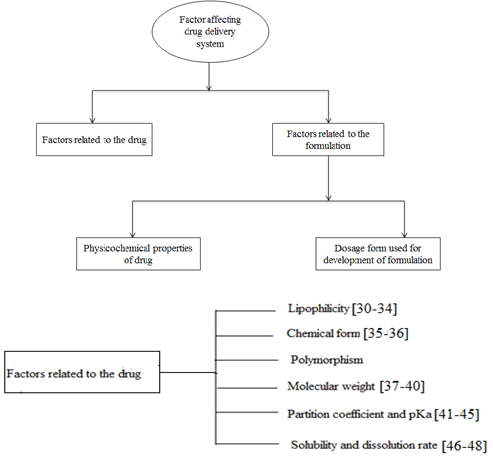
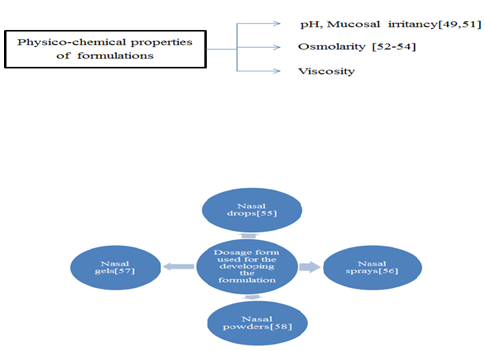
Figure-: Various dosage form of Nasal Drug delivery System
Nasal absorption improvement aspects[59]:-
On the basis of researchers work and literature review can say that absorption of nasal drug improved by various methods. These methods can be divided in two classes.
1. Physicochemical effects:- in which method drug absorption can be improved by modification of physicochemical properties of drugs. And by using absorption enhancers. This method includes drug solubility, drug partition, weak ionic interactions etc.
2. Membrane effects: - nasal mucosal surface also be helpful in improving nasal absorption.
Following approaches also have been used for improvement of nasal drug absorption [60-65].
* Prodrugs
* Enzymatic inhibitors
* Absorption enhancers
* Mucoadhesive drug delivery system
* Novel formulation forms
These approaches are used in following problems:-following problem may take place in the nasal absorption or affect the bioavailability of drug-
1. Poor physiological properties of drug and/or formulation
2. Enzymatic degradation
3. Low permeability through nasal membrane
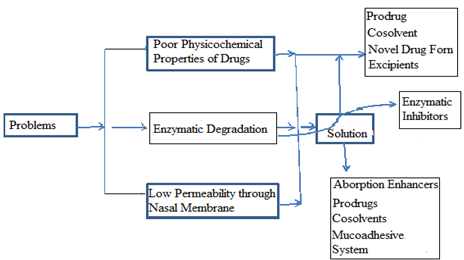
Figure-: Problems and their Solutions for Nasal improvement.
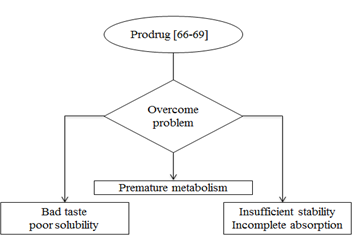
Figure-: Advantage of Prodrug formulations.
Enzymatic inhibitors[70-76]- nasal mucus layer and nasal mucosa act as enzymatic barriersin the nasal drug delivery. Enzymatic degradation can be avoided by use of protease and peptidases inhibitors.
Absorption enhancers[77-83]- various types of absorption enhancers are used in nasal drug delivery but mechanism of action is not known. Absorption enhancers are as surfactants, bile salts, fatty acids, and polymeric enhancers. These are change permeability of epithelial cell layer by modifying the phosphlipidic bilayer, and increasing paracellular transport because it opening the tight junction between epithelial cells. On the basis of researchers and reviews chitosan and cyclodextran are significantly used with low toxic effect[84-93].
Mucoadhesive drug delivery system[94-97]- by using mucoadhesive agent residence time of drug on mucosa increased. Nasal gel preparations are based on these agents. On the basis of resource these are grouped in two category namely natural mucoadhesive agent[98] and synthetic mucoadhesive[99-101].
Table No. 1- Natural Mucoadhesiveagent used in Nasal drug delivery.
|
S.NO. |
NATURAL MUCOADHESIVE AGENT |
DRUG |
SYNTHETIC MUCOADHESIVE AGENT |
DRUG
|
|
1. |
Ficus carica[102] |
Midazolam |
Sorbital |
Rizatriptan benzoate |
|
2. |
Pectin[103] |
Chlorpromazine |
PEG |
Metaclopromide Hcl |
|
3. |
Dellinia indica[104] |
Oxytocin |
HPMC[108] |
Salbutamol |
|
4. |
Linum usitatissimum[105] |
Midazolam |
Carbapol |
Naloxone |
|
5. |
Chitosan |
Indomethacin |
Carbapol[109] |
Progesterone |
|
6. |
Gellan gum[106] |
Mometasone |
Polyacrylic acid |
Oxymetazoline Hcl |
|
7. |
Albumin[107] |
Propranolol Hcl |
HPMC[110] |
Lidocaine Hcl |
Novel drug formulations:- liposomes, microspheres, and nanoparticles are widely used novel drug formulation. These formulation improved stability, increased bioavailability, so gives better result than other formulations.
1. Liposomes-[111-119] these are phospholipid vesicles composed by bilayer enclosingone or more aqueous compartments, in these compartments drug can be entrapped or adsorbed.
2. Microspheres.-[120-121] microsphere has important role in nasal drug delivery with enhance absorption, sustained release, and also has great importance because it protects drug from enzymatic degradation.
3. Nanoparticles-[122-132].
Types of nasal drug delivery:-on the basis of anatomy and physiology, many researchers have been developed various nasal drug delivery systems. These systems depend on the conditions of disease such as acute and chronic. And other various factors are discussed in this review previously [133].
1. Local delivery:[134-140]
-Favourable for topical nasal disorder.
-Low dose required
-Minimizing toxic effects.
-Recently topical ant biotherapy has been used.
For example-
DrugsDisorders

NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
2. systemic delivery:
-Fast and extended drug absorption [141].
-Nasal formulation gives more system effect than other routes [142-143].
For example-
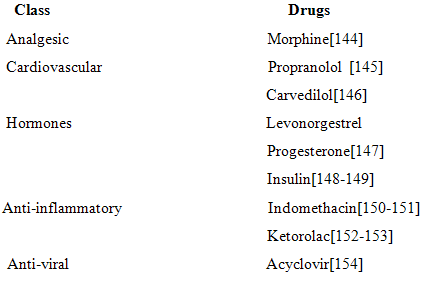
3. Nasal vaccines: nasal vaccines are generally used against respiratory infections. In vaccinationnasal route is the choice of route and also used as alternative of parenteral route because it increaseslevel of immunoglobulin G and immunoglobulin A [155-157]. Nasal vaccines are used against various infection such as-
a. Influenza A and B, proteasomes-influenza[158]
b. Adenovirus-vectored influenza[159]
c. Group B meningococcal native[160]
d. Attenuated respiratory syncytial virus[161]
e. Para influenza 3 virus[162]
f. Human immunodeficiency virus[163]
g. Hepatitis B virus[164]
4. CNS delivery through nasal route:
-Brain is the delicate organ and performs vital functions.
-Brain surrounding with tight junction of BBB, it has greater transendothelial electric resistance compared to other tissue such as skin, bladder, lungs [165].
-When drugs/xenobiotics pass through BBB reduce the brain exposure [166-167].
-Olfactory neuroepithelium delivers the drugs nose to CNS, paracellur, trascellular and/or neuronal transport take place [168].
-Olfactory pathways present potential to bypass BBB[169-172].
-Drug delivery into CNS via nasal route has been presented by many researchers in human as well as in animal [173-176].
For example- Animal model of Alzheimer’s disease[177-178].
-Brain tumours[179-180],epilepsy[181], pain, sleep disorder[182],
Greater brain exposure via nasal delivery, needleless of passage BBB but in that other cases lacking of evidence recorded[183-187].
Reference
1. Tyagi Shanu, Sharma Nitin, Sharma P.K., A review on application of natural bioadhesive polysaccharides for intranasal drug delivery, Int.J.A.PS.BMS, APR-JUNE.2012, Vol.1(2), 80-94.
2. JadhavR.K ,GambhireN.Ishaque, Nasal drug delivery system-factors affecting and applications current drug therapy, 2007,2,27-38.
3. Yamaya M, finkbeiner WE, Chun SY, Differentiated structure and function of cultures from human tracheal epithelium. Am J physiol, 1922; 262:L713-L724.
4. Romeo VD, Meireles J, Sileno AP, Pimplaskar HK, Behl CR. Effect of physicochemical properties and other factors on systemic nasal delivery. Adv Drug delivery Rev, 1998;29:89-116.
5. Illum L. Nasal drug delivery: new developments and strategies. Drug Discovery Today, 2002;7:1184-1189.
6. Graff LC, Pollock GM. Nasal drug administration: potential for targeted central nervous system delivery. J Pharm Sci, 2005; 94:1187-1195.
7. Vyas S.P, Khar R.K, Targeted and controlled drug delivery novel carrier system. 1st ed.CBS publishers and distributors. 2006, 173,249,331,417.
8. RathananandMahalaxmi, kumar D.S, Shirwaikar A, preparation of mucoadhesive microsphere for nasal delivery by spray drying. Indian journal of pharmaceutical science, 2007, 69(5),651-657.
9. Pontiroli A, Albertto M, Calderara A, absolute bioavailability of nicotine applied to different Nasal administration of glucagon and human calcitonin to nasal regions. Eur J ClinPharmacol 1991; 41:585-588.
10. Striebel HW, Pommerening J, Rieger A, Intranasal fentyl titration for postoperative pain management in an unselected population. Anaesthesia 1993; 48(9):753-757.
11. Talegaonkar S, Misra PR, Intranasal delivery: An approach to bypass the blood brain barrier. Indian J pharmacol 2004; 36(3):140-147.
12. Vyas SP, Talwar N, Jain NK. An erythrocyte based bioadhesive system for nasal delivery of propranolol. J.Control.Rel.1993;23:231-237.
13. Dekkar M, Encyclopaedia of pharmaceutical technology , Informa health Care, New York, USA, 2002.
14. Chien YW, chang SF, Intranasal drug delivery for systemic medications. Crit Rev The drug Carrier Syst, 1987;4:67-194
15. Wynsberghe DV, Noback R.C, Carola R, Human anatomy and physiology, Mcgraw-hillCompanie, UK,1994.
16. Stevens A, Lowe J, Human histology, Mosby, Philadelphia, USA, 1997.
17. Merkus FW, Verhoef JC, Schipper NG, Marttin E, nasal mucociliary clearance as a factor in nasal drug delivery. Adv Drug deliv Rev, 1998;29:13-38.
18. Kumar Dharmendra, BansalMayank, Nasal gel formulation, tgjpr 2012,1(3)303-310.
19. Michael IU, Remigius U, Agu, Norbert, Kinget, Nasal mucoadhesive drug delivery background, application, trends and future perspectives. Advanced Drug Delivery Reviews,2005,57:1640-1665.
20. Mygind N, Anggard A, Anatomy and physiology of the nose pathophysiology alterations in allergic rhinitis, clinical Rev Allergy, 1984,2,173-178
21. Mygind N, Dahl R, Anatomy,physiology and function of the nasal cavities in health and desease. Adv Drug Del Rev 1998;29:3-12
22. Ozer AY, The importance of intranasal route for application of drugs and nasal drug delivery systems, Pharmacia-JTPA 1990;30;136-147.
23. Huang C.H, Kimura R, Nassar R.B, Mechanism of nasal absorption of drugs, physicochemical parametres influencing the rate of in situ nasal absorption of drugs in rats. Journal of Pharmaceutical Science, 1985, 74(6),608-611
24. Illum L, Nasal drug delivery, possibilities, problems and solutions, Journal of Control Release, 2003;87:187-198.
25. Kimbell JS, Gross EA, Richardon RB, Conolly RB, Morgan KT, Correlation of regional formaldehyde flux predictions with the distribution of formaldehyde-induced squamous metaplasia in F344 rat nasal passages, Mutant Res, 1997,380,143-154.
26. Charlton S, Jones NS, Davis SS, Illum L, Distribution and clearance of bioadhesive formulations from the olfactory region in man, Effect of polymer type and nasal delivery device. Eur J Pharm Sci, 2007:30:295-302.
27. Illum L, Chickering DE, Bioadhesive formulations for nasal peptide delivery : Fundamentals, novel Approaches and Development. Marcel Dekker. New York 1999;507-539.
28. Aurora J, Development of nasal delivery system: A Review, Drug Delivery Technology 2002,2(7):1-8.
29. PiresAnaisa, Fortuna Ana, Alves Gilberto, Intranasal Drug Delivery: How, Why and What for ?. Journal of pharmacy and pharmaceutical science, 2009,12(3),288-311.
30. Corbo DC, drug absorption through mucosal membranes: effect of mucosal route and penetrant hydrophilicity . Pharm Res, 1989;6:848-852.
31. Hussain A, Kimura R, Huang CH, Kashihara T, nasal absorption of naloxone and buprenorphine in rats. Int J Pharm 1984;21:233-237.
32. Bawarshi RN, Hussain A, Crooks PA, nasal absorption of 17a-ethinyloestradiol in the rat. J Pharm Pharmacol 1989;41:214-215.
33. Hussain A, Hamadi et al, does increasing the lipophilicity of peptides enhance their nasal absorption. J Pharm Sci 1991;80:1180-1181.
34. Mc Martin C, Analysis of structural requirements for the absorption of drugs and macro molecule from the nasal cavity. J Pharm Sci, 1987;76:535-540.
35. Huang C, Kimura R, NassarA,Hussain A, Mechanism of nasal drug absorption of drug II: absorption of L-tyrosine and the effect of structural modification on its absorption. J Pharm Sci 1985;74:1298-1301.
36. Costantino HR, Illum L, Brandt G, Johnson PH, Quay SC, intranasal delivery: Physioco chemical and therapeutic aspects. Int J Pharm, 2007;337:1-24
37. Fisher A, Illum L, Davis S, Schacht E, Di-iodo-L-tyrosine labelled dextran as molecular size markers of nasal absorption in the rat. J Pharm pharmacol 1992,44:550-554.
38. Yamamoto A, Morita T, Hashida M, Sezaki H, Effect of absorption promoters on the nasal absorption of drugs with upper various molecular weight. Int J Pharm 1993;93:91-99.
39. Corbo DC, Characterisation of the barrier properties of mucosal membranes. J Pharm Sci 1990;79:202-206.
40. Donovan M, Flynn G, Amidon G, Absorption of polyethylene glycols 600 through 2000: the molecular weight dependence of gastro intestinal and nasal absorption. Pharm Res, 1990,7:863-868
41. Jiang XG, Lu X, Cui JB, Qiu L, Xi NZ. Studies on octanol-water partition coefficient and nasal drug absorption. Yao XueXueBao 1997;32:458-460.
42. Hirai S, Yashika T, Matsuzawa T, Mima H, Absorption of drugs from the nasal mucosa of rat. Int J pharm Sci 1985;74:608-611.
43. Shao Z, Park GB, Krishnamoorthy R, Mitra AK, The Physicochemical properties, plasma enzymatic hydrolysis, and nasal absorption of acyclovir and its 2’-ester prodrugs, Pharm Res , 1994,11:237-242.
44. Hussain A, Bawarshi-Nassar R, Huang CH, Transnasal Systemic Medications. Elsevier, msterdam, 1985.
45. Zaki NM, Awad GA, Mortada ND, Rapid-onset intranasal delivery of metoclopramide hydrochloride. Part I. Int J Parm,2006;327:89-96.
46. Arora P, Sharma S, Garg S. Permeability issues in nasal drug delivery. Drug Discovery Today, 2002;7:967-975.
47. Kao HD, Traboulsi A, Itoh S, Enhancement of the systemic and CNS specific delivery of L-dopa by the nasal administration of its water soluble prodrugs. Pharm Res 2000;17:978-984.
48. Hussain A, Al-Bayatti AA, Dakkur A, Okochi K, Hussain MA, Testosterone 17ß-N,N-Dimethylglycinate Hydrochloride: A prodrug with a potential for nasal delivery of testosterone. J Pharm Sci, 2002;91:785-789.
49. Dae-Duk Ki, drug absorption Studies: In situ, In vitro and In silicon models, chapter 9, Springer, USA, 2007.
50. Washington N, Steele RJ, Jackson SJ, Bush D, determination of baseline human nasal pH and the effect of intranasally administered buffers. Int Pharm, 2000;198:139-146.
51. Pujara CP, Shao Z, Duncan MR, Mitra AK, Effect of formulation variables on nasal epithelial cell integrity; Biochemical evaluations. Int J Pharm, 1995;114:421-430.
52. Ohwaki T, Ando H, Watanabe S, Miyake Y, Effect of dose, pH, and osmolarity on nasal absorption of secretine in rats. J Pharm Sci 1985;74:550-552.
53. Ohwaki T, Ando H, Karkimoto F, Miyake Y, Effect of dose, pH, and osmolarity on nasal absorption of secretine in rats II: Histological aspects of nasal mucosa in relation to the absorption variation due to effect of pH and osmolarity . J pharm Sci 1987;76:695-698.
54. Ohwaki T, Ishii M, Aoki S, Tatsuishi K, Kaman K, Effect of dose pH and osmolarity on nasal absorption of secretin in rats III, In vitro membrane permeation test and determination of apparent partition coefficient of secretion. Chem Pharm Bull (Tokyo)1989;37:3359-3362.
55. Patel RS, McGarry GW. Most patients overdose on topical nasal corticosteroid drops: an accurate delivery device is required. J LaryngolOtol 2001; 115: 633-635.
56. Ishikawa F, Katsura M, Tamai I, Tsuji A. Improved nasal bioavailability of elcatonin by insoluble powder formulation. Int J Pharm 2001; 224: 105-114.
57. Junginger HE. Mucoadhesive hydrogels. PharmazeutischeIndustrie 1956; 53: 1056-1065.
58. Davis SS, Illum L. Absorption enhancers for nasal drug delivery. ClinPharmacokinet 2003; 42(13): 1107-1128.
59. Behl CR, Pimplaskar HK, Sileno AP, Xia WJ et al. Optimization of systemic nasal drug delivery with pharmaceutical excipients. Adv Drug Del Rev 1998,29;117-133.
60. Upadhyay, Shivam , Joshi, Pratik et al Intranasal drug delivery system, A glimpse to become maestro, J APS, 2011,1,34-44.
61. Miyamoto, Misa, Improved nasal absorption of drugs using poly-arginine: effects of concentration and molecular weight of poly-arginine on the nasal absorption of fluorescein isothiocyanate-dextran in rats. European Journal of pharmaceutics and biopharmaceutics, 2001,52:21-30.
62. DhuriaShyeilla V, Leah R, Hanson, William H, Frey, Therapeutic consideration: intranasal delivery to the central nervous system: mechanisms and experimental considerations. Journal of Pharmaceutical Sciences, 2010,99,1654-1673.
63. Harshad, Parmar, Bhandari, Anand, Shah, Dushyant, Recent technique in nasal drug delivery: a review. International Journal of drug development & research,2011,3(1),99-106.
64. Bhowmik, Debjit, Kharel, Rakesh et al Innovative approaches for nasal drug delivery system and its challenges and opportunities. Annals of biological research, 2010,1(1)21-26.
65. Costatino, Henry, Brandt, Gordon, Paul et al Intranasal delivery ; physicochemical and therapeutic aspects, International journal of pharmaceutics,2007.337,1-24.
66. Albert A. Chemical aspects of selective molecular weight dependence of toxicity. Nature, 1958; 182:421-430.
67. Higuchi T., Stella V., Prodrugs as Novel Drug Delivery Systems, American Chemical Society, Washington, DC, 1975.
68. Wang H, Hussain AA, Wedlund PJ. Nipecotic Acid: Systemic availability and brain delivery after nasal administration of nipecotic acid. and n-Butyl nipecotate to rats. Pharm Res,2005; 22:556-562.
69. Yang C, Gao H, Mitra AK. Chemical stability, enzymatic hydrolysis, and nasal uptake of amino acid ester prodrugs of acyclovir. J Pharm Sci, 2001; 90:617-624.
70. Machida M. Effects of surfactants and protease inhibitors on nasal absorption of recombinant human granulocyte colonystimulating factor (rhG-CSF) in rats. Biol Pharm Bull, 1994; 17:1375-1378.
71. Morimoto K, Miyazaki M, Kakemi M. Effects of ofproteolytic enzyme inhibitors on nasal absorption of salmon calcitonin in rats. Int J Pharm, 1995; 113:1-8.
72. Bernkop-Schnurch A. Use of inhibitory agents to overcome the enzymatic barrier to perorally administered therapeutic peptides and proteins. J Control Release, 1998; 52:1-16.
73. Hussain MA. The use of alpha-aminoboronic acid derivatives to stabilize peptide drugs during their intranasal absorption. Pharm Res,1989; 6:186-189.
74. Hoang VD, Uchenna AR, Mark J, Renaat K, Norbert V. Characterization of human nasal primary culture systems to investigate peptide metabolism. Int J Pharm, 2002; 238:247-256.
75. O?Hagan DT. Nasal absorption enhancers for biosynthetic human growth hormone in rats. Pharm Res, 1990; 7:772-776.
76. Greimel A, Bernkop-Schnürch A, Del Curto MD, D'Antonio M. Transport characteristics of a beta sheet breaker peptide across excised bovine nasal mucosa. Drug Dev Pharm, 2007;33:71-77.
77. Wang J, Tabata Y, Morimoto K. Aminatedgelatin microspheres as a nasal delivery system for peptide drugs: Evaluation of in vivo insulin absorption in rats. J Control Release, 2006; 113:31-37.
78. Bogdanffy MS. Biotransformation enzymes in the rodent nasal mucosa: The value of a histochemical approach. Environ Health Perspect, 1990; 85:177-186.
79. Hersey SJ, Jackson RT. Effect of bile salts on nasal permeability in vitro. J Pharm Sci, 1987; 76:876-879.
80. Mishima M, Wakita Y, Nakano M. Studies on the promoting effects of medium chain fatty acid salts on the nasal absorption of insulin in rats. J Pharmacobiodyn, 1987; 10:624-631.
81. Baglioni C, Phipps RJ. Nasal absorption of interferon: enhancement by surfactant agents J Interferon Res, 1990; 10:497-504.
82. Illum L, Farraj NF, Davis SS. Chitosan as a novel nasal delivery system for peptide drugs. Pharm Res, 1994; 11:1186-1189.
83. Ekelund K, Osth K, Påhlstorp C, Björk E Ulvenlund S, Johansson F. Correlation between epithelial toxicity and surfactant structure as derived from the effects of polyethylynyoxide surfactants on caco-2 monolayers and pig nasal mucosa. J Pharm -Sci, 2005; 94:730-44.
84. Sinswat P, Tengamnuay P. Enhancing effect of chitosan on nasal absorption of salmon calcitonin in rats: comparison with hydroxypropyl- and dimethyl--cyclodextrins. Int J Pharm, 2003; 257:15-22.
85. Zaki NM, Mortada ND, Awad GA, AbdElHady SS. Rapid-onset intranasal delivery of metoclopramide hydrochloride Part II: Safety of various absorption enhancers and pharmacokinetic evaluation. Int J Pharm, 2006; 327:97-103.
86. Giunchedi P, Juliano C, Gavini E, Cossu M, Sorrenti M. Formulation and in vivo evaluation of chlorhexidinebuccal tablets prepared using drug loaded chitosan microspheres. Eur J Pharm Biopharm, 2002; 53:233-239.
87. Maestrelli F, Zerrouk N, Chemto C, Mura P.Influence of chitosan and hydrochloride salts on naproxen dissolution rate and permeation across Caco-2 cells. IntJ Pharm, 2004; 271:257-267.
88. Ravi Kumar MNV, Muzzarelli RAA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev, 2004; 104:6017-6084.
89. Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chem Rev, 1998; 98:1743-1754.
90. Duchêne D, Bochot A, Yu SC, Pépin C, Seiller M. Cyclodextrins and emulsions. IntJ Pharm, 2003; 266:85-90.
91. Asai K, Morishita M, Katsuta H, Hosoda S, Shinomiya K, Noro M, Nagai T, Takayama K. The effects of water-soluble cyclodextrins on the histological integrity of the rat nasal mucosa. Int J Pharm, 2002; 246:25-35.
92. Babu RJ, Dayal P, Singh M. Effect of cyclodextrins on the complexation and nasal permeation of melatonin. Drug Deliv, 2008;15:381-388.
93. Jug M, Becirevic-Lacan M. Development of a Cyclodextrin-Based Nasal Delivery System forLorazepam. Drug DevInd Pharm, 2008;34:817-826.
94. Gavini E, Rassu G, Sanna V, Cossu M, Giunchedi P. Mucoadhesive microspheres for nasal administartion of an antiemetic drug, metoclopramide: in-vitro/ex-vivo studies. J Pharm Pharmacol, 2005; 57:287-294.
95. Gavini E, Rassu G, Haukvik T, Lanni C, Racchi M, Giunchedi P. Mucoadhesive microspheres for nasal administration of cyclodextrins. J Drug Target, 2008; 17:168-179.
96. Patil SB, Sawant KK. Development, optimization and in vitro evaluation of alginatemucoadhesive microspheres of carvedilol for nasal delivery. J Microencapsul 2008; iFirst:1-12.
97. Ugwoke MI, Agu RU, Verbeke N, Kinget R. Nasal mucoadhesive drug delivery: Background, applications, trends and future perspectives. Adv Drug Deliv Rev, 2005; 57:1640-1665.
98. Jain, AnekantYashant, et al Perspectives of biodegradable Natural Polysaccharides for site specific Drug Delivery to the colon, Journal of Pharmaceutical science, 2007,10,86-128.
99. Jain K, Girish, Shah, Dhirendra et al Gums and mucilages versatile excipients for pharmaceutical formulations, Asian J P S2009,4:309-323.
100. Tommasina, Coviello, et al polysaccharide hydrogels for modified release formulations. Journal of controlled release,2007,119(1)5-24.
101. Crini, Gregoria , Recent developments in polysaccharide based materials used as adsorbents in waste water treatment. Progress in Polymer Science,2005,30,38-70.
102. BasuShyamoshree, bandyopadhyayAmalkumar, Development and characterization of Mucoadhesive In-situ Nasal Gel of Midazolam prepared with Ficuscarica Mucilage, Pharm Sci tech, 2010,11:1223-1231.
103. Luppi, Barbara, Angela et al freeze-dried chitosan/pectin nasal inserts for antipsychotic drug delivery, European Journal of Pharmaceutics and Biopharmaceutics, 2010,71(6)729-731.
104. Kuotsu, Ketousetuo, Amal et al, development of Oxytocin Nasal Gel using Natural mucoadhesive agent obtained from the fruits of delliniaindica, Science Asia, 2007,33,57-60.
105. Basu, Subrata, Amar et al Development and Evaluation of a mucoadhesive nasal gel of midazolam prepared with Linumusitatissimum, seed mucilage, Scinica Pharmaceutics,2009,77,899-910
106. Nirmal H, Bakliwal S, Pawr S, In-situ gel: New trends in Controlled and sustained Drug Delivery System, IJPTR,2010,2,1398-1482.
107. TabassiSayyed, AbolghassemSajadi, RazaviNaheed, Preparation and Characterisation of albumin microsphere encapsulated with propranolol Hcl. DARU Journal of Pharmaceutical science, 2003,11, 137-147.
108. Tanaji. Nandgude., Rahul. Thube.,Nitin. Jaiswal, Pradip. Deshmukh, VivekChatap, Formulation and evalution of pH induced in situ nasal gel of Salbutamol Sulphate
109. Grace rantham ,narayana.n,iiavarasam.R, preparation and evolution of carbopol based nasal gels for systemic delivery of progesterone. International journal of pharmaeutical Research & Development vol-2, 2005.
110. BasuS. and A.K Bandyopadhyay, Nasal Drug Delivery : An Overview International Journal of pharmaceutical Science& technology, vol-4, 2010.
111. Alpar, HO, Bowen JC, Brown MRW, Effectiveness of liposomes as adjuvants of orally and nasally administered tetanus toxoid. Int. J. Pharm. 1992,88:335-344.
112. Maitani Y, Asano S, Takahashi et al , Permeability of insulin entrapped in liposomes through the nasal mucosa of rabbits. Chem Pharm, Bull 1992,40,1569-1572.
113. Jain AK, Chalasani KB, Khar RK, Ahmed FJ Diwan PV. Muco-adhesive multivesicular liposomes as an effective carrier for transmucosal insulin delivery. J Drug Target, 2007;15:417-427.
114. Law SL, Huang KJ, Chou VHY, Cherng JY. Enhancement of nasal absorption of calcitonin loaded in liposomes. J Liposome Res, 2001; 11:164-174.
115. Murramatsu K, Maitani Y, Takayama K, Nagai T. The relationship between the rigidity of the liposomal membrane and the absorption of insulin after nasal administration of liposomes modified with an enhancer containing insulin in rabbits. Drug DevInd Pharm, 1999; 25:1099-1105.
116. Kato Y, Hosokawa T, Hayakawa E, Ito K. Influence of liposomes on tryptic digestion of insulin. Biol Pharm Bull, 1993; 16:457-461.
117. Lee VH, Yamamoto A, Kompella UB. Mucosal penetration enhancers for facilitation of peptide and protein drug absorption. Crit RevTher Drug Carrier Syst, 1991; 8:91-192.
118. Vyas SP, Goswami SK, Singh R. Liposomes based nasal delivery system of nifedipine: Development and characterization. Int J. Pharm, 1995; 118:23-30.
119. Ding WX, Qi XR, Fu Q, Piao HS, Pharmacokinetics and pharmacodynamics of sterylglucosidemodofied liposomes for levonorgestrel delivery via nasal route. Drug Delivery ,2007,14:101-104.
120. Alsarra IA, Hamed AY, Alanazi FK, Acyclovir liposomes for intra nasal systemic delivery: Development and pharmacokinetics evaluation. Drug Deliv, 2008,15:313-321.
121. Gavini E, Hegge AB, Rassu G, Sanna V. Testa C, Pirisino G, Karlsen J, Giunchedi P. Nasal administration of Carbamazepine using chitosan microspheres: In vitro/in vivo studies. Int J Pharm, 2006; 307:9-15.
122. Illum L, Jorgensen H, Bisgaard H, Rossing N, Bioadhesive microspheres as a potential nasal drug delivery system. Int J. Pharm 1987,39:189-199.
123. MistryA, Stolnik S, Illum L, Nanoparticles for direct nose-to-brain delivery of drugs. Int J. Pharm.2009,379:146-157.
124. Sundaram S, Roy SK, Ambati BK, Kompella UB. Surface functionalized nanoparticles for targeted gene delivery across nasal respiratory epithelium. FASEB J.2009,23:3752-3765.
125. Tiyaboonchai W. Chitosan Nanoparticles: A Promising System for Drug Delivery Naresuan University Journal, 2003; 11:51-66.
126. Fernandez-Urrusuno R, Calvo P, Remunan Lopez C, Vila-Jato JL, Alonso MJ. Enhancement of nasal absorption of insulin using chitosan nanoparticles. Pharm Res, 1999; 16:1576-1581.
127. Fernandez-Urrusuno R, Romani D, Calvo P, Vila-Jato JL, Alonso MJ. Development of a freeze dried formulation of insulin-loaded Chitosan nanoparticles intended for nasal administration. S.T.P Pharma Sciences, 1999;9: 429-436.
128. Dyer AM, Hinchcliffe M, Watts P, Castile J, Jabbal-Gill I, Nankervis R, Smith A, Illum L. Nasal delivery of insulin using novel chitosan based formulations: A comparative study in two animal models between simple chitosan formulations and chitosan nanoparticles. Pharm Res, 2002; 19:998-1008.
129. Mao S, Germershaus O, Fischer D, Linn T, Schnepf R, Kissel T. Uptake and transport PEG-graft-trimethyl-chitosan copolymer-insulin nanocomplexes by epithelial cells. Pharm Res, 2005;22:2058-2068.
130. Simon M, Wittmar M, Kissel T, Linn T. Insulin containing nanocomplexes formed by self-assembly from biodegradable amine-modified poly(vinyl alcohol)-graft-poly(L-lactide): Bioavailability and nasal tolerability in rats. Pharm Res, 2005; 22:1879-1886.
131. Jung T, Kamm W, Breitenbach A, Hungerer KL, Hundt E, Kissel T. Tetanus toxoid loaded nanoparticles from sulfobutylated poly(vinyl alcohol)- graft-poly(lactide-co-glycolide): Evaluation of antibody response after oral and nasal application in mice. Pharm Res, 2001;18:352-360.
132. Debin A, Kravtzoff R, Vaz Santiago J, Cazales L, Sperandino S, Melber K, Janowics Z, Betbeder D, Moynier M. Intranasal immunization with recombinant antigens associated with new cationic particles induces strong mucosal as well as systemic antibody and CTL responses. Vaccine, 2002; 20:2752-2763.
133. Nagamoto T, Hattori Y, Takayama K, Maitani Y. Novel chitosan particles and chitosan-coated emulsions inducing immune response via intranasal vaccine delivery. Pharm Res,2004; 21:671-674.
134. Wattanakumtornkul S, Pinto AB, Williams DB. Intranasal hormone replacement therapy. Menopause, 2003; 10:88-98.
135. Salib RJ, Howarth PH. Safety and tolerability profiles of intranasal antihistamines and intranasal corticosteroids in the treatment of allergic rhinitis. Drug Saf, 2003; 26:863?893.
136. Desrosiers MY, Salas-Prato M. Treatment of chronicrhinosinusitis refractory to other treatments with topical antibiotic therapy delivered by means of a large-particle nebulizer: Results of a controlled trial. Otolaryngol Head Neck Surg, 2001; 125:265-269.
137. Vaughan WC, Carvalho G. Use of nebulized antibiotics for acute infections in chronic sinusitis. Otolaryngol Head Neck Surg, 2002;127:558-568.
138. Ramadan HH, Sanclement JA, Thomas JG. Chronic rhinosinusitis and biofilms. Otolaryngol Head Neck Surg, 2005; 132:414-417.
139. Sanderson AR, Leid JG, Hunsaker D. Bacterial biofilms on the sinus mucosa of human subjects with chronic rhinosinusitis. Laryngoscope, 2006; 116:1121-1126.
140. Desrosiers M, Bendouah Z, Barbeau J. Effectiveness of topical antibiotics on Staphylococcus aureus biofilm in vitro. Am J Rhinol, 2007; 21:149-153.
141. Lim M, Citardi MJ, Leong JL. Topical antimicrobials in the management of chronic rhinosinusitis: A systematic review. Am J Rhinol, 2008; 22:381-389.
142. Furubayashi T, Kamaguchi A, Kawaharada K, Masaoka Y, Kataoka M, Yamashita S, Higashi Y, Sakane T. Evaluation of the Contribution of the Nasal Cavity and Gastrointestinal Tract to Drug Absorption Following Nasal Application to Rats. Biol Pharm Bull, 2007; 30:608-611.
143. Heidari A, Sadrai H, Varshosaz J. Nasal delivery of insulin using bioadhesive chitosan gels. Drug Deliv, 2006; 13:31-38.
144. Ugwoke MI, Kaufmann G, Verbeke N, Kinget R. Intranasal bioavailability of apomorphine from carboxymethylcellulose-based drug delivery systems. Int J Pharm, 2000; 202:125-131.
145. Stoke DG, Reber KR, Waltzman LS, Erns, Hamilton D, Gawareck D, Mermelstein F, McNicol E, Wright C, Carr DB. Analgesic efficacy and safety of morphine-chitosan nasal solution in patients with moderate to severe pain following orthopedicsurgery.Pain Med, 2008; 9:3-12.
146. Kilian N, Müller DG. The effect of a viscosity and an absorption enhancer on the intra nasal absorption of metoprolol in rats. Int J Pharm, 1998; 163:211-217.
147. Patil SB, Sawant KK. Development, optimization and in vitro evaluation of alginate mucoadhesive microspheres of carvedilol for nasal delivery. J Microencapsul, 2008; 9:1-12.
148. Rathnam G, Narayanan N, Ilavarasan R. Carbopol-based gels for nasal delivery of progesterone. AAPS Pharm Sci Tech, 2008; 9:1078-1082.
149. Yu S, Zhao Y, Wu F, Zhang X, Lü W, Zhang H, Zhang Q. Nasal insulin delivery in the chitosan solution: in vitro and in vivo studieInt J Pharm, 2004; 281:11-23.
150. Varshosaz J, Sadrai H, Heidari A. Nasal delivery of insulin using bioadhesive chitosan gels. Drug Deliv, 2006; 13:31-38.
151. Karasulu E, Yavasolu A, Evrensanal Z, Uyanikgil Y, Karasulu HY. Permeation studies and histological examination of sheep nasal mucosa following administration of different nasal formulations with or without absorption enhancers. Drug Deliv, 2008; 15:219-225.
152. Onischuk AA, Tolstikova TG, Sorokina IV. Anti-inflammatory effect from indomethacin nanoparticles inhaled by male mice. J Aerosol Med Pulm Drug Deliv, 2008; 21:231-243.
153. Leykin Y, Casati A, Rapotec A. A prospective, randomized, double-blind comparison between parecoxib and ketorolac for early postoperative analgesia following nasal surgery. Minerva Anestesiol, 2008; 74:475-479.
154. Moodie JE, Brown CR, Bisley EJ. The safety and analgesic efficacy of intranasal ketorolac in patients with postoperative pain. AnesthAnalg., 2008; 107:2025-2031.
155. Alsarra IA, Hamed AY, Mahrous GM, El Maghraby GM, Al-Robayan AA, Alanazi FK. Mucoadhesive polymeric hydrogels for nasal delivery of Acyclovir. Drug DevInd Pharm, 2009; 35:352-62.
156. Huang J, Garmise RJ, Crowder TM, Mar K, Hwang CR, Hickey AJ, Mikszta JA, Sullivan VJ. A novel dry powder influenza vaccine and intranasal delivery technology: induction of systemic and mucosal immune responses in rats. Vaccine, 2004; 23:794-801.
157. Sharma S, Mukkur TKS, Benson HA, Chen Y. Pharmaceutical aspects of intranasal delivery of vaccines using particulate systems. J Pharm Sci, 2009; 98:812-43.
158. Slutter B, Hagenaars N, Jiskoot W. Rational design of nasal vaccines. J Drug Target, 2008;16:1-17.
159.&nbs











