{ DOWNLOAD AS PDF }
ABOUT AUHTORS
Suleman S. khoja 1 , Sohil S. khoja 1,
Farhad S. Khoja 2,Shamim Khoja2,Narmin Pirani2
1)Resource person in pharmaceutical quality assurance and Audit Compliance,VAPI 2) Registered Pharmacist , Gujarat
Suleman salim khoja
Email: premukhoja@gmail.com
Scope
Human Error is commonly defined as “a failure of a planned action to achieve a desired outcome”. GMPs clearly state in CFR 211.22 that “[the quality control unit has]…the authority to review Production records to assure that no errors have occurred or, if errors have occurred, that they have been fully investigated.” Let’s analyze this statement. If the FDA expects that errors be fully investigated, it is safe to assume that the term error is NOT a root cause. That’s why it needs to be fully investigated, hence determine the root cause of the human error. In order to successfully achieve this goal, we have to understand how to improve the way we deal with these types of situations. review article accurately how to accurately identify human errors, determine when a deviation or nonconformance requires CAPA, and get started using human performance improvement tools and processes in your organization.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2463
|
PharmaTutor (ISSN: 2347 - 7881) Volume 5, Issue 2 Received On: 21/09/2016; Accepted On: 17/10/2016; Published On: 01/02/2017 How to cite this article: Khoja SS, Khoja S, Khoja FS, Khoja S, Pirani N; Impact and management tool for identification and reduction of human Errors in pharmaceuticals Industry; PharmaTutor; 2017; 5(2); 7-13 |
Introduction
Human Error is commonly defined as “a failure of a planned action to achieve a desired outcome”. According to Irish Medicines boards “Human errors is frequently cited as primary cause of quality defect issues that have led to batch recalls.” Its report suggest that nearly 25% of all quality defects-such as deviations, laboratory errors, complaints and inspection issues – are attributed to human error
Which includes
-Failing to follow procedures correctly
-Using technical dossiers to support batch releases that do not correctly reflect the contents of the marketing authorisation
-Poor line clearance, resulting in rogues being left on a processing line
-Failing to implement variations following their approval by the competent authority
90% of recalls relating to packaging and labelling are attributed to human error and that quality defects are often attributed to human error without scientific evidence. Human behaviour is complex and just like equipment, product, and process it needs to be Analyzed in depth
Types of Human Error
A) Stressor Error- Stressor RIFs (pressure causing a feeling of stress)
B) Structural Error - Structural RIFs (inherent weakness in activity) RIFs can be grouped into ‘families’ of issues that might affect the risk of human error, e.g. process, information, resource, competence, organisation and stressors (PIRCOS).
Stressor Error - RIFs increase the probability of human error. They are often temporary but may re-occur, i.e. if they are caused by fatigue or very tight work deadlines. Therefore, they are subjective as they depend on the person within the team making an error. They also; however, expose structural vulnerability, which will need further investigation.
Structural Error - RIFs are relatively persistent in nature but are often not immediately obvious, unless triggered by stressors. Structural RIFs occur across a company and can be caused in any one of a number of areas: for example, processes where several concurrent activities are competing for attention; information giving (poor layout of batch manufacturing record); resource characteristics such as environmental conditions causing distraction; and organisational planning, including when shift arrangements undermine vigilance.
Reducing Human Error by LEADERSHIP & TEAMWORK
To ensure a true understanding of human error and its contributor factors, a strategic approach should be taken. It is extremely important to create awareness and understanding of risks in an organisation by analysing its processes and understanding human error risks. To do this, Study Approach QRM (quality risk management) approach is recommended, which includes risk assessment, risk control, risk review and risk communication. It must also include routine tracking, evaluation and analysis of human error metrics.(Details and Tracking with Effective Evaluation )
Proactive Approach
Proactively assess potential risks, situations occur where retrospective analysis is necessary. There are a number of key activities that should be undertaken in each situation. For retrospective risk identification, you will need to identify how and where in the process human error is likely to occur through the use of a scientific approach: -Review process flow and information flow
-Identify the RIFs;
-Identify the issues with which RIFs are commonly associated (PIRCOS);
-Plan your processes to minimise and control these risks.
- For retrospective analysis, you will need to capture the event in real time, taking note of environmental and other factors that may be relevant to a thorough evaluation of the issue.
You will also need to consider the level of the RIF effect:
Individual – affects a single individual;
Local – within a limited physical area, relevant to specific activity, affects a finite number of people;
Generic – this could be common, where the risks are shared across numerous instances of a common problem (e.g. misreading a table in a document used widely in the company); or independent, where there are several risks in different parts of an organisation (e.g. misreading a typeface used in numerous documents only used locally). Then you should analyse whether your con-compliances associated with human error can provide further insight (e.g. from QMS, deviations and CAPA complaints management system software). There are number of root-cause analysis (RCA) tools and techniques that can be used, such as Brainstorming sessions, fishbone diagrams, ‘five whys’ and fault-tree analysis
Blame Approach –Not Useful and Must Avoided
Despite the industry’s awareness of human errors, companies still frequently fail to substantively and correctly address errors. The typical response to a human error is retraining but this often fails to produce the desired result. Training (or lack thereof) is responsible for only about ten percent of the human errors that occur, since it basically takes care of issues related to lack of knowledge, skill, or ability. If the error was not due to one of these then training is practically useless. Also, many companies are still taking the “blame” approach to human error, which appeals to the individual’s sense of fear. The blame perspective only leads to less trust from people to bring up issues that can lead to failures, which in turn results in management being less aware of system weaknesses that eventually result in more mistakes. A systemic view (for human error), instead, assumes that some degree of error is inevitable and puts systems in place to detect, prevent, and correct it.
How Can we Improve – Area to evaluate Human Errors
-Management System: documentation control, investigation management, risk management and project management are important to set the bases for the rest of the operation.
-Procedures: these need to be accurate, human-engineered, available and enforceable.
-Human factors : Work areas need to be designed with human factors and human capabilities in mind. Excessive monitoring, mental calculations, housekeeping, and work layout, among others, become the main reasons for error in this category.
-Training: training needs to include the whys as well as the whats and hows. Also, on the job training and qualification (OJT) is necessary, especially for critical tasks and activities. -Immediate supervision: pre-job briefs, walkthroughs, presence and instructions to workers are necessary. We need to have supervisors on the floor, not in the office.
-Communication: between groups, shifts, radio communication rules and training. Employees should know what needs to be achieved daily and the proper way to do it.
-Individual Performance: need to evaluate conditions that could potentially create cognitive overload that creates attention and memory failures. Some of these conditions include available time for the job,fittness for duty or fatigue management, and complexity and task
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Accurately determine when and where error may occur
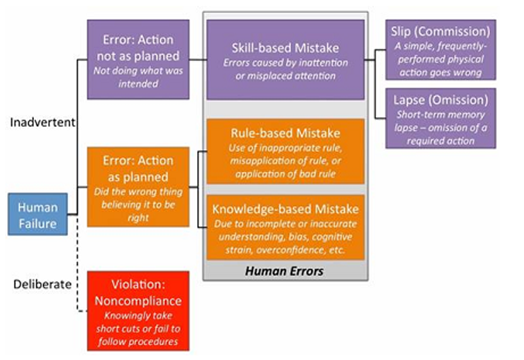
Figure-1: Skill,Rules knowledge (SRK)/Generic Error Modelling System (GEMS)
The model identifies that human failures present either as someone intentionally (deliberate) or unintentionally (inadvertent) doing the wrong thing. The inadvertent errors are considered to be human errors, which fall into one of three categories: skill-based, rule- based, or knowledge-based mistakes. Skill-based mistakes can be further broken down into slips or lapses, both of which occur due to a lack of attention to the task at hand.
Case Study -A Situation That May Be A True Human Error:
An Analyst performs a task that requires manually calculating and then mentally rounding a value and recording it in a Analytical worksheet . He performs the calculation, mentally rounds the result, and has in his head what he needs to write down. A co-worker arrives, interrupting him and diverting his attention from what he’s doing. He then writes other value rather than a rounded value.
The analyst then asks the another analyst to verify the step. He hands the documentation to the verifier but continues talking to him, which distracts the verifier and causes him to miss the rounding error. Processing continues, using the incorrect result. Later, QA identifies the rounding error in the worksheet (Raw Data), which alters every calculation from that point forward. In this instance, the impact on the batch was minimal — no limits or specifications were exceeded.
Upon investigation, the supervisor identified no gaps or issues in the process — the calculation is clearly and correctly defined in the procedure. He verified both people had successfully completed training on the rounding procedure and had training on the current version of the process procedure. Additionally, both successfully completed and checked this calculation many times in the past with no errors. The supervisor addressed the error with each individual, and both said they hadn’t been paying full attention to the task and did not realize an error had occurred
In this example, we have what may be a true human error — a one-time event where
trained personnel executing a well-defined process had their attention diverted from the task, resulting in a mistake due to inattention.
If we can justify this as the situation, having identified that no other process/system issues existed, then why not call it a slip or lapse, as appropriate? Again, it can happen. It just shouldn’t happen frequently. However, even if it is a true human error, we shouldn’t necessarily assume that only the involved personnel are at fault. When rule- or knowledge-based errors occur, we should also look at other processes — training and oversight, in particular — to identify factors that may have contributed to the mistake.
For example, a rule-based mistake occurs: A new Analyst misapplies a rounding rule and rounds all values instead of just the final value, which generates an out-of- specification (OOS) result. When this error is addressed, we should also question why the person wasn’t appropriately prepared for the task. Did the training not provide them enough practice? Did practice examples reflect the operational situations the person would face? Does the procedure specify that only the final value should be rounded?With knowledge-based mistakes, cognitive strain errors can occur when a person is multitasking — it may require more focus than they can provide to mentally manage the information required to accomplish multiple tasks at once. In this situation, we should identify why the person was multitasking. Were they assigned to perform too many tasks at once? Is the department under-resourced? Was the supervisor aware that the person is multitasking and is unable to function at the required level?
These are only two examples of how other processes should be considered when investigating human errors. Others may require addressing difficult procedure formats, non-user-friendly equipment, and other issues that may indicate problems in other areas.
#Does every deviation/Non Compliance require investigation and corrective action — even the lowrisk ones?
Addressing the CAPA part of the question first: Would a CAPA be necessary in the rounding error example? May be not. A risk assessment may classify it as a low-risk situation because:
Severity is low: The error had no batch impact, and future, similar rounding errors likely would not have a significant batch impact either. Detectability is high: The calculations are checked at least twice — by the verifier and QA and the checks caught the error that occurred.
Frequency is low: Both operators had correctly performed the task several times before with no errors.
We could use this to justify requiring no action beyond the supervisor pointing out the error to the involved analyst . For individuals who simply had an off day, a discussion with their supervisor may be enough to set things right. If further action is deemed necessary, requiring follow-up checks monitoring the operator and verifier to ensure a similar error isn’t made may be appropriate.
However, from a different perspective this error shows that, while this process is often correctly executed, it has possible failure points: Both the operator and the verifier missed the rounding error. Manual calculations or other manual operations can, and on occasion will, fail. So a CAPA could be identified to protect against the failure occurring again in the future. Automating the performance of the calculations and rounding would reduce the possibility of future errors.
We also don’t know whether other analyst /verifiers have made the same mistake. The example only discusses the two operators involved in this specific situation. Looking at a broader data set may identify several similar errors in manual calculations. In this case a CAPA should be considered, because it’s a broader failure than initially identified.
To loop back to the first half of the question and tie all of this together, I do believe that every deviation/NC requires some level of investigation, root cause analysis (RCA), and corrective action. Risk analysis shows that some deviations/NCs pose higher risks than others. And those that pose product quality or patient safety risks require thorough investigation, RCA, and CAPAs.
However, minor/low-risk situations still resulted from a process issue/problem, and they should not be ignored or excluded from the process simply because they didn’t pose a significant/high-risk impact to the process this time. Disregarding minor/low-risk situations allows the problem to continue, which will likely create a larger problem the next time it occurs. May be the better way to state it is that for low-risk situations, a less formal approach to investigation, RCA, and CAPA is appropriate.
Consider our “low-risk” rounding error example. It went through a simple investigation, RCA, and CAPA process. When the error was identified, it was assessed to determine batch impact. The supervisor investigated whether the involved operators were trained, how the error occurred, and whether their prior performance was acceptable. This led to identifying human error as the root cause. The supervisor took corrective action by addressing the error with the involved personnel, which hopefully leads to a behaviour adjustment. So all three elements were, in fact, done, but in a less formal manner that befitted the situation.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
What are some first steps to begin using human performance improvement tools and Processes?
Use these tools and processes where you have existing human errors/performance issues to correct, or to help identify ways to continuously improve performance. In the case of identified human errors/performance issues, these are the gaps that would be identified in the Gap Analysis step on the Human Performance Technology (HPT) Model (Figure: 2). From there, specifically define how Actual Performance differs from Desired Performance, and investigate to understand the existing situation (Environmental Analysis) and identify possible causes that could be contributing to the errors/issues that are occurring (Cause Analysis). -GAP Analysis
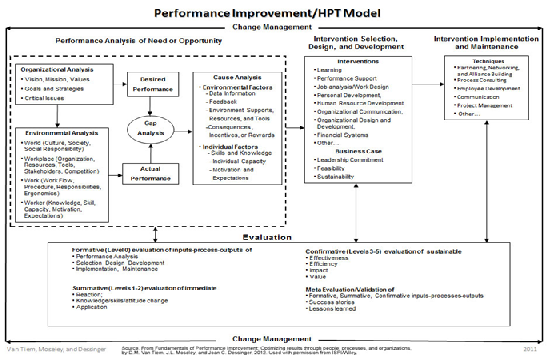
Figure.2: Performance Improvement / Human Performance Technology Mode
During the investigation, talk to the involved analyst (Person Involved) about the error/issue, and ask how/why it happened, how the error could be avoided or eliminated, what is needed to enable correct performance, and whether they understand the expectations for the task. Observe the task(s) and identify where distractions or multitasking occur, verify personnel are performing as expected, and verify that the procedures have enough information to guide task performance.
Look for answers to the questions presented in the Behavioural Engineering Model (Figure3):
• Do personnel know what’s expected of them and what the priorities are, and have they received feedback on their performance against those expectations?
•Do they have the correct resources (equipment and procedures, work environment,
time), and have they been designed to enhance performance?
• What incentives do they have to perform the task correctly? Is correct performance
rewarded and poor performance addressed?
• Do they have the knowledge and skills to perform the task as expected?
• Are they physically capable of performing the assigned task, and is the task scheduled
at optimum times for performance?
• Does the culture support proper performance of the task? Are personnel motives and company incentives aligned?

Figure 3:- Gilbert’s Behavioural Engineering Model
Alternatively, to use this in a continuous improvement manner, when no known performance issue exists, start at the Organizational Analysis step in the HPT Model. Identify the specific situation to be improved, and then define Desired Performance and what is needed to support it. Next, in the Environmental Analysis step, identify what currently exists in the organization and how well it’s functioning to define Actual Performance. Then, in the Gap Analysis step, identify any gaps between what’s needed (Desired Performance) and what exists (Actual Performance) that could lead to performance issues.
One starting point for the process can be addressing undesired behaviors. These should be relatively obvious (poor attitude, poor performance, etc.) and they are gaps that should be addressed where they exist. Or, select a small group and try the process in a desired improvement initiative in that group. Either approach should illustrate how the process works and what kind of results can be achieved in a microcosm of the larger company environment.
Conclusion
Human Error reduction with effective study and using study model, Effective Implementation of SOP ,Generation of quality products pharmaceutical and Help Industry to optimize the parameter from Moving Beyond Retraining as good and Justifiable FDA Response risk-influencing factors (RIFs).
Reference
1.https://www.nopsema.gov.au/resources/human-factors/human-error/ ,Acessed on Sep 20, 2016-00:30 Hrs
2.http://www.ispi.org/images/ISPI/About%20ISPI%20Images/HPT-Model-2012.jpg , Acessed on Sep 21, 2016 -01:46 Hrs
3.http://www.engineersjournal.ie/2013/12/12/irish-pharmachem-industry-fights-to-maintain-its-global-position/
4.http://learnaboutgmp.com/the-top-7-how-to-reduce-manufacturing-human-error/
5.http://www.fda.gov/downloads/drugs/developmentapprovalprocess/manufacturing/ucm324354.pdf
6. http://www.biopharminternational.com/reducing-human-error
7. http://www.process-improvement-institute.com/human-error-prevention-training/
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









