 About Authors: Inder Kumar Makhija
About Authors: Inder Kumar Makhija
Department of Pharmacognosy,
Manipal College of Pharmaceutical Sciences,
Manipal University, Manipal-576 104,
Karnataka
ABSTRACT
The world is endowed with a rich heritage of medicinal plants. The use of medicinal agents presumably predates the earliest recorded history. Medicinal plants are widely used by traditional practitioners for various ailments. Lawsonia inermis (Lythraceae) commonly known as ‘Henna’is a well-known plant used in the Indian medicine. Various parts of this plant have been used in traditional Indian medicine. The plant has wide range of phytochemicals including lawsone, isoplumbagin lawsoniaside, lalioside, lawsoniaside B, syringinoside, daphneside, daphnorin, agrimonolide 6-O-β-D-glucopyranoside, (+)-syringaresinol O-β-D-glucopyranoside, (+)-pinoresinol di-O-β-D-glucopyranoside, syringaresinol di-O-β-D-glucopyranoside, isoscutellarin3β, hennadiol, (20S)-3β, 30-dihydroxylupane, lawnermis acid, 3-methyl-nonacosan-1-ol, laxanthones I, II, III and lacoumarin etc. The various in-vitro and in-vivo studies of L. inermis reported the plant to have antibacterial, antifungal, antiparasitic, antiviral, anticancer, antidiabetic, tuberculostatic, anti-inflammatory, antifertility and wound healing properties. This review discusses on the botany, traditional use, phytochemistry and pharmacological data of the plant.
[adsense:336x280:8701650588]
INTRODUCTION
In the past, medicinal plants were used intensively in folkloric medicine for treatment of various disorders. Today, it is estimated that about 80 % of people in developing countries rely on traditional medicines for their primary health care. Traditional medicines are becoming popular, due to high toxicity and adverse effects of orthodox medicaments. This has led to sudden increase in the number of herbal industries in the drug market. Several plant species are used by various indigenous systems such as Siddha, Ayurveda, Unani and Allopathy for the treatment of different ailments (1-7). This review emphasizes the traditional uses and biological properties of L. inermis.
Lawsonia inermisLinn (Lythraceae) is a perennial plant commonly called as Henna, having different vernacular names in India viz., Mehndi in Hindi, Mendika, Rakigarbha in Sanskrit, Mailanchi in Malayalam, Muruthani in Tamil, Benjati in Oriya, Mayilanchi in Kannada and Mehedi in Bengali (8). It is native to North Africa and South East Asia, and often cultivated as an ornamental plant throughout India, Persia, and along the African coast of the Mediterranean Sea (9). Henna grows better in tropical savannah and tropical arid zones, in latitude between 15° and 25° N and S, produces highest dye content in temperature between 35-45°C. The optimal soil temperature range for germination is 25-30°C. Henna leaves are very popular natural dye to colour hand, finger, nails and hair. The dye molecule, lawsone is the chief constituents of the plant; its highest concentration is detected in the petioles (0.5-1.5 %). In folk medicines, henna has been used as astringent, anti-hemorrhagic, intestinal antineoplastic, cardio-inhibitory, hypotensive, sedative and also as therapeutic against amoebiasis, headache, jaundice and leprosy (10-11).
MORPHOLOGY
Lawsonia inermisis a glabrous branched shrub or small tree (2 to 6 m in height). Leaves are small, opposite, entire margin elliptical to broadly lanceolate, sub-sessile, about 1.5 to 5 cm long, 0.5 to 2 cm wide, greenish brown to dull green, petiole short and glabrous acute or obtuse apex with tapering base. New branches are green in colour and quadrangular, turn red with age. Young barks are greyish brown, older plants have spine-tipped branchlets (Fig. 1). Inflorescence has large pyramid shaped cyme. Flowers are small, numerous, aromatic, white or red coloured with four crumbled petals. Calyx has 0.2 cm tube and 0.3 cm spread lobes. The fruits are small, brown globose capsule, opening irregularly and split into four sections with a permanent style. Seeds have typical, pyramidal, hard and thick seed coat with brownish coloration (12-14).
Figure 1: Morphology of Lawsonia inermis plant

ETHNOPHARMACOLOGY
Lawsonia inermisis a well known ethnomedicinal plant used cosmetically and medicinally for over 9,000 years. Its use in the Indian traditional folk medicines is well documented. Table 1 indicates the use of different parts of L. inermis in traditional system of medicines.
Table 1: Ethnomedicinal uses of different parts of L. inermis
|
Plant Parts |
Traditional Uses (as/in) |
|
Root |
Bitter, depurative, diuretic, emmenagogue, abortifacient, burning sensation, leprosy, skin diseases, amenorrhoea, dysmenorrhoea and premature greying of hair (15).
|
|
Leaves |
Bitter, astringent, acrid, diuretic, emetic, edema, expectorant, anodyne, anti-inflammatory, constipating, depurative, liver tonic, haematinic, styptic, febrifuge, trichogenous, wound, ulcers, strangury, cough, bronchitis, burning sensation, cephalalgia, hemicranias, lumbago, rheumatalgia, inflammations, diarrhoea, dysentery, leprosy, leucoderma, scabies, boils, hepatopathy, splenopathy, anemia, hemorrhages, hemoptysis, fever, ophthalmia, amenorrhoea, falling of hair, greyness of hair, jaundice (15-18).
|
|
Flowers |
Cardiotonic, refrigerant, soporific, febrifuge, tonic, cephalalgia, burning sensation, cardiopathy, amentia, insomnia, fever (15).
|
|
Seeds |
Antipyretic, intellect promoting, constipating, intermittent fevers, insanity, amentia, diarrhoea, dysentery and gastropathy (15). |
PHYTOCHEMISTRY OF L. INERMIS
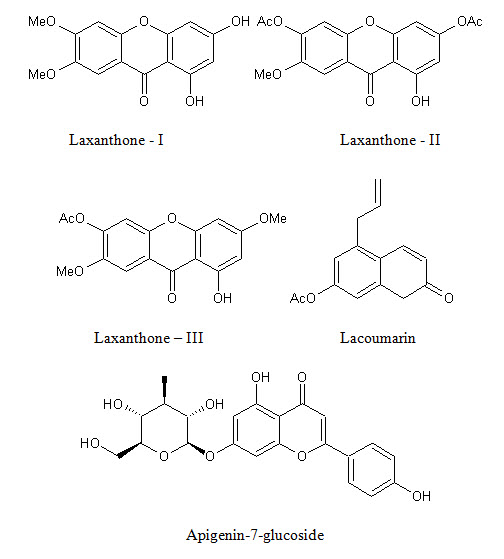
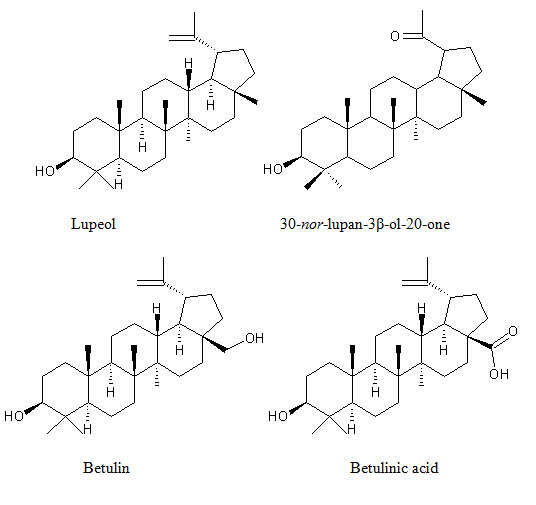
Much work is done in the field of phytochemical investigation of the plant. The chemical constituents isolated from L. inermis are napthoquinone derivatives, phenolic compounds, terpenoids, sterols, aliphatic derivatives, xanthones, coumarin, fatty acids, amino acids and other constituents. Phytochemicals reported in L. inermis L. are listed in (Table 2), with their structures in (Fig. 2).
Table 2: Phytochemicals found in various parts of L. inermis
|
Compounds |
Plant Parts |
References |
|
Napthoquinone derivatives Lawsone (2-hydroxy 1,4-naphthoquinone) |
Leaves |
(19) |
|
1,3-dihydroxy naphthalene, 1,4-napthaquinone, 1,2-dihydroxy-4-glucosylnaphthalene |
Leaves |
(20) |
|
Isoplumbagin |
Stem bark |
(21) |
|
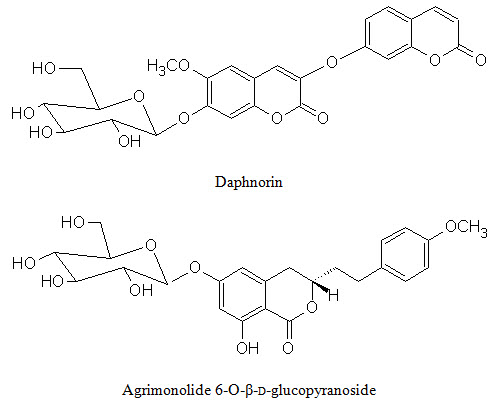
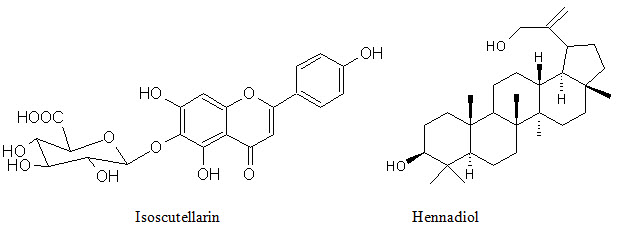
Phenolic conpounds Lawsoniaside (1,3,4-trihydroxynaphthalene 1,4-di-β-D-gluco-pyronoside), Lalioside (2,3,4,6-tetrahydroxyacetoxy-2-β-D-glucopyranoside) Lawsoniaside B (3-(4-O-a-D-glucopyranosyl-3,5-dimethoxy) phenyl-2E-propenol), syringinoside, daphneside, daphnorin, agrimonolide 6-O-β-D-glucopyranoside, (+)-syringaresinol O-β-D-glucopyranoside, (+)-pinoresinol di-O-β-D-glucopyranoside, syringaresinol di-O-β-D-glucopyranoside, isoscutellarin |
Bark, Leaves |
(22,23) |
|
Terpenoids 3β, 30-dihydroxylup-20(29)-ene (hennadiol), (20S)-3β, 30-dihydroxylupane, Lupeol, 30-nor-lupan-3β-ol-20-one, betulin, betulinic acid, lawnermis acid (3β-28β-hydroxy-urs-12,20-diene-28-oic acid) and its methyl ester |
Bark, Seeds |
(24, 25)
|
|
Sterols Lawsaritol ( 24β-ethycholest-4-en-3β-ol) Stigmasterol and β-sitosterol |
Roots, Leaves |
(26) |
|
Aliphatic constituents 3-methyl-nonacosan-1-ol, n-tricontyl n-tridecanoate |
Stem bark |
(27, 28) |
|
Xanthones Laxanthone I (1,3 dihydroxy-6,7 dimethoxy xanthone), Laxanthone II (1-hydroxy-3,6 diacetoxy-7-methoxyxanthone), Laxanthone III ( 1-hydroxy-6-acetoxy xanthone) |
Whole plant |
(29,30) |
|
Coumarins Lacoumarin (5-allyoxy-7-hydroxycoumarin) |
Whole plant |
(31) |
|
Flavonoids Apigenin-7-glucoside, apigenin-4-glycoside, luteolin-7-glucoside, luteolin-3-glucoside |
Leaves |
(32) |
|
Essential oil -(Z)-2-hexenol, linalool, α ionone, β ionone, α-terpineol, terpinolene, δ-3-carene and γ-terpineol |
Leaves
|
(33, 34) |
|
Other chemical constituents Hennotannic acid, glucose, gallic acid, amino acid Trace metal – Cu, Ni, Mo, V, Mn, Sr, Ba, Fe and Al Minerals – Na2O, CaO and K2O |
Whole plant |
(35) |
Figure 2: Shows biologically active constituents present in L. inermis

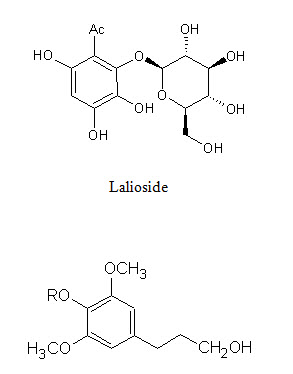
Lawsoniaside B, R = α-D-glucopyranoside
Syringinoside, R = β-D-glucopyranosyl-(1 6)-β-D-glucopyranoside

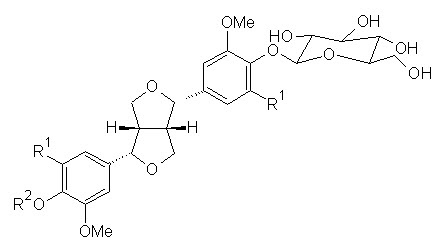
(+)-Syringaresinol O-β-D-glucopyranoside, R1 = OCH3, R2 = H
(+)-Pinoresinol di-O-β-D-glucopyranoside, R1 = H, R2 = β-D-glucopyranoside
Syringaresinol di-O-β-D-glucopyranoside, R1 = OCH3, R2 = β-D-glucopyranoside

PHARMACOLOGICAL ACTIVITY OF L. INERMIS
Several researchers have reported the different biological actions of L. inermis in various in-vitro and in-vivo test models. Henna leaves, flower, seeds, stem bark, roots have been found to exhibit antioxidant, antidiabetic, hepatoprotective, hypoglycemic, antimicrobial, anticancer and wound healing properties. These are described in greater details in the following sections.
Antioxidant activity
Lawsone (2-hydroxy-1, 4 napthoquinone) is the mainingredient of L. inermis. On oxidation of 100 µM phenanthridine by guinea pigs aldehyde oxidase, superoxide anion and hydrogen peroxide formation was found to be 6-10 % and 85-90 % respectively. The mechanism of action was believed to be flavin semiquinone (FADH). The IC50 value of lawsone was 9.3 ± 1.1 µm, which in excess of 15 fold of maximal plasma concentration of lawsone, indicating high degree of safety margin (36). The modulator effect of 80 % ethanol extract of leaves of L. inermis were studied on drug metabolizing phase-I and phase-II enzymes, antioxidant enzymes, glutathione content, lactate dehydrogenase and lipid peroxidation in the liver of swiss albino mice. The hepatic glutathione S-transferase and DT-diaphorase specific activities were elevated above basal level by L. inermis extract treatment. With reference to antioxidant enzymes the investigated doses were effective in increasing the hepatic glutathione reductase (GR), superoxide dismutase (SOD) and catalase activities significantly at both the dose levels. Reduced glutathione (GSH) measured as non-protein sulphydryl was found to be significantly elevated in liver. Within the extrahepatic organs examined (forestomach, kidney and lung) glutathione S-transferase and DT-diaphorase level were increased in a dose independent manner (37). Another study was carried out to assess the effect of aqueous and methanolic extracts of L. inermis extract on chromium (VI)–induced cellular and DNA toxicity. The extracts showed significant (P<0.05) potential in scavenging free radicals of DPPH, ABTS, Fe3+ and also in inhibition of lipid peroxidation. Extracts also showed significantly properties of DNA and cyto-protection (38). Different constituents isolated from the leaves of L. inermis were tested for their antioxidant activity using ABTS. The IC50 value of henna constituents are p-coumaric acid (2.6 mM), cosmosiin (2.9 mM) apiin (1.6 mM) respectively. These fundings depicted that all isolated compounds exhibited antioxidant activity comparable to that of ascorbic acid (2.5 mM) (39).
Antibacterial activity
Forty-five species of 29 plant families used in the traditional medicine by Iranian people showed antibacterial activities against bacterial species, Bacillus cereus, Bacillus pumilus, Bordetella bronchiseptica, Escherichia coli, Kiebsiella pneumonia, Micrococcus luteus, Pseudomonas aeruginosa, Pseudomonus fluorescens, Serratia marcescens, Staphylococcus aureus and Staphylococcus epidermidis. Hennashowed strong against Bordetella bronchiseptica. These findings indicated that L. inermis can be used in the treatment of bacterial infections (40). Crude extracts of fresh dry leaves and seeds were investigated for their antimicrobial activity against three standard strains and the eleven patients’s isolated strains. Henna dry leaves demonstrated the best in-vitro antimicrobial activity and in particular against Shigella sonnei (41).Ethanol extracts of 20 plants species used by Yemeni traditional healers to treat infectious diseases were screened for their antibacterial activity. The ethyl acetate extract of L. inermis was found to be the most active against all the bacteria in the test system (42).Genotoxic studies on main constituent of henna suggested that it was a weak bacterial mutagen for Salmonella typhimurium strain TA98 and was more mutagenic for strain TA2637. It suggested that hydroxyl napthaquinone have no genotoxic risk to the consumer (43). Primary invaders of burn wounds viz Staphylococcus aureus, Pseudomonas aeruginosa, Candida albicans, Fusarium oxysporum andAspergillus niger were treated with aqueous and chloroform extract obtained from leaves ofL. inermis, using in-vitro agar incorporation and well diffusion methods. Extract inhibit growth pattern of all microbes except C. albicans. Overall, study suggested that henna may be effective in the management of wound infections (35). The antibacterial activity of methanolic extract of L. inermis was investigated by agar well diffusion method using S. aureus (MTCC 087), E. coli (MTCC 729), K. pneumonia (MTCC 432), P. aeruginosa (MTCC 1688) and P. mirablis (MTCC 425). According to results in the lowest tested concentration of 62.5 μg/ml and 125 μg/ml, 7.2 % of the plant extract were active, 5 % active in the concentration of 250 μg/ml, 75.7 % active in the concentration of 500 μg/ml and 92.8 % active at the concentration of 1000 μg/ml in a dose dependent manner (44).
Antifungal activity
Sensitivity toward henna was strong in Trichophyton mentagrophytes, T. rubrum, T. tonsurans, T. violaceum, T. verrocosum, T. schoenleinii, Epidermophyton floccosum, Microsporum ferrugineum, M. canis, sporotrichum schenckii Ethanol, methanol and aqueous extract of leaves of L. inermis are involved in defensive mechanism against spore germination of Drechslera oryzae (45). During screening of barks of 30 plant species for activity against Microsporum gypseum and Trichophyton mentagrophytes, only L. inermis extract exhibited absolute toxicity. The extract showed broad fungitoxic spectrum when tested against 13 other dermatophytes. Further the fungitoxicity of the extract remained unaltered at high temperature on autoclaving and after long storage (46). Aqueous extract of leaves of L. inermis was tested for the antifungal potential against eight important species of Aspergillus which isolated from sorghum, maize and paddy seed samples. A. flavus recorded high susceptibility and hence solvent extracts viz., petroleum ether, benzene, chloroform, methanol and ethanol extract of the plant showed significant antifungal activity (47). These finding suggested that henna extract could be used as alternative source of antifungal agents for protection of plants or crops against fungal infection.
Hepatoprotective activity
Alcoholic extract of the bark of L. inermis showed hepatoprotective effect against the CCl4. Extract cause elevation in serum marker enzymes (GOT and GPT), serum bilirubin, liver lipid peroxidation and reduction in total serum protein, liver glutathione, glutathione peroxidase, glutathione-s-transferase, glycogen, superoxide dismutase and catalase activity (16,48,49). The hepatoprotective activity of the ethanolic extract of the dried leaves of L. inermis and its fractions (petroleum ether, ethyl acetate, butanol and butanone fractions) was evaluated against CCl4 induced hepatotoxicity in mice. The ethanolic extract and its fractions significantly reduced the total bilirubin content and SGOT, SGPT and SAL activities, and reduced liver weight compared to LIV-52 used as control (50,51).
Antidiabetic activity
Ethanol (70 %) extract of L. inermis showed significant hypoglycemic and hypolipidemic activities in alloxan induced diabetic mice after oral administration. The feeding of 0.8 g/kg of L. inermis extract decreased the concentration of glucose, cholesterol and triglycerides to normal (52).
Protein glycation inhibitory activity
Ethanol extract of the plant tissues was evaluated in-vitro for protein glycation inhibitory activity using the model system of bovine serum albumin and glucose. The extract and its components showed significant effect on protein damage induced by a free radical generator during in-vitro assay system. It was found that the alcoholic extract, lawsone and gallic acid showed significant inhibition of Advanced Glycated End Products (AGEs) formation and exhibit 77.95 %, 79.10 % and 66.98 % inhibition at a concentration of 1500 μg/mL, 1000 μg/mL and 1000 μM respectively. L. inermis constituents were found to be glycation inhibitors with IC50 82.06±0.13 μg/mL, 67.42±1.46 μM and 401.7±6.23 μM respectively (53).
Wound Healing activity
Ethanol extract of the plant (200 mg/kg/) was used to evaluate the wound healing activity on rats using excision, incision and dead space wound models. Topical application was made in the case of excision wound model. Whereas, oral treatment was done with incision and dead space wound model. Extract of L. inermis showed high rate of wound contraction, a decrease in the period of epithelialization, high skin breaking strength, a significant increase in the granulation tissue weight and hydroxyproline content. Histological studies of the tissue showed increased well organized bands of collagen, more fibroblasts and few inflammatory cells when compared with the controls which showed inflammatory cells, scanty collagen fibres and fibroblasts. These fundings suggest the use of L. inermis in the management of wound healing (54). Chloroform and aqueous extracts of leaves of the plant were capable of inhibiting the growth of microorganisms that are involved in causing burn wound infections (55,35).
Anti-inflammatory activity
Isoplumbagin and lawsaritol, isolated from stem bark and root of L. inermis screened for anti-inflammatory activity against carrageenan induced paw edema in rats. The compounds phenylbutazone, isoplumbagin and lawsaritol at the oral dose of 100 mg/kg exhibited 61, 60 and 40 % inhibition respectively. The results showed that isoplumbagin exhibited significant activity, was compared to that of phenylbutazone (56). Butanol and chloroform fractions showed potent anti-inflammatory, analgesic and antipyretic effects that aqueous fraction of crude ethanol extract of L. inermis in a dose dependent manner (57).
Tuberculostatic activity
The tuberculostatic activity of henna was tested in-vitro and in-vivo using Lowenstein Jensen medium, the growth of Tubercle bacilli from sputum and of Mycobacterium tuberculosis H37Rv was inhibited by 6 μg/ml of the herb. In-vivo studies on guinea pigs and mice showed that the plant at a dose of 5 mg/kg body weight led to a significant resolution of experimental tuberculosis following infection with M. tuberculosis H37Rv (58).
Antiviral activity
The ethanol soluble fraction of L. inermis fruits displayed highly potent activity against Sembiki forest virus (SFV) in swiss mice and chick embryo models exhibiting 100 to 65 % activities after 10 to 25 days of virus challenge (59).
Anticancer activity
The anticarcinogenic activity of chloroform extract L. inermis leaves was carried using microculture tetrazolium salt assay on the human breast (MCF-7), colon (Caco-2), liver (HepG2) carcinoma cell lines and normal human liver cell lines (Chang Liver). The preliminary results showed that the henna extract displayed the cytotoxic effects against HepG2 and MCF-7 and IC-value of 0.3 and 24.85 µg/ml respectively (60). Isoplumbagin at a concentration of 10.5–10.8 M, the compound typically produced LC50 – level responses against a majority of the melanoma and colon cancer cell lines as well as against several of the non-small cell lungs, colon, CNS, and renal cell lines. Isoplumbagin showed an interesting profile of cytotoxic activity (61).
The antitumour activity of L. inermis leaf extract was studied on 7,12-dimethylbenzanthracene (DMBA) induced 2-stage skin carcinogenesis and B16F10 melanoma tumour model using swiss albino mice. Topical application of L. inermis leaf extract at a dose level of 1000 mg/kg body weight was found to be effective in reducing the number of the papillomas. The tumor yield was significantly decreased 1.6 respectively as compared with the DMBA treated control group 3.5. In other experiment the effect of cyclophosphamide (CP) alone and in combination with L. inermis was studied in B16F10 melanoma tumour bearing mice. The Inhibition rate was 25.9% in the CP treated group but these increased to 35.14% with L. inermis. The life span time and volume of tumour doubling time were also increased. Results from two models showed that L. inermis extract have protective potential against skin tumour (62).
Molluscicidal activity
The molluscicidal activity of leaf, bark and seed of henna against Lymnaea acuminata and Indoplanorbis exustus were studied. Seed powder was more toxic than leaf and bark against L. exustus. Binary combinations of henna seed with Cedrus deodara Roxh and Azadirachta indica A Juss oil, or powdered Allium sativum, or Zingiber officinale rhizome oleoresin was more toxic to snails L. acuminata and I. exustus than their single treatment. The highest increase in the toxicity was observed when henna seeds powder and C. deodara oil (1:1) were tested against both the snails. The combination with neem oil was also more toxic than their individual components and other combinations (63).
Antitrypanosomal activity
Crude methanolic extract of leaf of L. inermis showed in-vitro activity against Trypanosoma brucei at concentration of 8.3 mg/ml of blood in mice but not in-vivo. The treatment tends to ameliorate the disease condition, but did not affect the level of parasitaemia and packed cell volume (64).
Antidermatophytic activity
The antidermatophytic activity of ethanol, ethyl acetate and hexane extracts of L. inermis were tested on 5 strains each of Tinea rubrum and Tinea mentagrophytes. All these extracts showed significant antidermatophytic properties in-vitro (65).
Antiparasitic activity
During an ethnopharmacological survey of antiparasitic medicinal plants used in Ivory Coast, 17 plants were identified and collected. Polar, non-polar and alkaloidal extracts of various parts of these species were evaluated in-vitro in an antiparasitic drug screening. Antimalarial, leishmanicidal, trypanocidal, antihelminthiasis and antiscabies activities were determined. Among the selected plants, leaves of L. inermis showed potential trypanocidal activities (66).
Antifertility activity
Ethanol extract prepared from the powdered seeds of L. inermis failed to show significant antifertility activity. However in subsequent studies it was observed that the powdered leaves of henna, when administered as suspension or incorporated into the diet inhibited the fertility of rats. The fertility induced appeared was found to be permanent (67).
Immunomodulatory activity
Methanol extract of henna leaves at 1 mg/ml concentration had displayed immunostimulant action as indicated by promotion of T-lymphocyte proliferative responses. Seven compounds were isolated adopting the lymphocyte transformation assay (LTA)-guided fractionation of the total methanolic extract of henna leaves (39). Naphthoquinone fraction obtained from leaves L. inermis showed significant immunomodulatory effect (68).
Nootropic activity
The effect of acetone soluble fraction of petroleum ether extract of L. inermis leaves was investigated on memory, anxiety and behaviour mediated via monoamine neurotransmitters using elevated plus maze and passive shock avoidance paradigms. The extract exhibited prominent nootropic activity, potentiated clonidine induced hypothermia and decreased lithium induced head twitches. However, the haloperidol induced catalepsy was not modified (69).
CONCLUSION
Lawsonia inermisis not only a colouring agent, but it also possesses various biological activities such as antioxidant, antimicrobial, antidiabetic, anticancer, anti-inflammatory, antiparasitic, antidermatophytic properties, anticancer, antiviral, wound healing, immunomodulatory, hepatoprotective, tuberculostatic, antifertility, protein glycation inhibitor properties. Scientific research on L. inermis suggested huge biological properties of the extracts might provide detailed evidence for the use of this plant in different medicines. Furthermore clinical trial on this plant needs to be conducted.
REFERENCES
1. Farnsworth NR, Akerele O, Bingel AS, Soejarto DD, Guo Z. Medicinal plants in therapy. Bulletin of the World Health Organization 1985:63(6):965-981.
2. Verpoorte R. Pharmacognosy in the new millennium: lead finding and biotechnology. Journal of Pharmacy & Pharmacology 2000:52(3):253-262.
3. Turner DM. Natural product source material use in the pharmaceutical industry: the Glaxo experience. Journal of Ethnopharmacology 1996:51(1-3):39-44.
4. Newman DJ, Cragg GM, Snader KM. The influence of natural products upon drug discovery. Natural Product Reports 2000:17(3):215-234.
5. Clark AM. Natural products as a resource for new drugs. Pharmaceutical Research 1996: 13(8):1133-1144.
6. Cragg GM, Newman DJ, Snader KM. Natural products in drug discovery and development. Journal of Natural Products 1997:60(1):52-60.
7. Cowan MM. Plant Products as antimicrobial agents. Clinical Microbiology Review 2009:12(4):564-582.
8. Kirtikar KR, Basu BD. Indian Medicinal Plants, Vol. 3. International book distributors, New Delhi, India, 1956.
9. Malekzadeh F. Antimicrobial Activity of Lawsonia inermis L. American Society for Microbiology 1968:16(4):663-634.
10. Rao SS, Regar PL, Singh YV. Agrotechniques for henna (Lawsonia inermis L.) cultivation, improvement and trade. Central Arid Zone Research Institute, Pali-Marwar, 2005, pp: 25-27.
11. Simon JE, Chadwick AF, Craker LE. In Herbs an indexed bibliography, The scientific literature on selected herbs aromatic and medicinal plants of the temperate zone. Archon Books, Hamden, 1984, pp: 1971-1980.
12. Sastri BN. The Wealth of India: Raw Materials, CSIR, New Delhi, 1962, pp: 47-50.
13. Chauhan MG, Pillai APG. Microscopic profile of powdered drug used in Indian system of medicine, Jamnagar, Gujarat, 2007, pp: 84-85.
14. Vasudevan TN, Laddha KS. Herbal drug microscopy, Yucca publishing house, Dombivli, 2003, pp: 68-69.
15. Warrier PK. Indian medicinal plants a compendium of 500 species, Vol. 3, Orient longman private limited, Chennai, 2004. pp: 303-304.
16. Ahmed S, Rahman A, Alam A, Saleem M, Athar M, Sultana S. Evaluation of the efficacy of Lawsonia alba in the alleviation of carbon tetrachloride induced oxidative stress. Journal of Ethnopharmacology 2000:69(2):157-164.
17. Bich DH, Chung DQ, Chuong BX, Dong NT, Dam DT, Hien PV, Lo VN, Mai PD, Man PK, Nhu DT, Tap N, Toan T. The Medicinal Plants and Animals in Vietnam, Vol. 2, Science and Technology Publishing House, Hanoi, Vietnam, 2004.
18. Reddy KR. Folk Medicine from Chittoor District, Andhra Pradesh, India, Used in the Treatment of Jaundice. International Journal of Crude Drug Research 1988:26(3):137-140.
19. Dixit SN, Srivastava HS, Tripathi RD. Lawsone, the antifungal antibiotic from the leaves of Lawsonia inermis and some aspects of its mode of action. Indian Phytopathological 1980:31:131-133.
20. Afzal M, Al-oriquat G, Al-Hassan JM, Muhammad N. Isolation of 1,2-Dihydroxy-4-glucosyloxynaphthalene from Lawsonia inermis. Heterocycles 1984:22(4):813.
21. Gupta S, Ali M, Alam MS. A napthaquinone from lawsonia inermis stem bark. Phytochemisty 1993:33(3):723-724.
22. Takeda Y, Fatope MO. New phenolic glucosides from Lawsonia inermis. Journal of Natural Products 1988:51(4):725-729.
23. Cuong NX, Nhiem NX, Thao NP, Nama NH, Dat NT, Anh HLT, Huong LM, Kiem PV, Minh CV, Wonc J, Chung W, Kim YH. Inhibitors of osteoclastogenesis from Lawsonia inermis leaves. Bioorganic & Medicinal Chemistry Letters 2010:20(16):4782-4784.
24. Chakrabartty T, Poddar G, St-Pyrek J. Isolation of dihydroxy lupene and dihydroxy lupane from the bark of Lawsonia inermis. Phytochemistry 1982:21(7):1814-1816.
25. Handa G, Kapil A, Sharma S, Jagdev S. Lawnermis acid a new anticomplementary tri-terpenoids from Lawsonia inermis seeds. Indian Journal of Chemistry1997:36(3):252-256.
26. Gupta S, M Ali M, Alam MS, Niwa M, Sakai T. 24β-ethylcholest-4-en-3-β-ol from the Roots of Lawsonia inermis. Phytochemistry 1992:31(7):2558-2560.
27. Gupta S, Ali M, Alam MS, Sakai T, Niwa M. A new aliphatic hydrocarbon from Lawsonia inermis bark. Indian Journal of Chemistry 1992:31:705-707.
28. Chakrabartty D, Poddar G, Deshmukh SK. Triterpenoids and other constituents of Lawsonia inermis. Indian Journal of Chemistry 1977:15:96-97.
29. Bhardwaj DK, Sheshadri TR, Singh R. Xanthones from Lawsonia inermis. Phytochemistry 1977:16(10):1616-1617.
30. Bhardwaj DK, Jain RK, Jain BC, Mehta CK. 1-hydroxy-3, 7- dimethoxy-6-acetoxy xanthone a new xanthone from Lawsonia inermis. Phytochemistry 1978:17(8):1440-1441.
31. Bhardwaj DK, Murari R, Sheshadri TR, Singh R. Lacoumarin from Lawsonia inermis. Phytochemistry 1976:15(11):1789.
32. Afzal M, Al-oriquat G, Al-Hassan JM, Muhammad N. Flavone glycosides from Lawsonia inermis. Heterocycles 1980:14:1973-1976.
33. Wong KC, Teng YE, Volatile Components of Lawsonia inermis L. Flowers. Journal of Essential Oil Research 1995:7(4):425-428.
34. Reichling VJ, Harkenthal M, Brandt H, Bayerl C. Temporare Henna Taottoos. Deutsche Apotheker Zeitung 1999:(139)33:35-41.
35. Muhammad HS, Muhammad S. The use of Lawsonia inermis linn. (Henna) in the management of burn wound infections. African Journal of Biotechnology 2005:4(9):934-937.
36. Omar MA. Effects of 2-hydroxy-1, 4-napthoquinone, a natural dye of henna, on aldehyde oxidase activity in guinea pigs. Journal of Medical Science 2005:5(3):163-168.
37. Dasgupta T, Rao AR, Yadava PK. Modulatory effect of Henna leaf (Lawsonia inermis) on drug metabolising phase I and phase II enzymes, antioxidant enzymes, lipid peroxidation and chemically induced skin and forestomach papillomagenesis in mice. Molecular & Cellular Biochemistry 2003:245(1-2):11-22.
38. Guha G, Rajkumar V, Kumar A, Mathew L. Antioxidant activity of Lawsonia inermis extracts inhibits chromium (VI)-induced cellular and DNA toxicity. Evidence-based Complementary and Alternative Medicine 2009:6(4):1-10.
39. Mikhaeil BR, Badria FA, Maatooq GT, Amer MM. Antioxidant and immunomodulatory constituents of henna leaves. Zeitschrift für Naturforschung C 2004:59:468-476.
40. Bonjar S. Evaluation of antibacterial properties of some medicinal plants used in Iran. Journal of Ethnopharmacology 2004:94(2-3):301-305.
41. Habbal OA, Ai-Jabri AA, El-Hag AH, Al-Mahrooqi ZH, Al-Hashmi NA. In-vitro antimicrobial activity of Lawsonia inermis Linn (henna) - A pilot study on the Omani henna. Saudi Medical Journal 2005:26(1):69-72.
42. Ali NAA, Julich WD, Kusnick C, Lindequist U. Screening of Yemeni medicinal plants for antibacterial and cytotoxic activities. Journal of Ethnopharmacology 2001:74(2):173-179.
43. Kirkland D, Marzin D. An assessment of the genotoxicity of 2-hydroxy-1, 4-naphthoquinone, the natural dye ingredient of Henna. Mutation Research 2003:537(2):183-199.
44. Arun P, Purushotham KG, Jayarani J, Kumari V. In vitro Antibacterial activity and Flavonoid contents of Lawsonia inermis (Henna). International Journal of PharmTech Research, 2010:2(2):1178-1181.
45. Natarajan MR, Lalitha DK. Leaf extracts of Lawsonia inermis as antifungal agent. Current Science 1987:56(19):1021-1022.
46. Singh VK, Pandey DK. Fungitoxic studies on bark extract of Lawsonia inermis against ringworm fungi. Hindustan antibiotics bulletin 1989:31(1-2):32-35.
47. Raveesha KA, Satish S, Mohana DC, Raghavendra MP. Antifungal activity of some plant extracts against important seed borne pathogens of Aspergillus sp. Journal of Agricultural Technology 2007:3(1):109-119.
48. Anand KK, Singh B, Chand D, Chandan, BK. An evaluation of Lawsonia alba extract as hepatoprotective agent. Planta Medica 1992:58(1):22-25.
49. Bhandarkar M, Khan A. Protective effect of Lawsonia alba against carbontetrachloride induced hepatic damage in albino rats. Indian Journal of Experimental Biology 2003:41(1): 85-87.
50. Hemalatha K, Natraj HN, Kiran AS. Hepatoprotective activity of leaves of Lawsonia alba. Indian Journal of Natural Product 2004:20(4): 14-17.
51. Latha PG, Suja SR, Shyamal S, Rajasekharan S. Some hepatoprotective garden plants. Natural Product Radiance 2005:4(4):278-279.
52. Syamsudin I, Winarno H. The effects of Inai (Lawsonia inermis) leave extract on blood sugar level: An Experimental Study. Researcj Journal of. Pharmacology 2008:2(2):20-23.
53. Sultana N, Choudhary MI, Khan AJ. Protein glycation inhibitory activities of Lawsonia inermis and its active principles. Journal of Enzyme Inhibition and Medicinal Chemistry 2009:24(1):257-261.
54. Nayak BS, Isitor G, Davis EM, Pillai GK. The evidence based wound healing activity of Lawsonia inermis Linn. Phytotherapy Research 2007:21(9):827-831.
55. Hamdi YP, Benazzouz M, Belkhiri H, Chari Z, Serakta M, Bensgni L. Healing effect of Lawsonia inermis L. (henna) as exemplified by the third degree burns. Revue de Medecines et Pharmacopees Africaines 1997:11-12:151-156.
56. Gupta S, Ali M, Pillai KK, Alam MS. Evaluation of anti-inflammatory activity of some constituents of Lawsonia inermis. Fitoterapia 1993:64(4):365-366.
57. Ali BH, Bashir AK, Tanira MOM. Antiinflammatory, antipyretic and analgesic effects of Lawsonia inermis L. (henna) in rats. International Journal of Experimental and Clinical Pharmacology 1995:51(6):356-363.
58. Sharma VK. Tuberculostatic activity of henna Lawsonia inermis Linn. Tubercle 1990:71(4): 293-296.
59. Khan MMAA, Jain DC, Bhakuni RS, Zaim M, Thakur RS. Occurrence of some antiviral sterols in Artemisia annua. Plant Science 1991:75(2):161-165.
60. Endrini S, Rahmat A, Ismail P, Taufiq-Yap YH. Comparing of the cytotoxicity properties and mechanism of Lawsonia inermis and Strobilanthes crispus extract against several cancer cell lines. Journal of Medical Sciences 2007:7(7):1098-1102.
61. Ali M, Grever MR. A cytotoxic napthoquinone from Lawsonia inermis. Fitoterapia 1998:69(2):181-183.
62. Wasim R, Agrawal RC, Ovais M. Chemopreventive action of Lawsonia inermis Leaf Extract on DMBA-induced skin papilloma and B16F10 Melanoma Tumour. Pharmacologyonline 2009:2:1243-1249.
63. Singh A, Singh DK. Molluscicidal activity of Lawsonia inermis and its binary and tertiary combinations with other plant derived molluscicides. Indian Journal Experimental Biology 2001:39(3):263-268.
64. Wurochekke AU, Chechet G, Nok AJ. In-vitro and In-vivo antitrypanosomal brucei infection in mice. Journal of Medical Science 2004:4(3):236-239.
65. Natarajan V, Mahendraraja S, Menon T. Antidermatophytic activities of Lawsonia alba. Biomedicine 2000:20(4):243-245.
66. Okpekon T, Yolou S, Gleye C, Roblot F, Loiseau P, Bories C, Grellier P, Frappier F, Laurens A, Hocquemiller R. Antiparasitic activities of medicinal plants used in Ivory Coast. Journal of Ethanopharmacology 2004:90(1):91-97.
67. Munshi SR, Shetye TA, Nair RK. Antifertility activity of three indigenous plant preparations. Planta Medica 1977:31(1):73-75.
68. Dikshit V, Dikshit J, Saraf M, Thakur V, Sainis K. Immunomodulatory activity of naphthoquinone fraction of Lawsonia inermis Linn. Phytomedicine 2000:7:102-103.
69. Iyer MR, Pal SC, Kasture VS, Kasture SB. Effect of Lawsonia inermis on memory and behaviour mediated via Monoamine neurotransmitters. Indian Journal of Pharmacology 1998:30(3):181-185.
Reference ID: PHARMATUTOR-ART-1022
FIND OUT MORE ARTICLES AT OUR DATABASE









