ABOUT AUTHORS:
Sarthak B. Dave*, Dipen K. Sureja
Department of Pharmaceutical Chemistry
Shree H. N. Shukla Institute of Pharmaceutical Education & Research,
B/H Marketing Yard, Near Lalpari Lake,
Amargadh (Bhichari), Rajkot-360002
davesarthak@ymail.com
ABSTRACT
Imidazole derivatives have occupied a unique place in the field of medicinal chemistry. It is the constituent of several natural compounds like histamine, histamine, biotin, alkaloids and nucleic acid and a very important class among the medicinal compounds. Large number of imidazole derivatives have been are being developed for different therapeutic actions, therefore this article aims to review the work reported on the synthesis of Imidazole derivatives using microwave reactions as a modern method for synthesis, to get better yield, economic and environment friendly reaction. Imidazole is an entity which is being synthesized in many of its derivative form from past few years; the entity is major source of interest for many of medicinal chemist to explore its various pharmacological potentials.
REFERENCE ID: PHARMATUTOR-ART-1938
INTRODUCTION:-
During the past decade, the concept of Imidazolines (I) receptors has been developed and gained consensus. Different rank order of affinity of ligands indicates the existence of at least two major classes of Imidazolines receptors I1 and I2.[1] Findings from different laboratories have been shown that they are widely distributed in different tissues and species and may participate in the regulation of various physiological functions. Therefore a more definite knowledge of the structure and function of this receptor system could help to the search for therapeutic agents useful for treating efficaciously a variety of disorders such as hypertension, diabetes mellitus, gastric ulcer, endogenous depression and stroke. Some Imidazole containing compounds like clonidine, guanfacine and newly synthesize lofexidine hydrochloride also act as α2agonists and clinically useful for the treatment of hypertension. α2 –adrenergic agonist also exhibit activity in human platelets and peripherally act in Ocular hypertension (glaucoma). [2]
Medicinal chemistry is the discipline concerned with determing the influence of chemical structure on biological activity and in the practice of medicinal chemistry developed from an empirical one involving organic synthesis of new compound based largely on the modification of structure and then identifies their biological activity.[3,4] Medicinal chemistry concerns with the discovery, development, interpretation and the identification of mechanism of action of biologically active compounds at the molecular level.[5] Various biologically active synthetic compounds have five-membered nitrogen-containing heterocyclic ring in their structures.[6]
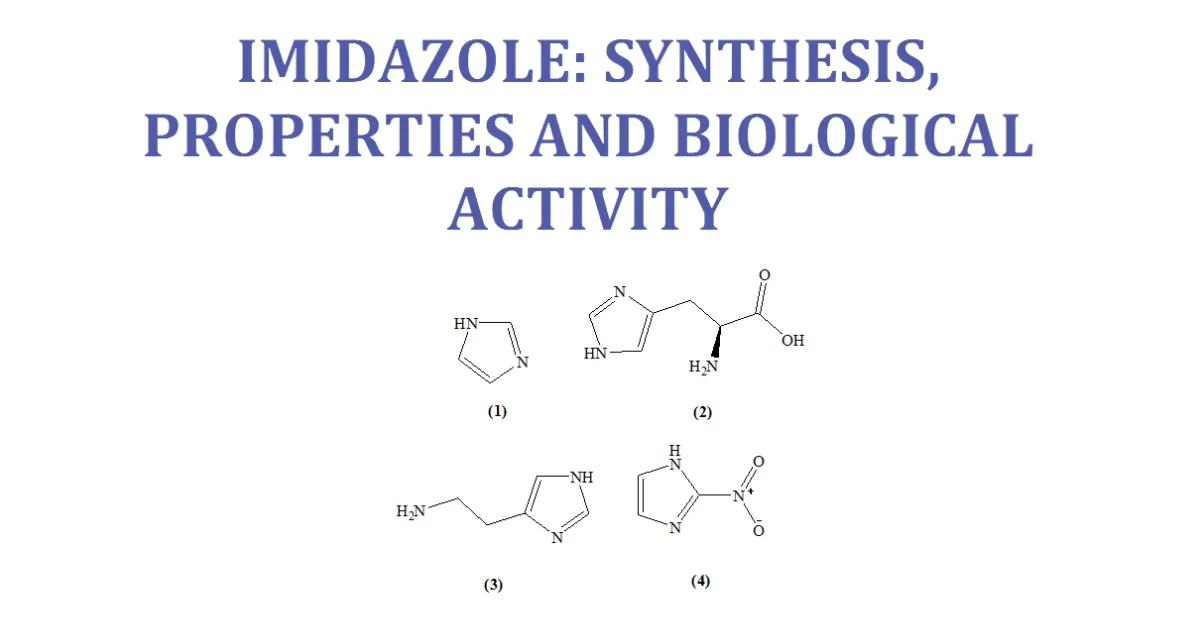
Imidazole is an organic compound with the formula C3H4N. This aromatic heterocyclic is a “1, 3-diazole” and is classified as an alkaloid. Imidazole (1) refers to the parent compound,whereas imidazoles are a class of heterocyclic with similar ring structure, but varying substituents. This ring system is present in important biological building blocks, such as histidine (2), and the related hormone histamine (3). Imidazole can serve as a base and as a weak acid. Many drugs contain an imidazole ring, such as antifungal drugs and Nitroimidazole (4). [7-11]
STRUCTURE AND PROPERTIES:-
Imidazole is a monoacidic base having the ability to form crystalline salts with acids. The melting point of number of characteristic imidazolium salts [12]. Imidazole is a 5-membered planar ring, which is soluble in water and other polar solvents. It exists in two equivalents Tautomeric forms, 1H-imidazole and 3H-imidazole, because the hydrogen atom can be located on either of the two nitrogen atoms. Imidazole is a highly polar compound, as evidenced by a calculated dipole of 3.61D, and is entirely soluble in water. The compound is classified as aromatic due to the presence of a sextet of p-electrons, consisting of a pair of electrons from the protonated nitrogen atom and one from each of the remaining four atoms of the ring. [13]
REACTIVITY:-
Imidazole can be considered as having properties similar to both pyrrole and pyridine. The electrophilic reagent would attack the unshared electron pair on N-3, but not that on the ‘pyrrole’ nitrogen since it is the part of thearomatic sextet. While the imidazole ring is rather susceptible to electrophilic attack on an annular carbon, it is much less likely to become involved in nucleophilic substitution reaction unless there is a strongly electron withdrawing substituent’s elsewhere in the ring. In the absence of such activation the position most prone to nucleophilic attack is C-2. The fused benzene ring in Benzimidazole provides sufficient electron with drawl to allow a variety of nucleophilic substitution reaction at C-2.
The overall reactivity of imidazoles and Benzimidazole is referred from sets of resonance structure in which the dipolar contributors have finite importance. These predict electrophilic attack in imidazole at N-3 or any ring carbon atom, nucleophilic attack at C-2 or C-1 and also the amphoteric nature of the molecule. In Benzimidazole the nucleophilic attack is predicted at C-2. The reactivity of Benzimidazole ion at the C-2 position with nucleophiles is enhanced compared with the neutral molecule. [14]
PHYSICAL PROPERTIES:-
It is colourless liquid having a high B.P. of 256oC than all other 5- membered heterocyclic compounds due to the intermolecular H-bonding, where there is linear association of molecule.
Imidazoles shows a large value of dipole moment of 4.8 D in dioxane. Imidazole show amphoteric properties and has pKa of 7.2 more than pyrazole and pyridine. Imidazoles are an aromatic compound possessing a resonance value of 14.2 K cal/ mol, which is almost half the value for pyrazole. The electrophillic substitution occurs frequently in imidazole and nucleophillic substitution happens in the presence of electron withdrawing group in its nucleus. Imidazoles have M.P. 90ºC, it is a weak base and tautomeric substance, since position 4 and 5 are equivalent. [15]
It’s spectroscopic parameters are λmax of 207 nm, I.R.=1550, 1492, 1451(cm-1), t = 2.30, 2.86, mass spectroscopy is studied for heterocyclic compounds containing one hetero-atom, in detail, not in case containing two or more heteroatom.
General Methods of Preparation:-
Imidazole can be synthesized by numerous methods. Many of these synthesis can also be applied to different substituted imidazoles and imidazole derivatives simply by varying the functional groups on the reactants. Several approaches are available for synthesis of imidazoles as, Debus synthesis, Radiszewski synthesis, dehydrogenation of imidazolines, from alpha halo ketones, Wallach synthesis, from aminonitrile and aldehyde and Marckwald synthesis [16]. Details of the synthetic procedures are given below.
1) Debus Synthesis:-
Debus Synthesised imidazole by using glyoxal (9) and formaldehyde (10) in ammonia. This synthesis, while producing relatively low yields, is still used for creating C-substituted imidazoles (11). [17]
2) Radiszewski Synthesis:
Radiszewski reported the condensation of a dicarbonyl compound, benzil (12) and a- ketoaldehyde, benzaldehyde (13) or a-diketones in the presence of ammonia, yield 2, 4, 5-triphenylimidazole (14). [18]
3) Dehydrogenation of Imidazoline:-
Knapp and coworkers have reported a milder reagent barium managanate for the conversion of imidazolines to imidazoles in presence of sulphur. Imidazolines obtained from (15) alkyl nitriles and (16) 1, 2 ethanediamine on reaction with BaMnO4NH yield (17) 2-substituted imidazoles. [19]
4) Wallach Synthesis:-
Wallach reported that when N, N- dimethyloxamide (18) is treated with phosphorus pentachloride, a chlorine containing compound (19) is obtained which on reduction with hydroiodic acid give N- methyl imidazole (20). Under the same condition N, N-diethyloxamide is converted to a chlorine compound, which on reduction gives 1- ethyl –2- methyl imidazole. [20]
5) By the formation of one bond:-
The (1,5) or (3,4) bond can be formed by the reaction of an imidate (22) and an a-aminoaldehyde or a-aminoacetal (21), resulting in the cyclization of an imidine (23) to imidazole (24). The example below applies to imidazole when R=R1=Hydrogen. [21]
6) Markwald Synthesis:-
The preparation of (27) 2- mercaptoimidazoles from (25) a- amino ketones or aldehyde and (26) potassium thiocyanate or alkylisothiocyanates is a common method for the synthesis of imidazoles. The sulphur can readily be removed by a variety of oxidative method to give the desired imidazoles. The starting compounds, a- amino aldehyde or ketone, are not readily available, and this is probably the chief limitation of the Markwald synthesis. [22]
SYNTHESIS OF IMIDAZOLE DERIVATIVES BY MICROWAVE REACTIONS:-
Qasim et al [23] synthesized 2- phenylimidazo [4,5-f] [1,10] Phenanthroline derivatives (30), by reacting dicarbonyl compound (28) and p-substituted benzaldehyde (29), this is a type of acid catalysed reaction with excellent yields in a neutral ionic liquid, 1-methyl-3-heptyl- imidazolium tetrafluoroborate [(HeMIM) BF4 ], under solvent free and microwave assistedconditions. This particular reaction accompanies all the merits of microwave reactions like easy workup, better yield, and environment friendly reaction.
Ermolat et al [24] synthesized mono and disubstituted-2-amino-1H imidazoles (32) via microwave assisted hydrazinolysis of substituted imidazo [1, 2, a] pyrimidines (31) is reported. This method avoids strong acidic conditions and is superior to the conventional cyclocondensation of a haloketones with N-acetyl guanidine.
Frank et al [25] synthesized 5-substituted-2-(2-methyl-4-nitroimidazomethyl)-1, 3, 4-oxadiazoles (33) containing the nitroimidazole moiety by microwave-assisted as well as conventional method was carried out and their antibacterial, antifungal and anti-inflammatory activity was reported.
Marek et al [26] synthesized via a facile 4-step reaction sequence starting from commercially available and inexpensive N-Cbz amino acids (34). The condensation of the corresponding abromoketones with formamidine acetate in liquid ammonia was revealed to be a useful method for the synthesis of such imidazole derivatives (35), derivatives thus prepared are structurally related to histamine.
Pathan et al [27] reported the reaction of alkyl cyanide (36) with ethylene diamine (37) in the presence of carbon disulphide give 2-substituted 2-imidazolines (38) under microwave irradiation. The yields of product obtained using this protocol is significantly high and the reaction time is reduced.
Na Zhao et al[28] reported an efficient and a quick microwave-assisted synthesis of Benzimidazole and trisubstituted imidazoles (41). Three Benzimidazole were obtained as a result of the condensation of 1,2phenylenediamine (39) with carboxylic acids and acetoacetic ester (40) without catalyst.
Kawashita et al [29] a variety of heteroaromatic compounds, 2-substituted imidazoles (43) were synthesized by oxidative aromatization of 2-substituted imidazolines (42) using the Activated carbon and molecular oxygen system.
Ermolat’ev et al [30]reported an efficient microwave-assisted one-pot two-step protocol was developed for the construction of disubstituted 2-amino-1H-imidazoles (46). This process involves the sequential formation of 2, 3-dihydro-2-hydroxy imidazo[1,2-a] pyrimidinium salts from readily available 2-aminopyrimidines (44) and a-bromoketones (45), followed by cleavage of the pyrimidine ring with hydrazine.
Lupsori et al[31] a series of 1-hydroxymethylazoles (50) were synthesised by condensation reaction of azoles (48) (pyrazole, imidazole, 3,5-dimethylpyrazole, 2methylimidazole and benzimidazole) with paraformaldehyde (49). The reactions were carried out under microwave irradiation conditions using tetrahydrofurane (THF) or dimethyl sulfoxide (DMSO) as solvents. Microwaves assisted procedure has noticeable advantages compared to classical methods: yield increase, substantial reduction of reaction time, solvents consumption and waste minimization.
Soh et al[33] developed a microwave-assisted protocol for the construction of di- and monosubstituted 2-aminoimidazoles. The two-step reaction involves the synthesis of N-(1Himidazol-2-yl) acetamides (52) from readily available alpha-haloketones (51) and N-acetyl guanidine, followed by deacetylation. Significant rate enhancement was observed for both steps of the protocol, and the overall reaction time was shortened to 20 min compared to 48 h of the conventional procedures. A representative set of di- and monosubstituted 2-aminoimidazoles was prepared using commercially available parallel reactors.
PHARMACOLOGICAL ACTIVITIES:-
Imidazole derivatives have a wide range of pharmacological activity, literature survey revealed that imidazole and its derivative are reported to have, analgesic and anti-inflammatory activity[33-36], cardiovascular activity [37-39] , anti-neoplastic activity, anti- fungal activity [40,41], enzyme inhibition activity [42,43] anti anthelmintic activity [44], anti-filarial agent, anti- viral activity and anti- ulcer activity. Other than their pharmacological actions they also function as dyestuffs catalysts and polymerizing agents. 2-nitro imidazole (azomycin) and 1-(2-hydroxyethyl)-2-methyl-5-nitroimidazole (metronidazole) are anti-bacterial agent with particular applications as trichomonacide. Along with metronidazole other nitroimidazoles (misonidazole, metrazole and clotrimazole) are important anti-cancer drugs. Two imidazolines, priscol and privine are valuable vasodialating and vasoconstricting drugs. 2- aminoimidazolines are among the class which are known for Fungicidal action. The modern scientific searches aim at discovering more effective and better-tolerated imidazole derivatives.
1. Antifungal and anti bacterial activity:-
Ramya v et al [46] synthesized a series of (53) novel 5-(nitro/bromo)-styryl-2-benzimidazole derivatives and tested for the antibacterial activity against Staphylococcus aureus, Escherichia coli, Enterococcus faecalis, and Klebsiella pneumoniae and anti fungal activity against Candida albicans and Aspergillus fumigates. This was comparable with ciprofloxacin.
Deepika Sharma et al [47] have synthesized (54) 2-(substituted phenyl)-1H-imidazole and (substituted phenyl)-[2-(substituted phenyl)-imidazol-1-yl]-menthanone analogues and screened for antimicrobial activity against gram positive, Gram negative, and fungal species. Norfloxacin used as standard and following compound is most potent.
Daniele Zampieri et al [47] synthesized (55) bis-imidazole derivatives and screened for antifungal and anti mycobacterial activity. All compounds showed moderate to good activity against Candida albicans and Candida glabrata. Miconazole used as reference drug.
Shreenivas et al [48] Compounds were screened for their in-vitro antibacterial activity against S. aureus and B. subtilis employing cup-plate method at the concentration of 100µg/ml in nutrient agar media and also for in-vitro antifungal activity against C. albicans and A. Niger by cup plate method at 100µg/ml. concentration using sabouraud-dextrose agar. DMSO was used as solvent control for antimicrobial activity. Streptomycin was used as standard for antimicrobial. The area of inhibition of zone measured in cm. (56)
2. Anti -inflammatory and analgesic activity:-
Puratchikody A. et al [49] studies on (57) 2-substituted-4, 5-diphenyl-1H-imidazoles and checked the anti-inflammatory activity based on Carrageenan-induced paw edema method. This compound shows maximum activity and indomethacin used as reference drug.
Kavitha C.S.et al [50] has synthesized a series of (58) 2-methylaminibenzimidazole derivatives and newly synthesized compounds were screened for analgesic and anti-inflammatory activities. This compound shows analgesic activity and compared with standard nimesulide drug.
3. Anticancer activity:-
Yusuf Ozkay et al [51] synthesized many novel (59) imidazole-(Benz) azole and imidazole epiperazine derivatives in order to investigate the anticancer activity. Anticancer activity screening results revealed that these were the most active compounds in the series. Cisplatin was used as reference drug.
Hanan M. Refaat [52] synthesized various series of 2-substituted benzimidazole. Several of the synthesized products were subjected for anticancer screening which revealed that all the tested compounds exhibited antitumor activity against human hepatocellular carcinoma, breast, adenocarcinoma, and human colon carcinoma. 3a (60) and 4a (61) showed the highest potency against human hepatocellular carcinoma.
Cenzo congiu et al [53] synthesized a series of (62) 1, 4-diarylimidazole-2(3H)-one derivatives and their 2-thione analogues and evaluated antitumor activity. This Compound show potent antitumor activity.
4. Antitubercular activity:-
Ramya V et al [54] synthesizedseries of novel (63) 5-(nitro/bromo)-styryl-2-benzimidazoles (1–12) derivatives and screened for in vitro anti-tubercular activity against Mycobacterium tuberculosis, and these compounds showed good antitubercular activities. Streptomycin was used as reference drug.
Preeti Gupta et al [55] describe anti-mycobacterium tuberculosis activities of ring substituted –(64) 1H imidazole-4-carboxylic acid derivatives and 3-(2-alkyl-1H-imidazole-4-yl)-propionic acid derivatives against durg-sensetive and durg- resistent M. tuberculosis strains. 2f and 2h compounds were most potent compound.
Jyoti Pandey et al [56] synthesized a series of (65) imidazole derivatives and compounds were screened against M .tuberculosis where this compound showed good antitubercular activity.
5. Antidepressant activity:-
Farzin Hadizadeh et al [57] synthesized (66) moclobemide analogues by replacing moclobemide phenyl ring with substituted imidazole and studied for the antidepressant activity using forced swimming test. Analogues 7a-c was found to be more potent than moclobemide.
6. Antiviral activity:
Deepika Sharma et al [58] synthesized imidazole derivatives and the antiviral screening of (67) (substituted phenyl)- [2-(substituted phenyl)-imidazol-1-yl]-methanones against viral strains indicated that compounds A and B selected as the most potent antiviral agents. Ribavirin was used as standard drug.
Michele Tonelli et al [59] synthesized seventy six 2-phenylbenzimidazole derivatives and evaluated for cytotoxicity and anti viral activity against a panel of RNA and DNA viruses. (68) Compound ([5,6-dichloro-2-(4-nitrophenyl) benzimidazole]) exhibited a high activity Resulting more potent than reference drugs smycophenolic acid and 6-azauridine.
7. Antilishmanial activity:-
Kalpana bhandari et al [60] synthesized a series of (69) substituted aryloxy alkyl and aryloxy aryl alkyl imidazole and evaluated in vitro as antileishmanial against Leshmania donovani. Among all compounds exhibited 94–100% inhibition.
|
Compounds |
R |
R1 |
R2 |
R3 |
|
A |
Ph |
H |
CF3 |
H |
|
B |
CH3 |
H |
CF3 |
H |
|
C |
CH3 |
H |
NO2 |
H |
|
D |
CF3 |
H |
NO2 |
H |
|
E |
CH3 |
NO2 |
H |
H |
|
F |
CH3 |
CH3 |
NO2 |
H |
8. Anticonvulsant Activity:-
Bhragual et al [61]evaluated the anticonvulsant activity by Maximal Electroshock Method (MES). Substitution of chloro and nitro group at 2nd position in the substituted ring (70) showed significant anticonvulsant activity without neurotoxicity while hydrogen and 4-nitro substitution does not showed the anticonvulsant activity.
Many other amino substituted xantheno[1,2-d]imidazoles derivatives had also been synthesized with cell growth inhibitory activity specifically against breast cancer cell lines, insertion of two basic side chains at 2- and 5positions in this moiety, exhibited a strong dose-dependent ant proliferative activity. [62]
Again some specific moiety like 5-Arylamino-1H-benzo[d]imidazole-4,7-diones were synthesized for their inhibitory activities on the proliferation of human umbilical vein endothelial cells (HUVECs) and the smooth muscle cells (SMCs). Among them, several 1-H benzo[d]imidazole-4, 7-diones exhibited the selective antiproliferative activity on the HUVECs. [63]
APPLICATIONS OF IMIDAZOLES:-
One of the applications of Imidazole is in the purification of His tagged proteins in immobilized metal affinity chromatography (IMAC). Imidazole is used to elute tagged proteins bound to Ni ions attached to the surface of beads in the chromatography column. An excess of imidazole is passed through the column, displaces the His-tagged from nickel coordination and free the His-tagged proteins. Imidazole can be used to prepare buffers in the pH range of 6.2-7.8 at room temperature. It is recommended as a component of a buffer for assay of horseradish peroxides. It is also used as a chelator for the binding of different divalent cations. [64]
The oral administration of Imidazole shows beneficial effects on psoriasis and seborrhoea dermatitis. In psoriasis the improvement begins after a period of one and a half to three months. In seborrhoea dermatitis the patients begin from less redness, itchiness, and scaling within a period of four to six weeks. The benefits of this treatment occur without the need for applications of ointments or other topical applications.
The Imidazole nucleus is an important synthetic strategy in drug discovery. Many imidazoles have been prepared as pharmacological agents Azomycine, Clotrimazole, Miconazole, Ergothionine, Clonidine and Moxonidine. One of the most important applications of imidazole derivatives is their used as material for treatment of denture stomatities. Imidazole has become an important part of many pharmaceuticals. Synthetic Imidazoles are present in many fungicides and antifungal, antiprotozoal, and antihypertensive medications. Imidazole is part of the theophylline molecule, found in tea leaves and coffee beans, which stimulates the central nervous system. It is present in the anticancer medication mercaptopurine, which used in leukemia by interfering with DNA activities Imidazole also used in industry as a corrosion inhibitor on certain transition metals, such as copper. Conductivity of the copper decreases due to corrosion. Many compounds of industrial and technological importance contain imidazole derivatives. The thermostable polybenzimidazole imidazole fused to a benzene ring and acts as a fire retardant. Imidazole can also be found in various compounds which are used for photography and electronics. [65-66]
CONCLUSION:
Vast number of imidazole containing compounds have been synthesized and evaluated for their biological activity.Microwave reactions are extremely attractive to synthetic organic chemists owing to their ability to improve region and/or chemoselectivity and for ecofriendly & lesser reaction times. In case of imidazoles derivatives which are proved to be having great potential for the different pharmacological activities, therefore synthesis of these by using microwave techniques are found to be further advantageous.
Imidazole moiety have been most frequently studied, many of its analogs are active against various pathologicalconditions, which are discussed in brief in this article. Imidazole is an entity which has interesting physical and chemical properties, in the present article focus lies on analysis of these properties which in turn may be exploited for different pharmacological activities, like compounds having a 3,4,5-trimethoxyphenyl ring linked to either N-1 or C-4 position of the imidazole entity gave an interesting profile of cytotoxicity with specific activity against leukemia cell lines, combination of indole-imidazole compounds formed demonstrated substantial in vitro anti proliferative activities against cancer cell lines, effective substitutions are also made in the entity which resembles structures of various natural compounds whose anti cancerous activity has already been examined. Substitutions are discussed in pharmacological actions as anti neoplastic agent.
On the basis of various literature survey imidazole derivatives show various activity against antimicrobial, anti-inflammatory, analgesic, antitubercular, anticancer etc. The possible improvements in the activity can be further achieved by slight modifications in the substituents on the basic imidazole nucleus. Having structural similarity with histidine imidazole compound can bind with protein molecules with ease compared to the some other heterocyclic moieties. Thus imidazole offers better pharmacodynamic characteristics. Furthermore, some imidazoles drugs, at high concentrations, could exert direct inhibitory effects on membranes, without interference with sterols and sterol esters. Various recent new drugs developments in imidazoles derivatives show better effect and less toxicity.
Thus can say Imidazole is a moiety which had been exploited in the past years for synthesizing various compounds having diverse pharmacological activities, and still it can be further utilized for future prospective against various pathological conditions and other uses.
REFERENCES:-
[1] Q Wilma and B Pascal, J. Med. Chem., 1999, 42, 2737.
[2] A Stephen, D Munk, A Harcourt, J. Med. Chem, 1997, 40, 18.
[3] D. A. Williams and T. L. Lemke; Foye’s Principles of medicinal chemistry, Williams and Wilkins, Lippincott, 2002, 5, 36.
[4] S. N. Pandeya, A Text Book of medicinal chemistry, SG publisher, Grantham, 2004, 1(3), 2-3.
[5] H. Singh and. V.K. Kapoor, Medicinal and Pharmaceutical Chemistry, Vallabh Prakashan, Delhi, 2008, 2, 1 -2.
[6] Lednicer D, Mitscher L.A, In Organic Chemistry of Drug Synthesis, Wiley Interscience NewYork, 1997, 1, 226.
[7] A.R. Katritzky, Rees; Comprehensive Heterocyclic Chemistry. Pergamon press, Oxford, 1984, 5, 469-498.
[8] M.R. Grimmett; Imidazole and Benzimidazole Synthesis, Academic Press, New york, 1997, 2, 228.
[9] E.G. Brown; Ring Nitrogen and Key Bio molecules, Kluwer Academic Press, Oxford, 1998, 8, 190.
[10] A.F. Pozharskii; Heterocycles in Life and Society, Chichester, Newyork, 1997, 301
[11] TL Gilchrist, Heterocyclic Chemistry, John Wiley & Sons The Bath press, New york 1985.
[12] The Chemistry of Heterocyclic Compounds, Imidazole and Its Derivatives, John Wiley and Sons, New York, 2006, 13.
[13] Bhatnagar A, Sharma P,K, Kumar N, Inter. J. Pharm Tech Res., 2011, 3( 1), 268.
[14] Kartaraski B, Comp.Hetro, Chem., 1984, 5, 345
[15] M.K. Jain, S.C. Sharma, Modern Organic Chemistry, Vishal publication, Kolkata, 2005,809.
[16] Bhatnagar A, Sharma P,K, Kumar N, Inter. J. Pharm Tech Res., 2011, 3( 1), 268.
[17] Baroniya S, Anwer Z, Sharma P.K, Dudhe R, Kumar N, Der Pharmacia Sinica., 2010, 1 (3), 172-182.
[18] E. Lunt ,C.G. Newton ,C. Smith,G.P. Stevens, J. Med. Chem.,1987, 30 (2), 357- 66.
[19] Robert C, Elderfield, Heterocyclic compound, 1957, 5, 744.
[20] Wallach Ber, 1881, 14,735, Wallach 7 Strickerber, 1880, 13, 51, Wallach & Schulze, Ber, 1880, 13, 1514
[21] I.L.Finar, Stereochemistry and chemistry of natural products, Organic chemistry, Pearson Education, South Asia, 2006, 2, 622, 629.
[22] Robert C, Elderfield, Heterocyclic compound, 1957, 5, 744.
[23] S Sultan, S. S Ali, Der Pharma Chem, 2011, 3(1), 518-522.
[24] D.S. Ermolat , E.P. Svidritsky, E.V. Babaev , E.V. Eycken , Tetrahedron Lett., 2009, 5218–5220.
[25] P.V.Frank, K.S. Girish, B. kalluraya, J. Chem. Sci., 2007, 119, 1, 41–46.
[26] A.Marek, J.Kulhanek, M.Ludwig F.Bures, B.Sirivennela, S.Smarani, H.P.Kumar, R. Suthakaran, Molecules., 2007, 12, 1183-1190.
[27] M.Y. Pathan, V.V. Paike, P.R. Pachmase, S.P. More, S.S. Ardhapure, R.P. Pawar, ARKIVOC., 2006, 205-210.
[28] N. Zhao, Y. Wang , J Yang , J. of the Chinese Chem. Soci., 2005, 52 , 535-538.
[29] Y. Kawashita, M. Hayashi, Molecules, 2009, 14, 3073-3093.
[30] D.S. Ermolat , B. Savaliya , A. Shah, E. Van der., Mol Divers., 2011, 15(2), 491
[31] S. lupsor, I. Tarcomnicu, F. Aonofriesei, M. Iovu, N.putochin, J. Ber., 1922, 55, 2749.
[32] M.D.Le Bas, D.F. O'Shea, J Comb Chem., 2005, 7(6), 947-51.
[33] M. Suzuki, S Madesa, K. Matsumot, Boll chem farm, 1986, 34(8), 3111-3120.
[34] F Suzuki, T Kuroda, T Tamru, J. Med. Chem., 1992, 35(15), 2863-2870.
[35] S.A. El – Feky, Z.K. Abdel – Samii, Pharmazie, 1995, 5, 341-343.
[36] L.Isikdag, A Meric, Boll chem Farm. 1999, 138(1), 24-29.
[37] D.W. Robertson, E.E. Beedle, J.H. Krushinski, G.D. Pollock, H. Willson, J.S. Wyssvl, J. Med. Chem.,1985, 28(6), 717-27.
[38] P.W. Erhardt, A.A Hagdon, D. Davey, C.A., Venepalli, Griffin C.W., Gomez R.P., Wiggins J.R., Ingebretsen W.R., Pang D, J.Med. Chem., 1989, 32 (6), 1173-6.
[39] R.A. Johnson, S.M. Huong, E.S. Huang, Anti viral research. 1999, 41 (3), 101-111.
[40] M.D. Brewer, R.J. Dorgan, B.R. Manger, P. Mamalis, R.A. Webster, J. Med. Chem., 1987, 30 (10),1848-53 .
[41] J.A. Nathanson, Mol. Pharmacol., 1985, 28(3), 254-68.
[42] L.I. Kruse, C. Kaiser, J.S. Frazee, E. Garvey, E.L. Hilbert, W.A. Faulkner, K.E. Flaim, J. Med. Chem. 1986, 29(12) , 2465-72.
[43] H.S. Liyk Hsu, H. Kiyota, M. Segawa, J. Bio chem.., 1998, 23(3), 416-422.
[44] E. Lunt, C.G. Newton, C. Smith, G.P. Stevens, M.F. Stevens, C.G. Straw, R.J. Walsh, J. Med. Chem.,1987, 30(2), 357- 66.
[45] R. V. Shingalapur, K. M. Hosamani, R.S. Keri, European Journal of Medicinal Chemistry., 2009, 44, 4244–4248.
[46] D. Sharma, B. Narasimhan, P. Kumar, V. Judge, R. Narang, E. De Clercq, J. Balzarini, European Journal of Medicinal Chemistry., 2009, 44, 2347–2353.
[47] D. Zampieri, M. G. Mamolo, L. Vio, E. Banfi, G. Scialino, M. Fermeglia, M. Ferrone and S. Pricl, Bioorganic & Medicinal Chemistry., 2007, 15, 7444–7458.
[48] M.T. Shreenivas, B.E. Kumara Swamy,G.R. Srinivasa,B.S. Sherigara , Der Pharma Chemica., 2011, 3(2),156-161.
[49] A. Puratchikodya and M. Doble, Bioorganic & Medicinal Chemistry, 2007, 15, 1083–1090.
[50] K. C.S. Achar, K. M. Hosamani, H. R. Seetharamareddy, European Journal of Medicinal Chemistry., 2010, 45, 2048–2054.
[51] Y. Özkay , I. Iskar , Z. Incesu , G. e. Akalin , European Journal of Medicinal Chemistry., 2010, xxx , 1-9.
[52] H. M. Refaat, European Journal of Medicinal Chemistry., 2010, 45, 2949-2956.
[53] C. Congiu, M. T. Cocco and V. Onnis, Bioorganic & Medicinal Chemistry Letters, 2008, 18, 989–993.
[54] R. V. Shingalapur, K. M. Hosamani, R.S. Keri, European Journal of Medicinal Chemistry, 2009, 44, 4244–4248.
[55] P. Gupta, S. Hameed, R. Jain, European Journal of Medicinal Chemistry, 2004, 39,805– 814.
[56] P. jyoti, T. K. Vinod, V. S.Shyam, C. Vinita, S. Bhatnagar , S Sinha ,A.N. Gaikwad and R. P. Tripathi, European Journal of Medicinal Chemistry, 2009, 44, 3350-3355.
[57] F.Hadizadeh , H. Hosseinzadeh, Vahideh-Sadat Motamed-Shariaty M. Seifib, S. Kazemi, Iranian J. of Pharm. Res., 2008, 7 (1), 29-33.
[58] D. Sharma, B. Narasimhan, P. Kumar, V. Judge, R. Narang, E. De Clercq, J. Balzarini, European Journal of Medicinal Chemistry., 2009, 44, 2347–2353.
[59] M. Tonelli , M. Simone , B. Tasso , F. Novelli , V. Boido , Bioorganic & Medicinal Chemistry, 2010, 18, 2937–2953.
[60] K. Bhandari , N. Srinivas , V. K. Marrapu , A. Verma , S. Srivastava ,S. Gupta, Bioorganic & Medicinal Chemistry Letters., 2010, 20, 291–293.
[61] D.D. Bhragual, N. Kumar, S. Drabu , J. Chem. Pharm. Res., 2010, 2(2), 345-349.
[62] I.K. Kostakis, N. Pouli, P. Marakos, O.C. Kousidou, A. Roussidis, G.N. Tzanakakisc and N.K. Karamanos, Bioorganic & Medicinal Chemistry., 2008, 16, 3445–3455.
[63] K.H. Chung, S.Y. Hong, H.J. You, R.E. Parka and C.K. Ryua, Bioorganic & Medicinal Chemistry, 2006, 14, 5795–5801.
[64] B. Storrie, E.A. Madden, Meth. Enzymol. 1990, 182, 217.
[65] Ü. Uçucu, N. Gündogdu and I. Isikadag, IL Farmaco., 2001, 56, 285-290.
[66] Al-Azzawi R. W. Master thesis. Baghdad University (Baghdad, 2007).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE