ABOUT AUTHOR:
Vivek P. Chavda
Department of Pharmaceutics, B.K. Mody Government Pharmacy College,
Rajkot – 360003, Gujarat (India)
vivek7chavda@gmail.com
ABSTRACT
Cancer is one of most cogent culprit for causes of mortality in society. Chemotherapy, radiation therapy and surgery are the elements which are endorsed to combat it. Nowadays antibodies are also gaining much attention in cancer treatment. Antibodies are found to be important and most promising and target specific therapeutic agents for cancer due to their epitope specific interaction. Many clinically useful antibodies can manipulate tumour-related signaling. In addition, antibodies exhibit various immunomodulatory properties and, by directly activating or inhibiting molecules of the immune system, antibodies can promote the induction of antitumour immune responses. Antibody-drug conjugates prove to be more promising in the treatment.
REFERENCE ID: PHARMATUTOR-ART-1937
INTRODUCTION
Antibodies are complex protein molecules made by the immune system to identify and neutralize foreign objects like bacteria and viruses. Each antibody recognizes a specific antigen unique to its target. Antibodies belong to a family of globular proteins called immunoglobulin’s.[1, 2] The basic structural unit of most mammalian antibodies is a glycoprotein (MW ~150,000 daltons) comprising four polypeptide chains—two light chains and two heavy chains, which are connected by disulfide bonds (Figure 1). Each light chain has a molecular weight of ~25,000 daltons and is composed of two domains, one variable domain (VL) and one constant domain (CL). There are two types of light chains, lambda (λ) and kappa (κ). In humans, 60% of the light chains are κ, and 40% are λ, whereas in mice, 95% of the light chains are κ and only 5% are λ. A single antibody molecule contains either κ light chains or λ light chains, but never both.[3-9]
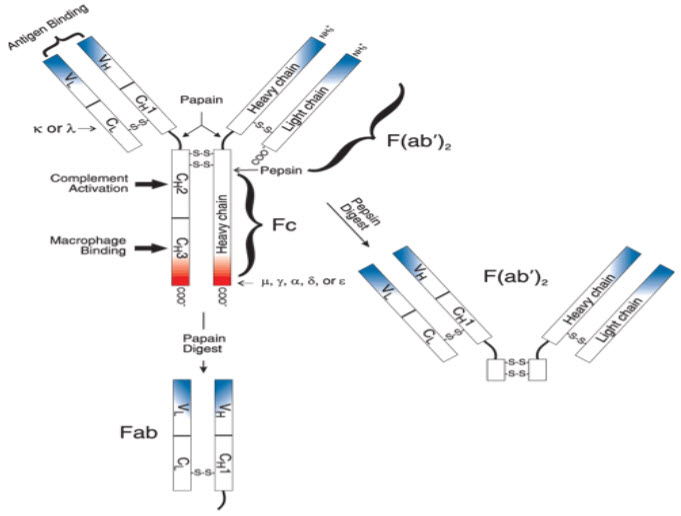
Figure 1: Schematic representation of an antibody molecule[3]
The Antibody’s antigenic determinants
The Antibody’s antigenic determinants are called Isotypes, Allotypes, and Idiotypes which determine the variability in Antibody structure. Isotypes are variants present in all members of a species, allotypes are variants caused by intraspecies genetic differences and idiotypes are variants caused by structural heterogeneity in the Antibody V regions.[10]
Monoclonal antibodies (mAb)
Monoclonal antibodies (mAb) are antibodies that are identical because they were produced by one type of immune cell (B cell), all clones of a single parent cell. Monoclonal antibodies have monovalent affinity. That is, they bind to the same epitope/site. Monoclonal antibodies have monovalent affinity, in that they bind to the same epitope. Given almost any substance, it is possible to produce monoclonal antibodies that specifically bind to that substance; they can then serve to detect or purify that substance. This has become an important tool in biochemistry, molecular biology and medicine.[11]
Production[11]
Monoclonal antibodies are typically made by fusing myeloma cells with the spleen cells from a mouse that has been immunized with the desired antigen. However, recent advances have allowed the use of rabbit B-cells to form a Rabbit Hybridoma. Four basic steps are involved in this; 1. Immunization, 2.Fusion, 3.Clonal selection and 4. Clone propagation. Polyethylene glycol is used to fuse adjacent plasma membranes, but the success rate is low so a selective medium in which only fused cells can grow is used. This is possible because myeloma cells have lost the ability to synthesize hypoxanthine-guanine-phosphoribosyltransferase (HGPRT), an enzyme necessary for the salvage synthesis of nucleic acids. The absence of HGPRT is not a problem for these cells unless the de novo purine synthesis pathway is also disrupted. By exposing cells to aminopterin (a folic acid analogue, which inhibits dihydrofolatereductase, DHFR), they are unable to use the de novo pathway and become fully auxotrophic for nucleic acids requiring supplementation to survive. The selective culture medium is called HAT medium because it contains hypoxanthine, aminopterin, and thymidine. This medium is selective for fused (hybridoma) cells. Unfused myeloma cells cannot grow because they lack HGPRT, and thus cannot replicate their DNA. Unfused spleen cells cannot grow indefinitely because of their limited life span. Only fused hybrid cells, referred to as hybridomas, are able to grow indefinitely in the media because the spleen cell partner supplies HGPRT and the myeloma partner has traits that make it immortal (similar to a cancer cell). This mixture of cells is then diluted and clones are grown from single parent cells on microtitre wells. The antibodies secreted by the different clones are then assayed for their ability to bind to the antigen (with a test such as ELISA or Antigen Microarray Assay) or immuno-dot blot. The most productive and stable clone is then selected for future use.
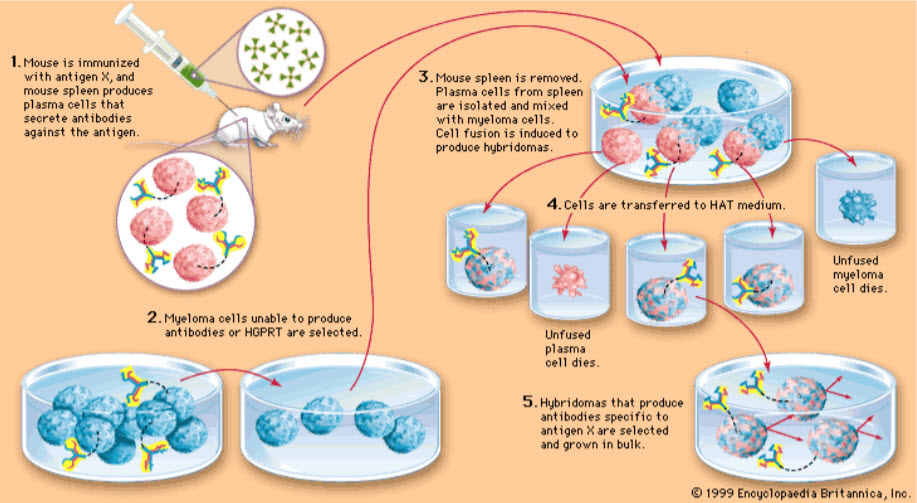
Figure 2: Hybridoma technology (Adopted from drugsfield)
Rationale
* These are antibodies with high avidity and specificity
* East to radio label
* mAb as efficient carriers for delivery of anti-tumor agents
* Enhanced vascular permeability of circulating macromolecules for tumor tissue and subsequent accumulation in solid tumors.
* Normal tissue: blood vessels have intact endothelial layer that permits passage of small molecules but not permits entry of macromolecules (like mAb).
* Tumor tissue: blood vessels leaky, so small and large molecules have access to malignant tissues.
* Tumor tissues generally do not have a lymphatic drainage system; therefore, macromolecules are retained and can accumulate in solid tumors.
* The applications developed at present have been primarily in cancer chemotherapy, where the greatest need arises for site-specific drug delivery.
Limitations
Monoclonal antibodies are not without problems: (1) the affinity for antigen may be low, (2) individual species of monoclonal antibodies do not readily activate complement or precipitate or agglutinate antigens in vitro, and (3) monoclonalsare difficult to use in vivo in humans because of the difficulty of producing human, as opposed to mouse, hybridomas.
Types of mAb’s designed
The four types of mAb’s are designed dependent upon their source for therapeutic use.[12]
1) Murine mAb’s
2) Chimeric mAb’s
3) Humanized mAb’s
4) Human mAb’s
Chemotherapy
Chemotherapy (often abbreviated to chemo) is the treatment of cancer with one or more cytotoxic antineoplastic drugs ("chemotherapeutic agents") as part of a standardized regimen. Chemotherapy may be given with a curative intent or it may aim to prolong life or to palliate symptoms. It is often used in conjunction with other cancer treatments, such as radiation therapy or surgery. Certain chemotherapeutic agents also have a role in the treatment of other conditions, including ankylosing spondylitis, multiple sclerosis, Crohn's disease, psoriasis, psoriatic arthritis, systemic lupus erythematosus, rheumatoid arthritis, and scleroderma.[13]Chemotherapy does not always work, and even when it is useful, it may not completely destroy the cancer. Patients frequently fail to understand its limitations. In one study of patients who had been newly diagnosed with incurable, stage 4 cancer, more than two-thirds of patients with lung cancer and more than four-fifths of patients with colorectal cancer still believed that chemotherapy was likely to cure their cancer.[14] Damage to specific organs is possible: Cardiotoxicity (heart damage), Hepatotoxicity (liver damage), Nephrotoxicity (kidney damage), Ototoxicity (damage to the inner ear), producing vertigo and Encephalopathy (brain dysfunction). The mechanism of anti-proliferation on cells cycle, rather than specific toxicity directed towards particular cancer cell.Host toxicity is seen after treatment discontinued, most of them had bad side-effects, such as no appetites, omit, and lose hair. One of biggest disadvantages is lack of in vivo selectivity of such chemotherapeutic agent.
Monoclonal antibodies for cancer treatment
One possible treatment for cancer involves monoclonal antibodies that bind only to cancer cell-specific antigens and induce an immunological response against the target cancer cell. Such mAb could also be modified for delivery of a toxin, radioisotope, cytokine or other active conjugate; it is also possible to design bispecific antibodies that can bind with their Fab regions both to target antigen and to a conjugate or effector cell. In fact, every intact antibody can bind to cell receptors or other proteins with its Fc region. The concept of specific molecular targeting has been applied to the development of innovative cancer-treatment strategies. At present, two main approaches are available for use in clinical practice: therapeutic monoclonal antibodies (mAbs) and small-molecule agents. Three mechanisms could be responsible for the cancer treatment.[15, 16]Monoclonal antibodies may
A. Trigger the immune system to attack cancer cells
B. Stop cancer cells from taking up proteins
C. Carry cancer drugs or radiation to cancer cells
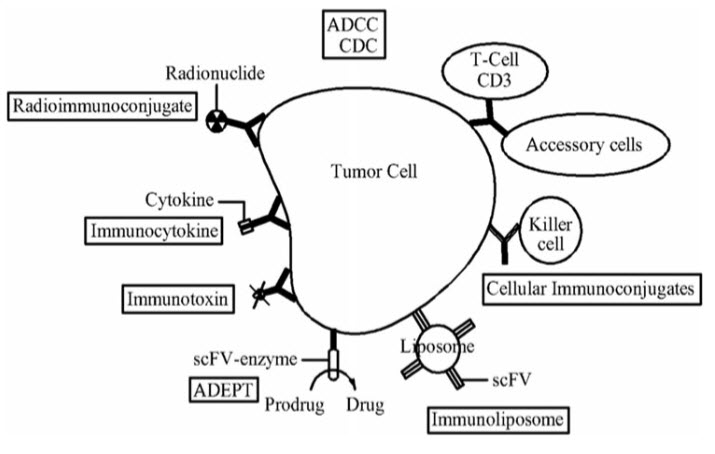
Figure 3: Monoclonal antibodies for cancer therapy.[17]
The immune system defends the body by attacking\foreign invaders that enter our body from the outside, but it does not always recognize cancer cells inside as enemies. Monoclonal antibodies can bind to the cancer cells having the tumor antigens on their surface and make the cancer cells more detectable to the immune system.[18]
Table 1: Therapeutic monoclonal antibodies approved or in review in the European Union or United States[19]
|
International non-proprietary name |
Trade name |
Type |
Indication first approved |
First EU (US) approval year |
|
Muromonab-CD3 |
Orthoclone Okt3 |
Anti-CD3; Murine IgG2a |
Reversal of kidney transplant rejection |
1986* (1986#) |
|
Abciximab |
Reopro |
Anti-GPIIb/IIIa; Chimeric IgG1 Fab |
Prevention of blood clots in angioplasty |
1995* (1994) |
|
Rituximab |
MabThera, Rituxan |
Anti-CD20; Chimeric IgG1 |
Non-Hodgkin's lymphoma |
1998 (1997) |
|
Basiliximab |
Simulect |
Anti-IL2R; Chimeric IgG1 |
Prevention of kidney transplant rejection |
1998 (1998) |
|
Daclizumab |
Zenapax |
Anti-IL2R; Humanized IgG1 |
Prevention of kidney transplant rejection |
1999 (1997); # |
|
Palivizumab |
Synagis |
Anti-RSV; Humanized IgG1 |
Prevention of respiratory syncytial virus infection |
1999 (1998) |
|
Infliximab |
Remicade |
Anti-TNF; Chimeric IgG1 |
Crohn disease |
1999 (1998) |
|
Trastuzumab |
Herceptin |
Anti-HER2; Humanized IgG1 |
Breast cancer |
2000 (1998) |
|
Gemtuzumabozogamicin |
Mylotarg |
Anti-CD33; Humanized IgG4 |
Acute myeloid leukemia |
NA (2000#) |
|
Alemtuzumab |
MabCampath, Campath-1H |
Anti-CD52; Humanized IgG1 |
Chronic myeloid leukemia |
2001 (2001) |
|
Adalimumab |
Humira |
Anti-TNF; Human IgG1 |
Rheumatoid arthritis |
2003 (2002) |
|
Tositumomab-I131 |
Bexxar |
Anti-CD20; Murine IgG2a |
Non-Hodgkin lymphoma |
NA (2003) |
|
Efalizumab |
Raptiva |
Anti-CD11a; Humanized IgG1 |
Psoriasis |
2004 (2003); # |
|
Cetuximab |
Erbitux |
Anti-EGFR; Chimeric IgG1 |
Colorectal cancer |
2004 (2004) |
|
Ibritumomabtiuxetan |
Zevalin |
Anti-CD20; Murine IgG1 |
Non-Hodgkin's lymphoma |
2004 (2002) |
|
Omalizumab |
Xolair |
Anti-IgE; Humanized IgG1 |
Asthma |
2005 (2003) |
|
Bevacizumab |
Avastin |
Anti-VEGF; Humanized IgG1 |
Colorectal cancer |
2005 (2004) |
|
Natalizumab |
Tysabri |
Anti-a4 integrin; Humanized IgG4 |
Multiple sclerosis |
2006 (2004) |
|
Ranibizumab |
Lucentis |
Anti-VEGF; Humanized IgG1 Fab |
Macular degeneration |
2007 (2006) |
|
Panitumumab |
Vectibix |
Anti-EGFR; Human IgG2 |
Colorectal cancer |
2007 (2006) |
|
Eculizumab |
Soliris |
Anti-C5; Humanized IgG2/4 |
Paroxysmal nocturnal hemoglobinuria |
2007 (2007) |
|
Certolizumabpegol |
Cimzia |
Anti-TNF; Humanized Fab, pegylated |
Crohn disease |
2009 (2008) |
|
Golimumab |
Simponi |
Anti-TNF; Human IgG1 |
Rheumatoid and psoriatic arthritis, ankylosing spondylitis |
2009 (2009) |
|
Canakinumab |
Ilaris |
Anti-IL1b; Human IgG1 |
Muckle-Wells syndrome |
2009 (2009) |
|
Catumaxomab |
Removab |
Anti-EPCAM/CD3;Rat/mouse bispecificmAb |
Malignant ascites |
2009 (NA) |
|
Ustekinumab |
Stelara |
Anti-IL12/23; Human IgG1 |
Psoriasis |
2009 (2009) |
|
Tocilizumab |
RoActemra, Actemra |
Anti-IL6R; Humanized IgG1 |
Rheumatoid arthritis |
2009 (2010) |
|
Ofatumumab |
Arzerra |
Anti-CD20; Human IgG1 |
Chronic lymphocytic leukemia |
2010 (2009) |
|
Denosumab |
Prolia |
Anti-RANK-L; Human IgG2 |
Bone Loss |
2010 (2010) |
|
Belimumab |
Benlysta |
Anti-BLyS; Human IgG1 |
Systemic lupus erythematosus |
2011 (2011) |
|
Ipilimumab |
Yervoy |
Anti-CTLA-4; Human IgG1 |
Metastatic melanoma |
2011 (2011) |
|
Brentuximabvedotin |
Adcetris |
Anti-CD30; Chimeric IgG1; immunoconjugate |
Hodgkin lymphoma |
2012 (2011) |
|
Pertuzumab |
Perjeta |
Anti-HER2; humanized IgG1 |
Breast Cancer |
2013 (2012) |
|
Raxibacumab |
(Pending) |
Anti-B. anthrasis PA; Human IgG1 |
Anthrax infection |
NA (2012) |
|
Trastuzumabemtansine |
Kadcyla |
Anti-HER2; humanized IgG1; immunoconjugate |
Breast cancer |
In review (2013) |
|
Vedolizumab |
(Pending) |
Anti-alpha4beta7 integrin; humanized IgG1 |
Ulcerative colitis, Crohn disease |
In review (NA) |
*Country-specific approval; approved under concertation procedure;
#Voluntarily withdrawn from market.
BLyS, B lymphocyte stimulator; C5, complement 5; CD, cluster of differentiation; CTLA-4, cytotoxic T lymphocyte antigen 4; EGFR, epidermal growth factor receptor; EPCAM, epithelial cell adhesion molecule; GP, glycoprotein; IL, interleukin; NA, not approved; PA, protective antigen; RANK-L, receptor activator of NFkb ligand; RSV, respiratory syncytial virus; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor
Antibody-drug conjugates(ADC’s)[20]
ADC’s are a unique combination of a precise and targeted monoclonal antibody, a stable linker, and a potent cytotoxic and are designed to deliver potent anticancer agents to tumors in a targeted manner to limit systemic exposure.[21-23] Antibody Drug Conjugates or ADCs are a new class of highly potent biopharmaceutical drugs designed as a Targeted therapy for the treatment of people with cancer.[20, 24-27] ADCs are complex molecules composed of an antibody (a whole mAb or an antibody fragment such as a single-chain variable fragment [scFv]) linked, via a stable, chemical, linker with labile bonds, to a biological active cytotoxic (anticancer) payload or drug.Antibody Drug Conjugates are examples of bioconjugates and immunoconjugates. By combining the unique targeting capabilities of a monoclonal antibodies with the cancer-killing ability of a cytotoxic drugs, antibody-drug conjugates allow sensitive discrimination between healthy and diseased tissue. This means that, in contrast to traditional chemotherapeutic agents, antibody-drug conjugates target and attack the cancer cell so that healthy cells are less severely affected.[28] The three basic parts of ADC are;
• Monoclonal antibody
• Cytotoxic agent
• Linker
A stable link between the antibody and cytotoxic (anti-cancer) agent is a crucial aspect of an ADC. Linkers are based on chemical motifs including disulfides, hydrazones or peptides (cleavable), or thioethers (noncleavable) and control the distribution and delivery of the cytotoxic agent to the target cell. Cleavable and noncleavable types of linkers have been proven to be safe in preclinical and clinical trials. Brentuximabvedotin includes an enzyme-sensitive cleavable linker that delivers the potent and highly toxic antimicrotubule agent Monomethylauristatin E or MMAE, a synthetic antineoplastic agent, to human specific CD30-positive malignant cells. Because of its high toxicity MMAE, which inhibits cell division by blocking the polymerization of tubulin, cannot be used as a single-agent chemotherapeutic drug. However, the combination of MMAE linked to an anti-CD30 monoclonal antibody (cAC10, a cell membrane protein of the Tumor necrosis factor or TNF-receptor proved to be stable in extracellular fluid, cleavable by cathepsin and safe for therapy. Trastuzumabemtansine, the other approved ADC, is a combination of the microtubule-formation inhibitor mertansine (DM-1), a derivative of the Maytansine, and antibody trastuzumab (Herceptin®/ Genentech/Roche) attached by a stable, non-cleavable linker. The availability of better and more stable linkers has changed the function of the chemical bond. The type of linker, cleavable or noncleavable, lends specific properties to the cytotoxic (anti-cancer) drug. For example, a non-cleavable linker keeps the drug within the cell. As a result, the entire antibody, linker and cytotoxic (anti-cancer) agent enter the targeted cancer cell where the antibody is degraded to the level of an amino acid. The resulting complex – amino acid, linker and cytotoxic agent – now becomes the active drug. In contrast, cleavable linkers are catalyzed by enzymes in the cancer cell where it releases the cytotoxic agent. The difference is that the cytotoxic payload delivered via a cleavable linker can escape from the targeted cell and, in a process called “bystander killing,” attack neighboring cancer cells.[29]Another type of cleavable linker, currently in development, adds an extra molecule between the cytotoxic drug and the cleavage site. This linker technology allows researchers to create ADCs with more flexibility without worrying about changing cleavage kinetics. Researchers are also developing a new method of peptide cleavage based on Edman degradation, a method of sequencing amino acids in a peptide.[30]Future direction in the development of ADCs also include the development of site-specific conjugation (TDCs)[31]to further improve stability and therapeutic index and α emitting immunoconjugates[32]and antibody-conjugated nanoparticles.[33]
References
[1] Litman GW, Rast JP, Shamblott MJ, Haire RN, Hulst M, Roess W, Litman RT, Hinds-Frey KR, Zilch A, Amemiya CT (January 1993). "Phylogenetic diversification of immunoglobulin genes and the antibody repertoire". Mol. Biol. Evol. 10 (1): 60–72. .
[2] Charles Janeway (2001). Immunobiology. (5th ed.). Garland Publishing.
[3] Antibody Structure and Classification. invitrogencom/site/us/en/home/References/Molecular-Probes-The-Handbook/Technical-Notes-andProductHighlights/Antibody-Structure-and-Classificationhtml Accessed on july 25th, 2013.
[4] Pier GB, Lyczak JB, Wetzler LM (2004). Immunology, Infection, and Immunity. ASM Press.
[5] Borghesi L, Milcarek C (2006). "From B cell to plasma cell: regulation of V(D)J recombination and antibody secretion". Immunol. Res. 36 (1–3): 27–32.
[6] Parker D (1993). "T cell-dependent B cell activation". Annu Rev Immunol 11 (1): 331–360.
[7] Rhoades RA, Pflanzer RG (2002). Human Physiology (4th ed.). Thomson Learning.
[8] Market E, Papavasiliou FN (October 2003). "V(D)J recombination and the evolution of the adaptive immune system". PLoS Biol. 1 (1): E16. doi:10.1371/journal.pbio.0000016. PMC 212695. PMID 14551913.
[9] Antibody. enwikipediaorg/wiki/Antibody Accessed on 25th july, 2013.
[10] Klaus D. Elgert, Textbook of Immunology: Understanding the immune system, Chapter 4; Antibody structure and function, Wiley and sons, Inc, 1998:58-78.
[11] Monoclonal antibodies. enwikipediaorg/wiki/Monoclonal_antibodies.
[12] Tsipi Ben-Kasus, Bilha Schechter, Michael Sela, Yosef Yarden, Cancer therapeutic antibodies come of age: Targeting minimal residual disease, Molecular oncology, 2007:42-54.
[13] Chemotherapy. enwikipediaorg/wiki/Chemotherapy Accessed on 25th july, 2013.
[14] Weeks JC, Catalano PJ, Cronin A, et al. (October 2012). "Patients' expectations about effects of chemotherapy for advanced cancer". N. Engl. J. Med. 367 (17): 1616–25.
[15] Jaracz S, Chen J, Kuznetsova LV, Ojima I, Recent advances in tumor-targeting anticancer drug conjugates, Bioorg Med Chem, 2005;13:5043-5054.
[16] Junutula J, Raab H, Clark S, et al, Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index, Nat Biotechnol, 2008;26:925-932.
[17] GUO YT, HOU QY. Monoclonal Antibodies in Cancer Therapy. Clin Oncol Cancer Res 2011;8:215–9.
[18] Ignacio M, Sandra H, Martin G. Immunostimulatory monoclonal antibodies for cancer therapy. Nature 2007; 7: 95–106.
[19] Reichert JM. Therapeutic monoclonal antibodies approved or in review in the European Union or United States. The antibody society Accessed on 25th july,2013; antibodysociety.org/news/approved_mabs.php:Adopted without modification.
[20] Antibody-drug conjugates. enwikipediaorg/wiki/Antibody-drug_conjugate 2013.
[21] The Next Advancements in Cancer Drug Development: Antibody-Drug Conjugates - Onco'Zine - The International Cancer Network, June 14, 2012
[22] Antibody-Drug Conjugates: Guided Missiles Deployed Against Cancerous Cells
[23] What are ADCs? "ADC Review / Journal of Antibody-drug Conjugates", - June 1, 2013
[24] Antibody Drug Conjugates: A Marriage of Biologics and Small Molecules - Pharmaceutical Technology. Pharmtech.findpharma.com. Retrieved on 2010-11-20.
[25] Ducry, Laurent; Stump, Bernhard (2010). "Antibody−Drug Conjugates: Linking Cytotoxic Payloads to Monoclonal Antibodies". Bioconjugate Chemistry 21 (1): 5–13.
[26] Kovtun, Y. V.; Audette, CA; Ye, Y; Xie, H; Ruberti, MF; Phinney, SJ; Leece, BA; Chittenden, T et al. (2006). "Antibody-Drug Conjugates Designed to Eradicate Tumors with Homogeneous and Heterogeneous Expression of the Target Antigen". Cancer Research 66 (6): 3214–21.
[27] Kovtun, YV; Goldmacher, VS (2007). "Cell killing by antibody-drug conjugates". Cancer letters 255 (2): 232–40.
[28] Mullard A. Maturing antibody–drug conjugate pipeline hits 30. Nature Reviews Drug Discovery 12, 329–332 (May 2013) doi:10.1038/nrd4009
[29] Kovtun YV, Goldmacher VS. Cell killing by antibody-drug conjugates. Cancer Lett. 2007 Oct 8;255(2):232-40. Epub 2007 Jun 5.
[30] B?chor R, Kluczyk A, Stefanowicz P, Szewczuk Z. New method of peptide cleavage based on Edman degradation. Mol Divers. 2013 May 21. (Epub ahead of print).
[31] Axup JY, Bajjuri KM, Ritland M, Hutchins BM, Kim CH, Kazane SA, Halder R, et al. Synthesis of site-specific antibody-drug conjugates using unnatural amino acids. Proc Natl Acad Sci U S A. 2012 Oct 2;109(40):16101-6. doi: 10.1073/pnas.1211023109. Epub 2012 Sep 17.
[32] Wulbrand C, Seidl C, Gaertner FC, Bruchertseifer F, Morgenstern A, Essler M, Senekowitsch-Schmidtke R. Alpha-Particle Emitting (213)Bi-Anti-EGFR Immunoconjugates Eradicate Tumor Cells Independent of Oxygenation. PLoS One. 2013 May 28;8(5):e64730. doi: 10.1371/journal.pone.0064730. Print 2013
[33] Cardoso MM, Peça IN, Roque AC. Antibody-conjugated nanoparticles for therapeutic applications. Curr Med Chem. 2012;19(19):3103-27.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
FIND OUT MORE ARTICLES AT OUR DATABASE









