 About author:
About author:
Ashish Vishwakarma
Department of Pharmaceutics,
Chandigarh College of Pharmacy,
Mohali-140307
ashish1931@gmail.com
ABSTRACT-
Acceptability of any drug dosage form mainly depends upon its taste i.e. mouth feel. In the formulation of oro-dispersible tablet of bitter drug, the main challenge is to mask the bitter taste of drug, because the drug is dispersed and released in mouth. This is more essential in the formulation for pediatric and geriatric, bed ribbon and non-cooperative patients. There are many methods of taste masking but the main objective of this article is to explore method, technology and evaluation to mask taste of bitter drug by ion-exchange resins. Here we will study the formulation and evaluation of various oro-dispersible tablets of bitter drug. This is more economical, easiest and efficient method for taste masking.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1383
INTRODUCTION-
In the case of pediatric & geriatric patients, unpleasant taste should be avoided & leading to noncompliance which result decrease therapeutic efficacy. In the present scenario, this unpleasant & bitter taste of drug clash to the pharmacist. Therefore taste masking of bitter drug is very important. [1]
ANATOMY & PHYSIOLOGY OF TASTE-
The biological definition of taste (Gustation) - It is a chemical reaction arising from sensory responses of the four main taste perceptions: sweet, bitter, salt & sour.
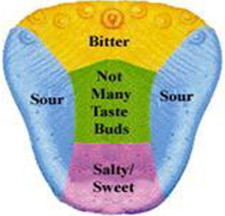
Fig-1
Taste sensation is arising by the signal transduction from the receptor organs for taste which are commonly known as taste buds. In mammals, taste buds are groups of 35-100 individual elongated “neutroepithelial” cells which are surrounded in exclusive structure in the adjacent epithelium, term as taste papillae. [2, 3]
Effect of age on taste buds-
Cells that make up the taste buds with age wear out , as a result taste buds begin to disappear from roof and the sides of the mouth excepttaste buds that’s are located over tongue. Remaining taste buds becomes less sensitive. Researchers have been proved that Smocking and eating of scalding food may damage to taste buds. This lacking of taste may lead to loss of appetite and poor nutrition.
Taste is a type of medium to experience the world of tastes for infants and young children. It is seen that children are more sensitive to certain taste than any adults, but because taste can be subjective. The mechanism that causes taste sensitivity in youngsters can be difficult to analyze. [4]
ORO-DISPERSIBLE TABLETS-
An orally disintegrating tablet (ODT) is defined as a solid dosage form that dissolves or disintegrates quickly in the oral cavity without the need for administration of water. The EP describes ODTs as »uncoated tablets intended to be placed in the mouth where they disperse rapidly before being swallowed« and as tablets which should disintegrate within 3 min. [5]
FDA defines ODT as »a solid dosage form which contains a medicinal substance or active ingredient which disintegrates rapidly within a matter of seconds when placed upon a tongue« [11]
ADVANTAGES OF ORO-DISPERSIBLE TABLETS-
* It has benefits like desired hardness, dosage uniformity, extremely easy administration & since no water is required for swallowing these tablets.
* These are suitable for geriatric, pediatric & travelling patients.
* These tablets display a fast & spontaneous de-aggregation in the mouth, soon after it comes in contact during deglutition. [5]
* The active ingredients in solution are more rapidly absorbed through the pre-gastric route from the mouth, pharynx & esophagus & through gastrointestinal epithelium to produce the desired effect. [6]
* ODTs are preferred for people suffering from dysphasia, institutional psychiatric patients as well as hospitalized patients suffering from a variety of disorders. [7,8]
* ODTs have also been found to be the dosage form of choice for patients suffering from nausea, vomiting or motion sickness. [9]
* Formulation of ODT has been studied as one of the ways to improve the bioavailability of poorly water soluble drugs & it has been observed that ODT increase the bioavailability of such drugs. [10]
[adsense:468x15:2204050025]
TASTE MASKING-
It is defined as the apparent reduction of an unpleasant taste by using suitable agent. Taste masking technologies are very important for improving Patient compliance & better therapeutic efficacy. Many oral drug delivery formulations have objectionable taste such as bitterness, saltiness or sourness. For those drugs taste masking is necessary.
METHODS OF TASTE MASKING-
1) Taste masking with flavors, sweeteners & amino acids.
2) Polymer coating of drug.
3) Formation of Inclusion complexes.
4) Ion- exchange resin complex.
5) Solid dispersion.Microencapsulation.
6) Mass extrusion.
7) Multiple emulsions.
8) Development of liposome.
9) Prodrug concept.
10) Spray drying technique.
11) Adsorption
12) By using lipophilic vehicles like lipids & recithins.
13) Formation of salt or derivatives.
14) Use of amino acids and protein hydrolysates.
15) By viscosity modification. [12]
There are many methods of taste masking of bitter drug which are written above but here we will study TASTE MASKING BY ION-EXCHAGE RESINS.
TASTE MASKING BY ION-EXCHANGE RESINS-
INTRODUCTION-
IERs are solid & suitably insoluble high molecular weight polyelectrolyte that can exchange their mobile ions of equal charge with the surrounding medium. [13] Synthetic ion exchange resins have been used in pharmacy & medicine for taste masking or controlled release of drug as early as 1950. [14] Bitter tasting drugs can be absorbed onto ion-exchange resins, thus effectively removing them from solution during the transit through the mouth, at salivary pH 6.7, remains in intact from making the drug unavailable for the taste sensation. Various studies have revealed that ion exchange resins are equally suitable for drug delivery technologies. [15]
CHEMISTRY-
An ion exchange resin is a polymer (normally styrene) with electrically charged sites at which one ion may replace another. Natural soils contain solids with charged sites that exchange ions, and certain minerals called zeolites are quite good exchangers. Ion exchange also takes place in living materials because cell walls, cell membranes and other structures have charges. In natural waters and in wastewaters, there are often undesirable ions and some of them may be worth recovering. For example, cadmium ion is dangerous to health but is usually not present at concentrations that would justify recovery. On the other hand, silver ion in photographic wastes is not a serious hazard, but its value is quite high. In either case, it makes sense to substitute a suitable ion such as sodium for the ion in the wastewater. Synthetic ion exchange resins are usually cast as porous beads with considerable external and pore surface where ions can attach. The resins are prepared as spherical beads 0.5 to 1.0 mm in diameter. These appear solid even under the microscope, but on a molecular scale the structure is quite open (Fig. 2). Whenever there is a great surface area, adsorption plays a role. If a substance is adsorbed to an ion exchange resin, no ion is liberated. Testing for ions in the effluent will distinguish between removal by adsorption and removal by ion exchange. Of course, both mechanisms may be significant in certain cases, and mass balances comparing moles removed with moles of ions liberated will quantify the amounts of adsorption and ion exchange. While there are numerous functional groups that have charge, only a few are commonly used for man-made ion exchange resins. These are:
• -COOH, which is weakly ionized to -COO¯
• -SO3H, which is strongly ionized to -SO3¯
• -NH2, which weakly attracts protons to form NH3+
• -secondary and tertiary amines that also attract protons weakly
• -NR3+, which has a strong, permanent charge (R stands for some organic group)
These groups are sufficient to allow selection of a resin with either weak or strong positive or negative charge. [15]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
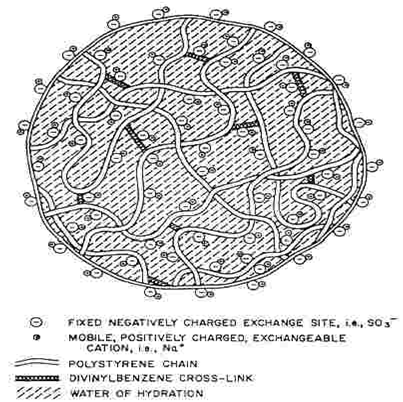
Fig-2 Expanded View of a Polystyrene Bed
CLASSIFICATION- Ion exchange resins are broadly classified into two main Categories, as Cation exchange resins and anion exchange
Resins (Fig. 3).
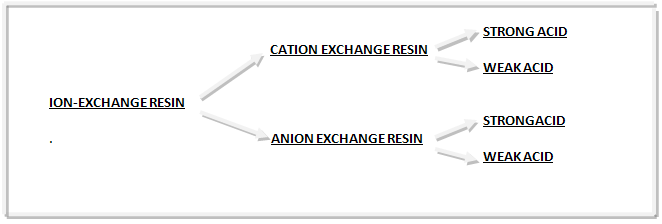
1. Cation Exchange Resins- whose exchangeable ions are positively charged: Cation exchange resins are prepared by the copolymerization of styrene and divinyl benzene and have sulfonic acid groups (-SO3H) introduced into most of the benzene rings. The mechanism of Cation exchange process can be represented by the following reaction:
Resin- - ex+ + C+ ®Resin- - C+ +ex+
Where, Resin- indicates a polymer with SO3-sites available for bonding with exchangeable Cation (ex+), and C+ indicates a Cation in the surrounding solution getting exchanged.
Cation exchange resins can be further classified into:
Strong Acid Cation Exchange Resins
Strong acid resins are so named because their chemical behavior is similar to that of a strong acid. These resins are highly ionized in both the acid (R-SO3H) and salt (RSO3Na) form of the sulfonic acid group (-SO3H). They can convert a metal salt to the corresponding acid by the reaction:
2(R-SO3 H) + NiCl2 ® (R-SO4) Ni + 2HCl
The hydrogen and sodium forms of strong acid resins are highly dissociated, and the exchangeable Na+ and H+ are readily available for exchange over the entire pH range. Consequently, the exchange capacity of strong acid resins is independent of the solution pH. These resins would be used in the hydrogen form for complete deionization; they are used in the sodium form for water softening (calcium and magnesium removal). After exhaustion, the resin is converted back to the hydrogen form (regenerated) by contact with a strong acid solution, or the resin can be convened to the sodium form with a sodium chloride solution. For the above reaction, hydrochloric acid (HCl) regeneration would result in a concentrated nickel chloride (NiCl2) solution.
Weak Acid Cation Exchange Resins
These resins behave similarly to weak organic acids that are weakly dissociated. In a weak acid resin the ionizable group is a carboxylic acid (COOH) as opposed to the sulfonic acid group (SO3H ) used in strong acid resins. The degree of dissociation of a weak acid resin is strongly influenced by the solution pH. Consequently, resin capacity depends in part on the solution pH. A typical weak acid resin has limited capacity below a pH of 6.0, making it unsuitable for deionizing acidic metal finishing wastewater.
2. Anion Exchange Resins- whose exchangeable ions are negatively charged: These are prepared by first chlormethylating the benzene rings of styrene-divinyl benzene copolymer to attach CH2Cl groups and then causing these to react with tertiary amines such as triethylamine. The mechanism of anion exchange process can be represented by the following reaction:
Resin+ - ex - + A- ® Resin+ - A- + ex-
Where, Resin+ indicates a polymer with N+ sites available for bonding with exchangeable anion (ex-), and A- indicates Cation in the surrounding solution getting exchanged.
Anion exchange resins can be further classified into:
Strong Base Anion Exchange Resins
Like strong acid resins, strong base resins are highly ionized and can be used over the entire pH range. These resins are used in the hydroxide (OH) form for water deionization. They will react with anions in solution and can convert an acid solution to pure water:
R-NH3OH + HCl ® R-NH3Cl + HOH
Regeneration with concentrated sodium hydroxide (NaOH) converts the exhausted resin to the OH form. [16]
METHOD OF IER-DRUG COMPLEX FORMATION-
Ionexchange resins may be supplied in case of cation exchangers as sodium, potassium or ammonium salts and of anion exchangers usually as the chloride. It is frequently necessary to convert a resin completely from one ionic form to another.
Charged drugs are normally loaded on to ion exchange resins by two methods, viz, column method and batch method.
Column method- In this method a highly concentrated drug solution is passedthrough a column of resin particles. Since the reaction is anequilibrium phenomenon, maximum potency and efficiency is bestobtained by the column method.
Batch method- In this method the drug solution is agitated with a quantity of resinparticles until equilibrium is established.The reaction involved during complexation of drug with resin maybe indicated as follows [19]
Re-COO-H+ + Basic drug+ → Re-COO- Drug++ H+
Re-N (CH3) +3Cl- + Acidic drug- → Re-N (CH3)+3Drug- +Cl-
Upon ingestion, drugs are most likely eluted from cation exchange resins by H+, Na+ or K+ ions and from anion exchange resins by Cl-, as these ions are most plentiful available in gastrointestinal secretions.
Typical reactions involved in the gastrointestinal fluids may be envisaged as follows:
In the stomach:
Re-COO- Drug + + HCl → Re-COOH + Drug Hydrochloride
Re-N (CH3)+3 Drug - + HCl → Re-N(CH3)3 Cl + Acidic drug
In the intestine:
Re-COO- Drug + + NaCl → Re-COONa + Drug Hydrochloride
Re-N (CH3)+3 Drug - + NaCl → Re-N(CH3)3 Cl + Sodium salt of drug
CONFIRMATION OF COMPEXATION-
FTIR studies-
Drug, Resin, and physical mixture of both and DRC are subjected to Fourier transform infrared spectroscopy (FTIR) studies. Samples are prepared using KBr disc method and spectra are recorded over the range 400 to 4,000 cm−1. Spectra are analyzed for drug–resin interactions and functional groups involved in the complexation process.
Powder X-ray diffraction studies-
X-ray diffractograms of Drug, Ion-exchange resin, and DRC are recorded using Philips PW 3710 deffractometer and analyzed for interactions between drug and resin and confirmation of complexation.
Thermal analysis-
Differential scanning calorimetry (DSC) is carried out using Mettler Toledo 823e instrument equipped with intracooler. Indium zinc standards are used to calibrate the temperature and enthalpy scale. The samples are hermetically sealed in aluminum pans and heated over the temperature range 30°C to 300°C with heating rate of 10°C/min. Inert atmosphere is provided by purging nitrogen gas flowing at 40 mL/min.
PROPERTIES OF ION-EXCHANGE RESINS-
1) PARTICLE SIZE & FORM-
The rate of ion exchange reaction depends on the size of the resin particles. Decreasing the size of the resin particles significantly decreases the time required for the reaction to reach the equilibrium with the surrounding medium; hence larger particle size affords a slower release pattern.
2) POROSITY & SWELLING-
Porosity is defined as the ratio of volume of the material to its mass. The limiting size of the ions, which can penetrate into a resin matrix, depends strongly on the porosity. The porosity depends upon the amount of cross-linking substance used in polymerization method. The amount of swelling is directly proportional to the number of hydrophilic functional groups attached to the polymer matrix and is inversely proportional to the degree of DVB cross linking present in the resin.
3) CROSS LINKING-
The percentage of cross-linking affects the physical structure of the resin particles. Resins with low degree of cross-linking can take up large quantity of water and swell into a structure that is soft and gelatinous. However resins with high DVB content swell very little and are hard and brittle. Cross-linkage has dramatic effect on loading efficiency. It affects porosity and swelling properties of resin. Low cross-linking agents remarkably upon hydration. Higher grade have finer pore structure thus reducing loading efficacy with increase in cross- linking. Low cross linkage increase loading efficacy but also increases release rates.(23)
4) MOISTURE CONTENT-
A physical property of the ion exchange resins that changes with changes in cross-linkage are the moisture content of the resin. For example, sulfonic acid groups (-SO3H) attract water, and this water is tenaciously held inside each resin particle. The quaternary ammonium groups of the anion resins also behave in a similar manner.(24)
5) EXCHANGE CAPACITY-
The exchange capacity refers to the number of ionic sites per unit weight or volume (mEq. Per gram or meq per ml). The weight basis values (mEq. per gm) are much higher than the volume based exchange capacity since the wet resin is highly hydrated. The exchange may limit the amount of drug that may be adsorbed on a resin, hence affect potency of the complex. Carboxylic acid resins derived from acrylic acid polymers have higher exchange capacities (10meq. /gm) than sulfonic acid (about 4meq. / gm) or amine resins because of bulkier ionic substituent and the polystyrene matrix. Therefore, higher drug percentages may often be achieved with carboxylic acid resins. (25)
6) ACID BASE STRENGTH-
It depends on various inorganic groups incorporated into resins. Resins containing sulphonic, phosphoric or carboxylic acid exchange groups have approximate pKa values of <1, 2, 3 and 4-6 respectively. Anionic exchangers are quaternary, tertiary or secondary ammonium groups having pKa values of >13, 7-9 or 5-9 respectively. The pKa values of resin will have significant influence on the rate at which the drug will be released in the gastric fluid.(26)
7) STABILITY-
The ion exchange resins are inert substances at ordinary temperature and excluding the more potent oxidizing agent are resistant to decomposition through chemical attack. These materials are indestructible. They get degraded and degenerated in presence of gamma rays.(23)
8) PURITY AND TOXICITY-
Since drug resin combination contains 60% or more of the resin, it is necessary to establish its toxicity. Commercial product cannot be used as such. Careful purification of resins is required. Resins are not absorbed by body tissue and are safe for human consumption. Test for toxicological tolerance showed that it does not have any pronounce physiological action at recommended dosage and is definitely non-toxic.(23)
9) SELECTIVITY OF THE RESINS FOR THE COUNTER-ION-
Resin selectivity is attributed to many factors. Since ion exchange involves electrostatic forces, selectivity at first glance should depend mainly on the relative change and the ionic radius of the (hydrated) ion competing for an exchange site. Factors other than size and charge also contribute to the selection of one counter ion in preference to another. The extent of adsorption increases with -
1. The counter ion that in addition to forming a normal ionic bond with the functional group of an exchanger also interacts through the influence of van der Waal forces with the resin matrix.
2. The counter ion at least affected by complex formation with its co-ion or non-exchange ion.
3. The counter ions that induce the greater polarization. These factors, together with the effect of the size and charge of an ion on exhibiting certain selectivity toward a resin, are at best only general rules, and as a consequence there are many exceptions to them. (24)
PHARMACEUTICAL GRADE ION-EXCHANGE RESINS-
|
NAME |
FUNCTIONALITY |
POLYMER BACKBONE |
|
AMBERLITETM IRP 64 |
WEAK ACID COO- |
CROSSLINKED POLYACRYLIC |
|
AMBERLITETM IRP 69 |
STRONG ACID SO3- |
SODIUM STYRENE-DIVINYL BENZENE |
|
AMBERLITETM IRP 88 |
WEAK ACID COO- |
CROSSLINKED POLYACRYLIC |
|
AMBERLITETM IR 120 |
STRONG ACID SO3H |
STYRENE-DIVINYL BENZENE |
|
AMBERLITETM IR C50 |
WEAK ACID COOH |
METHAACRYLIC ACID DIVINYL BENZENE |
|
AMBERLITETM IR 400 |
STRONG BASE N+R3 |
STYRENE-DIVINYL BENZENE |
|
AMBERLITETM IR4B |
WEAK BASE N+R2 |
STYRENE-DIVINYL BENZENE |
|
DOWEX 1 |
STRONG BASE N+R3 |
POLY STYRENE-DIVINYL BENZENE |
|
DOWEX2 |
WEAK BASE N+R2 |
POLY STYRENE-DIVINYL BENZENE |
|
DOWEX 50 |
STRONG ACID SO3H |
POLY STYRENE-DIVINYL BENZENE |
|
DOSHION P544(R) |
WEAK ACID COO- |
METHAACRYLIC ACID DIVINYL BENZENE |
|
DUOLITE AP 143 |
STRONG BASE N+R3 |
STYRENE-DIVINYL BENZENE |
|
KYRON T-104 |
WEAK ACID COO- |
METHAACRYLIC ACID DIVINYL BENZENE |
|
KYRON T-114 |
WEAK ACID COO- |
METHAACRYLIC ACID DIVINYL BENZENE |
|
KYRON T-134 |
WEAK ACID COO- |
METHAACRYLIC ACID DIVINYL BENZENE |
|
KYRON T-154 |
STRONG ACID SO3H |
STYRENE-DIVINYL BENZENE |
|
TULSION 335 |
WEAK ACID COOH |
METHAACRYLIC ACID DIVINYL BENZENE |
|
TULSION 339 |
WEAK ACID COOK |
METHAACRYLIC ACID DIVINYL BENZENE |
|
TULSION 344 |
STRONG ACID SO3Na |
SODIUM STYRENE-DIVINYL BENZENE |
|
PUROLITE C100 HMR |
STRONG ACID SO3H |
STYRENE-DIVINYL BENZENE |
|
PUROLITE C102 DR |
WEAK ACID COO- |
METHAACRYLIC ACID DIVINYL BENZENE |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
PHARMACEUTICAL GRADE IER- INDION-
|
PRODUCT NAME |
INDION 204 |
INDION 214 |
INDION 224 |
INDION 234 |
|
Applications |
Taste masking of bitter drugs such as Norfloxacin, Ofloxacin |
Taste masking of bitter drugs such as Azithromycin |
Sustained release agent in drug formulations |
Taste masking of bitter drugs such as Ciprofloxacin, Chloroquin phosphate
|
|
Matrix type |
Cross linked polyacrylic |
Cross linked polyacrylic |
Styrene DVB |
Cross linked polyacrylic |
|
Functional Group |
-coo- |
-coo- |
-SO3- |
-coo- |
|
Standard Ionic Form |
H+ |
H+ |
H+ |
K+ |
|
Particle size range, mm |
≤ 0.15 |
≤0.15 |
0.2 – 1.2 |
≤0.15 |
|
% Moisture |
≤ 5 |
≤ 5 |
≤ 3 |
≤ 10 |
|
Total Exchange Capacity meq/g, dry |
10.0 |
10.0 |
4.8 |
NA |
|
PRODUCT NAME
|
INDION 234S |
INDION 244 |
INDION 254 |
INDION 264 |
|
Applications |
Taste masking of bitter drugs as well as tablet disintegration |
Sustained release agent in drug formulations |
Sustained release agent in drug formulations. Product meets specs. Of Sodium Polystyrene sulfonate , USP
|
Stabilization of Vitamin B12 |
|
Matrix type |
Cross linked polyacrylic |
Styrene/DVB |
Styrene/DVB |
Cross linked polyacrylic
|
|
Functional Group |
-coo- |
-SO3- |
-SO3- |
-coo-
|
|
Standard Ionic Form |
K+ |
H+ |
Na+ |
H+ |
|
Particle size range, mm |
≤ 0.075 |
≤ 0.15 |
≤ 0.15 |
≤ 0.15 |
|
% Moisture |
≤ 10 |
≤ 10 |
≤0.15 |
≤ 5 |
|
Total Exchange Capacity meq/g, dry |
NA |
4.5* |
NA |
10.0* |
|
PRODUCT NAME
|
INDION 284 |
INDION 294 |
INDION 404 |
INDION 414 |
|
Applications |
Sustained release agent in drug formulations |
Tablet disintegrant/ Taste masking. Product meets specs. Of Polacrilin Potassium, USP
|
Treatment of hyperkalaemia. Product meets specs. Of Calcium polystyrene sulfonate, BP |
As superdisintegrant in mouth disperse tablets, iron & calcium pellets |
|
Matrix type |
Styrene/DVB |
Cross linked polyacrylic
|
Styrene/DVB |
Cross linked polyacrylic |
|
Functional Group
|
-SO3- |
-coo- |
-SO3- |
-coo- |
|
Standard Ionic Form |
Na+ |
K+ |
Ca++ |
K+ |
|
Particle size range, mm |
0.3 – 1.2 |
≤ 0.15 |
≤ 0.15 |
≤ 0.15 |
|
% Moisture |
≤ 70 |
≤ 10 |
≤ 8 |
≤ 10 |
|
Total Exchange Capacity meq/g,
|
1.0 |
NA |
NA |
NA |
|
PRODUCT NAME |
INDION 454 |
INDION 464 |
|
Applications |
Cholesterol reduction and taste masking of bitter drug |
Taste masking of bitter substances Ex. Nicotine |
|
Matrix type |
Cross linked Polystyrene |
Cross linked Polymethacrylic |
|
Functional Group |
- N+R3 |
- COO- |
|
Standard Ionic Form |
Chloride |
H+ |
|
Particle size range, mm |
≤0.15 |
≤ 0.15 |
|
% Moisture |
≤12 |
≤ 5 |
|
Total Exchange Capacity meq/g, |
N.A. |
9.5 |
Desired properties of pharmaceutical grade IERs-
a) Fine, free flowing powders
b) Particle size of 25 - 150 microns
c) Contain functional group that capable of exchanging ions and/or ionic groups
d) Insoluble in all solvents, all pH’s
e) Not absorbed by body
f) Do not have a defined molecular weight
Advantages of Ion Exchange Resin as a Taste Masking Agent in Orodispersible Tablet-
1. These method requires few and simple equipment.
2. The numbers of excipients required are less and are easily available.
3. The Bioavailability of drug is not altered.
4. The resins are easy to process and has high margin of safety.
5. The manufacturing can be carried out at room temperature and no other special experimental conditions are required
6. It has low cost of manufacturing. (23)
TASTE MASKED ORODISPERSIBLE TABLETS HAVING IERs AS A TASTE MASKING AGENT-
1. FORMULATION OF TASTE MASKED ORO-DISPERSIBLE TABLETS OF METFORMIN HYDROCHLORIDE-(27)
INTRODUCTION-
METFORMIN HYDROCHLORIDE is an oral biguinide agent, used in the management of non-insulin dependent (type-2) diabetes mellitus. The purpose of this article is to mask the taste of drug by complexation with indion-234 which is an IER & formulation of oro-dispersible tablet.
PREPARATION OF RESINATE-
Resinate was prepared using batch method. An accurately weighed amount of resin (100mg) was placed in a beaker containing 50 ml of demonized water. Accurately weighed100 mg of metformin hydrochloride was added to the resin solution and stirred for 180 min. The mixture was filtered through whatman filter paper and residue was washed with 50 ml of deionised water to remove any uncomplexed drug. Unbound drug in filtrate was estimated at 233.5 nm and drug-loading efficiency was calculated.
FORMULATION DESIGN-
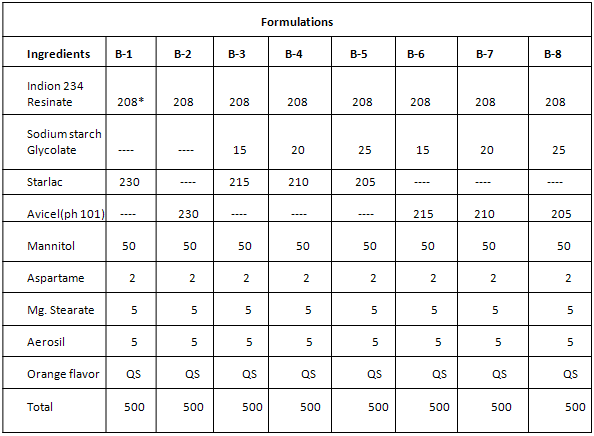
EVALUATION OF TABLETS-
EVALUATION OF PHYSICAL CHARACTERISTICS OF TABLETS (BATCH B-1-B-8)-
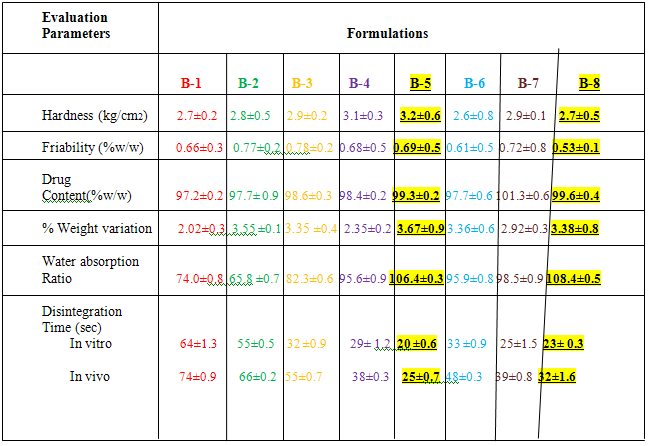
After evaluation Batch B-5 with 5% sodium starch glycolate and starlac and Batch B-8 with 5% sodium starch glycolate and avicel PH 101 was found to be optimum batch as it shows lowest disintegration time with desired friability values.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
2. FORMULATION OF TASTE MASKED ORO-DISPERSIBLE TABLETS OF ONDANSETRON HYDROCHLORIDE-(28)
INTRODUCTION-
Ondansetron hydrochlorideis commonly used as anti emetic. But it is a very bitter drug and slightly soluble in water. So in the work under taken an attempt was made to mask the taste and to formulate into a dispersible tablet by complexation with ion exchange resins, which also acts as super disintegrating agents. Since, these tablets can be swallowed in the form of dispersion; it is suitable dosage form for pediatric and geriatric patients. Cationic exchange resins like Indion-204, Indion-234 and Tulsion-335 were utilized for the sorption of drug.
PREPARATION OF DRUG-RESINATE COMPLEX-
Ion exchange resins like Indion- 204 Tulsion-335, Indion-234 were pretreated with 1N HCl and1N NaOH in order to remove impurities. Drug and resins were mixed in various ratios 1:1 to 1:6 on weight basis and stirred at magnetic stirrer for a period of 4 to 8 hours using deionised water. The resinate obtained was separated by filtration and dried. Non bitter complex was yielded at 1:6,1:5 and 1:6 drug to resin ratio using deionised water of pH 7 and also maximum percentage drug loading( 96.5, 99.1 & 98.8 %) was determined at the same ratio.
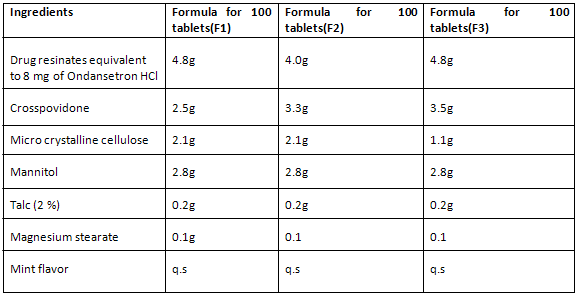
FORMULATION OF ORO-DISPERSIBLE TABLETS-
Formulation of tablets formulated with resins Indion-204 (F1), Indion-234 (F2) & Tulsion-335(F3)
EVALUATION OF TABLETS-
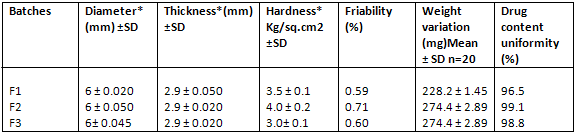
All the tablets passed weight variation test, as percentage weight variation was within the pharmacopoeial limits i.e.±7.5%. To be complied with the pharmacopoeial standards, dispersible tablets must disintegrate within 3 minutes. But formulated products have exhibited very less disintegrating time (i.e.14, 21 and 20 seconds), indicating that all three formulations were suitable for oro-dispersible tablets.
3.FORMULATION OF TASTE MASKED ORO-DISPERSIBLE TABLET OF METOCLOPRAMIDE HYDROCHLORIDE-(29)
INTRODUCTION-Metoclopramide hydrochloride a derivative of paraaminobenzoic acid is a commonly prescribed drug used for the management of gastrointestinal disorders such as gastric stasis, gastro esophageal reflux and for the prevention of cancer chemotherapy- induced emesis. In general, emesis is preceded with nausea and in such condition it is difficult to administer drug with a glass of water; hence it is beneficial to administer such drugs as ODTs. Metoclopramide HCl is an intensely bitter drug; hence, if it is incorporated directly into an ODT the main objective behind formulation of such a dosage form will definitely get futile.
Here we formulate oro-dispersible tablet by taste masking with Indion234.
PREPARATION OF DRUG RESIN COMPLEX-
Metoclopramide Hydrochloride resinate was prepared using a batch process. For preliminary study, we optimized the ratio of resin to drug at 1:3. Resin (3 g) was placed in an Erlenmeyer flask and then 250 ml of distilled water was added. The mixture was shaken in the water bath at 370C. The supernatant was collected and assayed spectrophotometrically at a wavelength of 273 nm (Techcomp, UV 2300, and Shanghai, Japan) to determine the drug-loading equilibrium time. Then, the Metoclopramide Hydrochloride resinates were separated from the filtrates by filtration, washed several times with distilled water, dried overnight at 500C and kept in a desiccators.
FORMULATION OF ORO-DISPERSIBLE TABLET-
|
Sr. No. |
Tablet Ingredient per Tablet (mg) |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
|
1 |
DRC |
43.3 |
43.3 |
43.3 |
43.3 |
43.3 |
43.3 |
43.3 |
|
2 |
Mannitol |
96 |
- |
48 |
64 |
72 |
32 |
24 |
|
3 |
MCC |
- |
96 |
48 |
32 |
24 |
64 |
72 |
|
4 |
Crosspovidone |
7 |
7 |
7 |
7 |
7 |
7 |
7 |
|
5 |
Aspartame |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
6 |
Magnesium Stearate |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
|
7 |
Flavor |
0.7 |
0.7 |
0.7 |
0.7 |
0.7 |
0.7 |
0.7 |
*-Drug resin complex (DRC) equivalent to 10 mg of Metoclopramide HCl.
EVALUATION OF TABLET-
|
BATCH NO. |
FRIAB * |
HARDNESS KG/CM2 ** |
THICK ** |
% WEIGHT # |
DISINTEGRA SEC* |
WETTING TIME (SEC)@ |
%WATER ABSORPTION RATIO @ |
|
F1 |
0.78± 0.12 |
4.13±0.24 |
2.40 |
148.44± |
57.52±0.41 |
10.04± 1.44 |
84.64 ± 0.34 |
|
F2 |
0.75± 0.34 |
4.23±0.30 |
2.49 ± 0.02 |
150.71± 1.85 |
37.50 ± 1.04 |
10.91± 0.90 |
82.64 ± 0.21 |
|
F3 |
0.73± 0.36 |
4.19±0.29 |
2.50 ± 0.08 |
150.64± 1.66 |
25.55 ± 1.32 |
10.95± 1.31 |
88.80 ± 0.15 |
|
F4 |
0.76± 0.22 |
4.14±0.21 |
2.42 ± 0.09 |
151.70± 1.33 |
30.37 ± 0.66 |
11.09± 0.61 |
83.75 ± 0.20 |
|
F5 |
0.75± 0.44 |
4.25±0.26 |
2.45 ± 0.04 |
149.12± 2.23 |
45.24 ± 0.75 |
11.60± 1.23 |
78.8 ± 0.35 |
|
F6 |
0.71± 0.24 |
4.10±0.27 |
2.47 ± 0.03 |
150.57± 1.79 |
49.94 ± 1.34 |
11.68± 0.40 |
79.50 ± 0.32 |
|
F7 |
0.72± 0.59 |
4.45±0.12 |
2.52 ± 0.07 |
150.94± 1.45 |
57.09 ± 0.76 |
13.34± 1.10 |
75.43 ± 0.60 |
*n=3, ** n=6, # n=20, @n=3
Among seven batches, batch F3 which contain MCC and Mannitol in 1:1 ratio along with 7 mg of Crosspovidone was selected as optimized batch because of its lowest disintegration time and highest drug release.
4. FORMULATION OF TASTE MASKED ORODISPERSIBLE TABLETS OF TRAMADOL –(30)
INTRODUCTION-
Tramadol is a centrally acting opioid analgesic structurally related to codeine and morphine used in the treatment of moderate to severe pain in diverse conditions. Combined with low dependence/abuse potential, it has proven to be of significant advantage over other agents, especially in the elderly.
Here bitter taste of Tramadol is masked by forming complex with Tulsion 335.
PREPARATION OF DRC-
Batch method was used to prepare drug–resin complex (DRC). Five grams of Tulsion 335 was allowed to swell for 90 min in a beaker containing 200 ml of deionized water of conductivity less than 2. Then, 5 g of Tramadol HCl was added to it and stirred for 300 min. The mixture was filtered through Whatman filter paper no. 41. The residue was washed with 100 ml of deionized water and dried.
FORMULATION OF ORO-DISPERSIBLE TABLET-
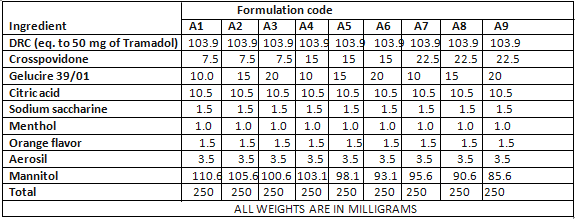
EVALUATION OF TABLETS-
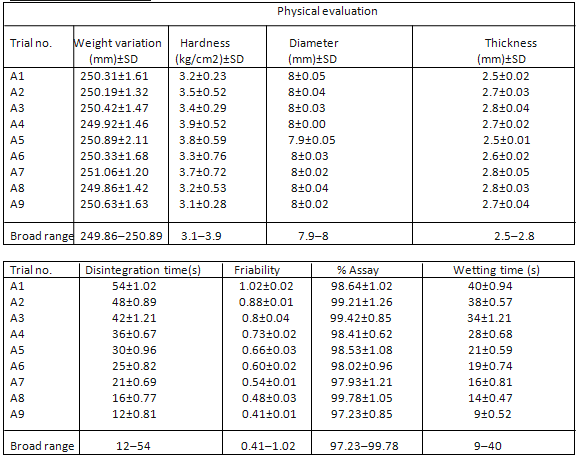
The outcomes of various evaluation parameters are shown in Tables. The dissolution studies indicated 100% drug release within 20 min from all batches. All batches showed DT less than a minute and friability less than 1.02%.
5. FORMULATION OF ORO-DISPERSIBLE TABLET OF AMBROXOL HYDROCHLORIDE-(31)
INTRODUCTION-
Ambroxol hydrochloride (HCL) is a potent mucolytic capable of inducing bronchial secretion. It is used in the treatment of asthma, bronchitis, and cough. But it is a very bitter drug and slightly soluble in water. Thus, in the work under taken, an attempt was made to mask the taste and to formulate into a oro-dispersible tablet by complexation with ion exchange resins (Indion-204 and Indion-234), which also acts as super disintegrating agents.
PREPARATION OF DRC-
Ion exchange resins like Indion 204 and Indion 234 were pretreated with 1N HCl and 1N NaOH in order to remove impurities. Drug and resins were mixed in various ratios 1:1 to 1:6 on weight basis and stirred at magnetic stirrer for a period of 4 to 8 h using deionized water. The resinate obtained was separated by filtration and dried. Non bitter complex was yielded at 1:5 and 1:6 drug to resin ratio using deionized water of pH 7 and also maximum percentage drug loading (96.5 and 98.8 percent) was determined at the same ratio.
FORMULATION OF ORO-DISPERSIBLE TABLET-
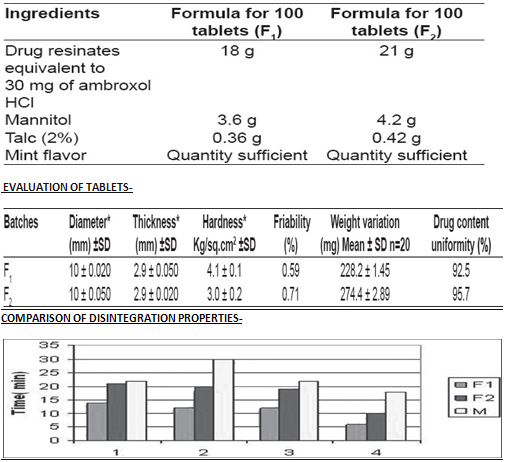
DRUG RELEASE PROFILES-
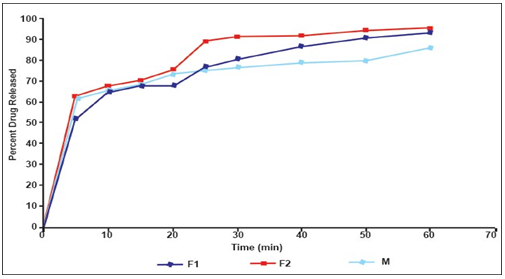
M stands for Marketed preparation
All the tablets passed weight variation test, as % weight variation was within the pharmacopoeial limits i.e., ±7.5%. To be compliance with the pharmacopoeial standards, dispersible tablets must disintegrate within 3 min. But, formulated products have exhibited very low disintegrating time (i.e., 14 and 21 seconds), indicating that both formulations were suitable for oro-dispersible tablets. In the in- vivo disintegration time, wetting time was observed to be very fast in F 1 formulation when compared to F 2 and marketed product.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
5. FORMULATION OF TASTEMASKED ORO-DISPERSIBLE TABLET OF DEXTROMETHORPHAN-(32)
INTRODUCTION-
Dextromethorphan hydrobromide is an antitussive drug widely used in the treatment of cough. The drug has a very bitter taste. It is given in doses of 15 mg every 4 h. The drug is commercially produced in various dosage forms including liquids, fast and sustained release tablets. These dosage forms have received poor compliance from geriatric and pediatric patients because of their bitter taste and difficulty for swallowing. In this regard, ODTs formulation of this drug is a challenge that will be an alternative dosage form to improve patient compliance. Therefore, this study was aimed at preparing and evaluating ODTs using a strongly cationic resin, Amberlite® IRP-69, to mask the bitter taste of dextromethorphan hydrobromide.
PREPARATION OF DRC-
A dextromethorphan hydrobromide resinate was prepared by a batch process. The resin, Amberlite® IRP-69, was weighed (30 g) and placed in a beaker that contained
1 l of 1.5% w/v dextromethorphan hydrobromide solution. The slurry was stirred on a magnetic stirrer for 24 h. In an initial experiment, this time period was proven to reach the equilibrium of drug loading. The resinate was collected by filtration, washed several times with copious amounts of deionized water, and dried at 50°C overnight in a hot air oven. The final resinate was kept in a tightly sealed container. Drug content in the resinate was determined by an elution method. A portion of the resinate (50 mg) was accurately weighed and placed in a volumetric flask that contained 200 mL of 2 N potassium chloride (KCl) solution. The mixture was stirred on a magnetic stirrer for 24 h and the eluted drug was assayed by UV spectroscopic at 278 nm (Perkin Elmer Lambda 2, Germany). The drug content (%w/w) in the resinate was calculated by 100 × (eluted drug/resinate).
FORMULATION OF ORO-DISPERSIBLE TABLET-
|
Compositions (% w/w) |
F1
|
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
|
Resinate (R) or physical mixture (P) equivalent to 15 mg drug |
11.8 (R) |
11.8 (P) |
11.8 (R) |
11.8 (P) |
11.8 (R) |
11.8 (P) |
11.8 (R) |
11.8 (P) |
|
Avicel® PH 102 |
_ |
_ |
_ |
_ |
20 |
20 |
25 |
25 |
|
Mannitol |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
|
Icing sugar |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
|
Magnesium stearate |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
|
Tablettose® q.s. to |
100 |
100 |
_ |
_ |
_ |
_ |
_ |
_ |
|
Super Tab® SD q.s. to |
_ |
_ |
100 |
100 |
100 |
100 |
100 |
100 |
Characteristics of the optimal formulations (F7 and F8)
|
Formula |
Thickness (mm) n = 20 |
Diameter (mm) n = 20 |
Weight (mg) n = 20 |
Friability (%) n = 20 |
In vitro DT (s) n = 6 |
Wetting time (s) n = 3
|
Wetting rate (mL/s) n = 6
|
Drug content (% LA) n = 3 |
|
F7 |
3.64 ± 0.01 |
9.61 ± 0.01 |
347.8 ± 0.6 |
0.76 |
20.7 ± 4.8 |
69.8 ± 15.8 |
0.0027 |
108.13
|
|
F8 |
3.56 ± 0.02 |
9.58 ± 0.01 |
344.8 ± 7.4 |
0.74 |
13.8 ± 1.2 |
31.4 ± 3.4 |
0.0044 |
94.00 |
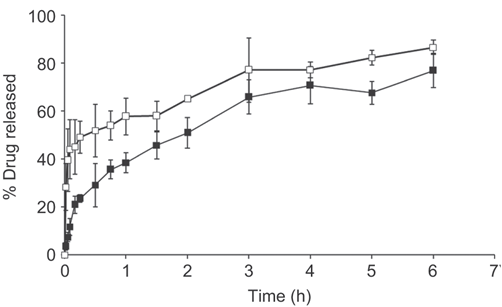
Release profile of dextromethorphan from the optimal formulations; (¤) F7 and (¤) F8 data are represented as mean ± SD (n = 3).
6. FORMULATION OF TASTE MASKED ORO-DISPERSIBLE TABLET OF DIPHENYHYDRAMINE HYDROCHLORIDE-(33)
INTRODUCTION-
An attempt was made to mask the taste by using Indion 234 and Tulsion 343 weak acid Cation exchange resins with a cross linked polyacrylic backbone and carboxylic functional groups and to formulate and evaluate effervescent and dispersible oral formulations of DPH. Diphenhydramine Hydrochloride (DPH HCl) an antihistaminic drug with bitter taste and low dose (25 mg)
PREPARATION OF DRC-
DPH was mixed separately with both the resins in the drug: resin ratio of 1:1, 1:2 and 1:3. Two hundred ml of distilled water was added to the mixtures and stirred continuously on magnetic stirrer (Whirlmatic-mega, Spectra lab, Mumbai, India), for 24 hours until the equilibrium was attained. Aliquots from the reaction mixture were withdrawn and filtered through Whatman filter paper no. 41 after every hour and were analyzed after appropriate dilution at 258 nm by UV/VIS spectrophotometer (JASCO-V530, Tokyo, Japan). The process was continued till the concentration values of two consecutive aliquots were almost constant. The readings were taken in triplicate. The resultant complex was filtered through Whatman filter paper no. 41, washed with water to remove the unreacted drug and oven dried at 50°C for 1 h and stored in air tight glass vial till the further use.
FORMULATION OF ORO-DISPERSIBLE TABLET-
|
|
|
BATCH CODE |
|
|
|
INGREDIENTS |
E1 (mg) |
E2 (mg) |
D1 (mg) |
D2 (mg) |
|
DRC equivalent to 25 mg with Tulsion 343 |
31.3 |
- |
31.3 |
- |
|
DRC equivalent to 25 mg with Indion 234 |
- |
35 |
- |
35 |
|
Sodium bicarbonate |
31 |
31 |
- |
- |
|
Tartaric acid |
20 |
20 |
- |
- |
|
Citric acid anhydrous |
9 |
9 |
- |
- |
|
Dicalcium phosphate |
92.4 |
88.4 |
- |
- |
|
Talc |
2.5 |
2.5 |
- |
- |
|
Avicel 101 |
- |
- |
57.4 |
57.5 |
|
Primojel |
- |
- |
8 |
8 |
|
Mannitol |
- |
- |
141.5 |
137.7 |
|
Aerosil |
- |
- |
1 |
1 |
|
Sodium Saccharin |
1.5 |
1.5 |
0.3 |
0.3 |
|
Poly vinyl pyrollidone (PVP K 30) |
8 |
8 |
8 |
8 |
|
Magnesium stearate |
2.5 |
2.5 |
2.5 |
2.5 |
|
Orange flavor |
1.8 |
2.1 |
q.s. |
q.s. |
|
Total |
200 |
200 |
250 |
250 |
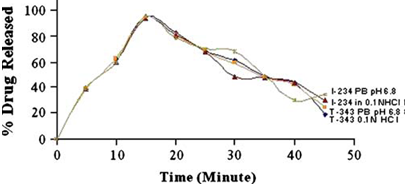
Drug release from Dispersible Tablets of T-343 and I-234 in 0.1 N HCl and Phosphate Buffer pH 6.8 (PB pH 6.8). Mean±SD for n=3
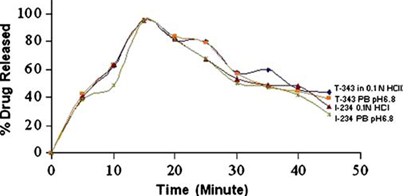
Drug release from Effervescent Tablets of T-343 and I-234 in 0.1 N HCl and Phosphate Buffer pH 6.8 (PB pH 6.8). Mean±SD for n=3.
EVALUATION OF TABLETS-
|
|
|
BATCH CODE |
|
|
|
Parameter |
E1 (T-343) |
E2 (I-234) |
D1 (T-343) |
D2(I-234) |
|
Uniformity of dispersion |
- |
- |
Passes through mesh no. 22 |
Passes through mesh no. 22 |
|
Disintegration time |
35±2 s |
40±2 s |
54±2 s |
59±2 s |
|
Drug release |
95.07% (0.1 N HCl) and 94.48% (phosphate buffer pH 6.8) of drug dissolved in 15 min
|
95.27% (0.1 N HCl) and 94.78% (phosphate buffer pH 6.8) of drug dissolved in 15 min
|
95.25% (0.1 N HCl) and 94.46% (phosphate buffer pH 6.8) of drug dissolved in 15 min
|
95.27% (0.1 N HCl) and 94.78% (phosphate buffer pH 6.8) of drug dissolved in 15 min |
The ratio of 1:2 for Indion 234 and 1:1 for Tulsion 343 were selected as the optimum and used for the further studies.
EVALUATION OF TASTE OF ORO-DISPERSIBLE TABLETS-(34)
Sensory evaluation
Taste, to think of, is a very subjective perception. Depending on individuals, the perceived taste may vary to different degrees. If we have well controlled experimental set up, it is possible to accurately and reproducibly measure taste thresholds. To quantitatively evaluate taste sensation, following methods have been reported in literature.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
• Panel testing (human subjects)
• Measurement of frog taste nerve responses.
• Multichannel taste sensor/ magic tongue
• Spectrophotometric evaluation/ D30’s value
Panel Testing
The panel testing is a psychophysical rating of the gustatory stimuli. In this method, a group of about 5?10 human volunteers is trained for taste evaluation by using reference solutions ranging in taste from tasteless to very bitter. Numerical values are then assigned to these levels of bitterness (eg.,0?5). Subsequently, test solution is tasted and rated on the same scale to assess its bitterness.
Measurement of Frog Taste Nerve Responses
In this method, adult bull frogs are anaesthetized intraperitoneally and the glossopharyngeal nerve is then located and dissected from the surrounding tissue and cut proximally. An ac?amplifier and an electronic integrator are used to respectively amplify and integrate the nerve impulses. The peak height of the integrated response is then taken as the magnitude of response.
Multichannel Taste Sensor / Magic tongue
This is an automated taste sensing device to detect the magnitude of bitterness of a drug substance. The device has a transducer which is composed of several kinds of lipid/polymer membranes with different characteristics that can detect taste in a manner similar to human gustatory sensation. Taste response is transferred into a pattern composed of electric signals of membrane potentials of the receptor part. Different response electric potential pattern are obtained for substance producing different taste qualities.
Spectrophotometric Method(35)
A known quantity of the taste?masked formulation is mixed with 10 ml of distilled water in 10 ml syringe by revolving the syringe, end to end, five times in 30 seconds. The test medium is then filtered through a membrane filter, followed by Spectrophotometric determination of the concentration of the drug in the filtrate. If this concentration is below the threshold concentration, it may be concluded that the bitter taste would be masked in vivo. This technique has been applied to evaluate the taste masked granules of sparfloxacin, with threshold concentration being 100μg/ml.
SUPPLIERS OF PHARMA GRADE ION EXCHANGE RESINS IN INDIA-
|
SR. NO. |
SUPPIER’S |
ADDRESS & WEBSITE/E-MAIL ID |
|
1 |
Ion Exchange (India) Ltd. |
Ion House, Dr. E. Moses Road, Mahalaxmi,Mumbai 400 011, India. E-mail: hocro@ionexchange.co.in |
|
2 |
·Potent Water Care Private Limited |
32, C.S.C. - 12,G - 29, D.D.A. Market, Sector - 3, Rohini, New Delhi, Delhi - 110 085, India |
|
3 |
Water Care Technology |
Shop No. 27, C. S. C. 12, G- 29, D. D. A. Market, Sector - 3, Rohini, New Delhi, Delhi - 110 085, India |
|
4 |
Doctor H2O |
No. 32, C. S. C. 12, G - 29, D. D. A. Market, Sector - 3, Rohini, Delhi, Delhi - 110 085, India |
|
5 |
Pool Tycoon |
Shop No. 32, C. S. C. 12, G - 29, D. D. A. Market, Sector - 3, Rohini, New Delhi, Delhi - 110 085, India |
|
6 |
·Triveni Interchem Pvt. Ltd. ( Group Of Triveni Chemicals) |
Pacharatna Char Rasta, G.I.D.C, Vapi, Gujarat - 396 195, India |
|
7 |
·Triveni Chemicals |
No. 135, Pancharatna Char Rasta, G. I. D. C., Vapi, Gujarat - 396 195, India |
|
8 |
·Aqua Ion Exchange Systems |
No. 20, Old No. 3, Dhanalakshmi Nagar, 100 Feet, New Scheme Road, New Sidhapudur, Coimbatore, Tamil Nadu - 641 044, India |
|
9 |
Kent Air Eco Corporation Limited LP |
No. J - 9, Silver Oak Estate, Gamma - 2, Greater Noida, Uttar Pradesh - 201 301, India |
|
10 |
·Hydrotherm Engineering Services |
34, Corner Market, Millenium Business Centre, Malviya Nagar, Delhi, Delhi - 110 017, India |
|
11 |
Aqua Chem Industries |
Shed No. L - 322/16/9, G. I. D. C. Estate, Office - 126 - A, City Center, Silvassa Road, Vapi, Valsad, Gujarat - 396 195, India |
|
12 |
Shri Krishna Enterprises |
217, Katra Peran, Tilak Bazar, Delhi, Delhi - 110 006, India |
|
13 |
Marcuras |
West- 194 B, S Block, MIDC Bhosari, Pune, Maharashtra - 411 026, India |
|
14 |
I - Con Trading Corporation |
38/3, B. T. Road, Kolkata, West Bengal - 700 002, India |
|
15 |
Noida Chemicals (A Unit Of Chemicals & Associates, New Delhi) |
Suite No. 27, Ashoka Chamber Opp Rachna Cinema Pusa Road New Delhi, New Delhi, Delhi - 110 060, India |
|
16 |
Ashi Inc (A Unit Of Chemicals & Associates, New Delhi) |
Chamber No. 27, 4th Floor, Ashoka Chambers, Opposite Metro Pillar No. 150, Pusa Road, New Delhi, Delhi - 110 060, India |
|
17 |
Clear Aqua Technologies Private Limited |
No. 13/6, Sri Nagar, T. V. Koil, Tiruchirapalli, Tamil Nadu - 620 005, India |
|
18 |
Unique Marketing |
No. 13, Karnavati Avenue, Near Silver Complex, Opposite Baroda Express Highway, C. T. M., Ahmedabad, Gujarat - 380 008, India |
|
19 |
Ion Robinson India |
D - 2, Yadav Nagar, Samaypur, Badli, New Delhi, Delhi - 110 042, India |
|
20 |
R. D. Engineering |
No. 130/7, Dum Dum Road, 1st Floor, Dum Dum, Kolkata, West Bengal - 700 074, India |
|
21 |
Maitreya Enterprises |
No. 1413, Sadashiv Peth, Shop No. 7 & 11, Avishkar Heights, Opposite Vidyarthigriha, Pune, Maharashtra - 411 030, India |
|
22 |
Aquaion Technology, Inc. |
No. 302, Nanadan Complex, Opposite Railway Crossing, Ellis Bridge, Ahmedabad, Gujarat - 380 006, India |
|
23 |
RK Chemical Works |
No. 3583, Netaji Subhash Marg, Darya Ganj, New Delhi, Delhi - 110 002, India |
|
24 |
Manas Watertech Engineers Private Limited |
No. 12, Kalpataru Industrial Estate, Near R Mall, Opp. Lawkim Company, Monorama Nagar, Thane West, Thane, Maharashtra - 400 607, India |
|
25 |
Soft Tech Ion Exchange Engineers |
No. 42/41, Parmeshwar Estate-2, Opp. Yamuna Estate Phase-1, G. I. D. C., Vatva, Ahmedabad, Gujarat - 382 445, India |
|
26 |
H2O Remediation Engineering |
Address: No. 12, Kalpataru Industrial Estate, Near R Mall, Opp. Lawkim Company, Monorama Nagar, Thane West, Thane, Maharashtra - 400 607, India |
|
27 |
Water Ions Purification |
A - 252, Pratap Vihar, Kalwar Road, Govindpura, Jaipur, Rajasthan - 302 012, India |
|
28 |
Aquatronix Engineers |
C - 1, Sharad Nagar, Behind L. G. Nagar Society, Nizampura, Vadodara, Gujarat - 390 002, India |
|
29 |
Tejasvi Group |
Shop No. 5, Banshidhar Complex, Ghanshayam Nagar, Main Road, Behind I. O. C. Quaters, Kalawad Road, Rajkot, Gujarat - 360 005, India |
|
30 |
Aetom Engineering Technologies Private Limited |
Plot No. 3039, Sector - 46, Gurgaon, Haryana - 122 002, India |
|
31 |
Aagam Chemicals |
K-43, Bharat Nagar., Nagpur, Maharashtra - 440 033, India |
|
32 |
Indus Chemical Private Limited |
No. 357, 3rd Floor, Aggarwal Modern Bazar, C - 33, Lawrence Road, Industrial Area, New Delhi, Delhi - 110 035, India |
|
33 |
Esbose Water Equipment Pvt. Ltd. |
5th Floor, Unit - 502, 18 - B, Ashutosh Mukherjee Road, Kolkata, West Bengal - 700 020, India |
|
34 |
Neel Operations |
G - 3, A - 9, Aurangabad Co - Operative Industrial Estate, M. I. D. C., Railway Station, Aurangabad, Maharashtra - 431 005, India |
|
35 |
N. M. Enterprises, Ahmedabad |
No. 406, Janpath Complex, Near Nehru Bridge, Ashram Road, Ahmedabad, Gujarat - 380 009, India |
|
36 |
Solu Tech Water Services |
Nisha Apartment, Plot No. 1, S. No. 56, Shevantiban Housing Society, Chinchwad, Pune, Maharashtra - 411 044, India |
|
37 |
Water Testing Services |
1, G. D. Bhutta House, Opposite Panchal Steel, Near Reliance Consultancy, Mogra Road, Mumbai, Maharashtra - 400 069, India |
|
38 |
Asha Resins Private Limited |
No. 1232, Bhawani Peth, Palkhi Vithoba Chowk, Pune, Maharashtra - 411 042, India |
|
39 |
N Shashikant |
No. 3, Indira Niwas, Peru Baug, Aarey Road, Goregaon East, Mumbai, Maharashtra - 400063, India |
|
40 |
Numatik Engineers Private Limited |
Shop No. 6, Bobby Pathak Avenue, Opposite Rotary Garden, Near Corporation Bank, Near Anand Nagar, Dahisar East, Mumbai, Maharashtra - 400 068, India |
|
41 |
Safe Water India |
201, Kalyan Complex, 426, Mangalwar Peth Near Narpatgiri Chowk, Pune, Maharashtra - 411 011, India |
|
42 |
Unitech Water Technologies |
401, Shanti House, Opposite Madhusoodan House, Near Sardar Patel Seve Samaj, C. G. Road, Ahmedabad, Gujarat - 380 006, India |
|
43 |
Amjey Chemicals |
: No. 402, Nanak Chambers, Opposite Fun Republic, Off New Link Road, Andheri West, Mumbai, Maharashtra - 400 069, India |
|
44 |
A To Z Chemicals |
No. 8/1, Lal Bazar Street, Mezzanine Floor, Room No. 10, Kolkata, West Bengal - 700 001, India |
|
45 |
Enviro-Chem Analyzers & Consultants |
B/106, Shiv Shakti Complex, S. V. Road, Dahisar East, Mumbai, Maharashtra - 400 068, India |
|
46 |
Aqualia Water Technologies |
Plot No. 101, Naga Gher, Ranipokhari, Dehradun, Uttarakhand, India |
|
47 |
Aquamatix Incorporation |
No. 38, 12th KM, Opposite J. C. Industrial Layout, Kanakapura Road, Bengaluru, Karnataka - 560 062, India |
|
48 |
Chemtech |
C-103, Maruti Industrial Estate, Phase-1, GIDC Vatva, Ahmedabad, Gujarat - 382 445, India |
|
49 |
Hira Engineering |
No. 19, Jai Ambe Cooperative Housing Society, Majaswadi, Near Raheja Brookheavan Building, Jogeshwari- Vikhroli Link Road, Jogeshwari, Mumbai, Maharashtra - 400 060, India |
|
50 |
Prime Water Systems |
Street -1071, Sai Saroja Nilayam, Sanathnagar, Hyderabad, Andhra Pradesh - 500 018, India |
|
51 |
Uniway Engineers Private Limited |
29, 48th Street, 9th Avenue, Ashok Nagar, Chennai, Tamil Nadu - 600083, India |
CONCLUSION-
THERE ARE MANY METHODS FOR TASTE MASKING OF BITTER DRUG TO FORMULATE AN ORO-DISPERSIBLE TABLET BUT ION EXCHANGE RESIN IS THE BEST SUITED FOR TASTE MASKINGOF BITTER DRUG. HERE WE DESCRIBE MANY EXAMPLES TO FORMULATE A TASTE MASKED ORO-DISPERSIBLE TABLET BY USING IERs.
REFERENCES-
1. Chatap, V.K. and W.J.R. Remington, 2002.The science and practice of pharmacy, Mack Publishing Company, 20, 1018-1020.
2. J.M. Lipari, T.C. Reiland, Flavor & Flavor Modifiers. Encyclopedia of Pharmaceutical Technology, 2010, 2, 1254-1263.
3. David, R., 2002, Signals and Perception of Taste Open University- Palgrave Macmillan.
4. Copper R.M., The Effect of age on taste sensitivity, J. Gerontology (1959) 14(1): 56-58.
5. Puttewar TY, Kshinsagar MD, Chandewar AV, Chikhale RV. Formulation and Evaluation of Oro-dispersible Tablet of taste masked doxylamino succinate using Ion-Exchange Resin. Journal of King & Saud University 2010, 22: 229-240.
6. P.Virely and R. Yarwood, Zydis- a novel fast dissolving dosage form, Manuf. Chem. 61 (1990) 36-37.
7. S.W. Avery and D.M. Dellarosa, Approaches to treating dysphagia in patients with brain injury, Am. J. occup. Ther. 48 (1994) 235-239.
8. P.J. Kahrilas, Anatomy, Physiology and Pathophysiology of dysphagia, Acta otorhinolaryngol. Belg 48 (1994) 97-117.
9. C.G. Wilson, N. Washington, J.Peach, G.R. Murray and J.Kennerley, The Behavior of a fast dissolving dosage form (Expidet) followed by r-scintigraphy, Int. J. Pharm 40 (1987) 119-123; DOI: 10.1016/0378-5173 (87) 90056-1.
10. I.S. Ahmed, M.M. Nafadi and F.A. Fatahalla, Formulation of Fast Dissolving Pcetoprofen tablet using freeze- drying in blisters technique, Drug Dev. Ind. Pharm.32 (2006) 437-442; DOI:1080/03639040500528913.
11. S.Bandari, R.K. Mittapalli and Y.M. Gannu Rao, Orodispersible tablet: An Overview, Asian J. Pharm.2 (2008) 2-11. DOI: 10.4103/0973-8398.41557.
12. Ahire, S.B.; Bankar, V.H.; Gayakwad P.D.; Pawar S.P. ; A Review: Taste masking Techniques in Pharmaceuticals; Pharma Science Monitor: An International Journal of Pharmaceutical Science (2011); ISSN: 0976-7928; 1645-1646.
13. Agarwal R, Mittal R and Singh A: Studies of Ion-Exchange Resin Complex of Chloroquine Phosphate. Drug Dev. Ind. Pharm. 2000; 6: 773-776.
14. Dorfner K: Ion Exchanger Properties and Applications, Third Edition, Ann Arbor Science Publisher1972; 2.
15. Bhalekar M, Avari JG, Jaiswal SB. Cation-Exchanger as pharmaceutical formulation. Ind J Pharm Sci38 (4): 184-187, 2004.
16. Jain N.K. Advanced Drug Delivery System. 1st ed., Aantares Pharma, NJ, USA2005. P.290-302.
17. Deasy. Ion exchange resin in microencapsulation. Newyork: Marcel Dekker Inc; 1980. P.150.
18. Bassett, Denney R.C., Jeffery G.H. Ion Exchange. In Vogel’s Textbook of Quantitative Inorganic Analysis. 4th edition. England: Longman Scientific and Technical; 1978. P.165-172.
19. Wen. B., Ramsay M.P., Antitussive drugs delivered by Ion Exchange Resins. U.S. Pat. No. 6,001,392 to warner-lambert Company; 1999.
20. Pisal S, Zainnuddin R, Nalawade P, Mahadik K, Kadam S. Molecular properties of ciprofloxacin–indion 234 complexes. AAPS PharmSciTech 2004; 5 (4):e62.
21. Kalsi P. Spectroscopic identification of organic compounds, 5th ed. New Delhi: New Age International Publishers; 2002. p. 84–102.
22. Skoog D, Holler F, Nieman T. The principles of instrumental analysis, 5th ed. Singapore: Thomson Book/Cole; 1998. p. 805–8.
23. Debjit Bhowmik; K.P. Sampath Kumar; Recent Trends in Ion Exchange Resins Used In Pharmaceutical Formulations- An Updates; The Pharma Research (T. Ph. Res.), (2010), 4; 138-148. Published on- 15 Dec 2010.
24. Borodkin SS, Swarbrick J, Boylon CJ. Encyclopedia of Pharmaceutical Technology. Vol. 8. New York: Marcel Dekker, Inc.; 1995. p. 203-310.
25. Jeong SH, Haddish NB, Haghighi K and Park K: Drug release properties of polymer coated ion-exchange resin complexes: experimental and theoretical evaluation. J. Pharm. Sci. 2007; 96: 618–632.
26. Anand V, Kandarapu R and Garg S: Ion-exchange resins: carrying drug delivery forward. Drug Discov. Today 2001; 6: 905–914.
27. Praveen K. Bhoyar, Jagdish R. Baheti, Shweta H. Mishra; Formulation and characterization of Taste Masked Oro-Dispersible Tablets of Metformin Hydrochloride; World Journal Of Pharmaceutical Research; Vol-1, Issue 2, 183-196; ISSN 2277-7105.
28. Venkatesh D.P.; Formulation Development and Evaluation of Taste Masked Oro-Dispersible Tablets of anti-emetic drug; Journal of Pharmacy Research 2009, 2(4), 606-609.
29. Jayashri G. Mahore,Kamlesh J. Wadher, Milind J. Umekar; Formulation and in vitro Evaluation of Taste Masked Orodispersible Tablet of Metoclopramide Hydrochloride; International Journal of Pharm Tech Research; Vol.2, No.3,pp 1827-1835.
30. Ashwini R. Madgunlkar, Mangesh R. Bhalekar, Rahul R. Padalkar; Formulation Design and Optimization of Novel Taste Masked Mouth-Dissolving Tablets of Tramadol Having Adequate Mechanical Strength; AAPS Pharm Sci Tech, Vol10, No.2, June 2009 DOi:10.1208/s 12249-009-9237-y.
31. DP Venkatesh, CG Geetha Rao; Formulation of taste masked oro-dispersible tables of Ambroxol hydrochloride; Asian Journal Of Pharmaceutics; Year-2008; Vol-2: Issue-4; pp 261-264.
32. Wipada Samprasit, Praneet Opanasopit, Prasert Akkaramongkolporn, Tanasait Ngawhirunpat, Kaewnapa Wongsermsin, and Suwannee Panomsuk; Preparation and evaluation of taste masked dextromethorphan oral disintegrating tablet; Pharmaceutical Development and Technology, 2012; 17(3): 315–320 © 2012 Informa Healthcare USA, Inc. ISSN 1083-7450 print/ISSN 1097-9867 online DOI: 10.3109/10837450.2010.535828.
33. Kiran Bhise, Shafi Shaikh, and Divyakumar Bora; Taste Mask, Design and Evaluation of an Oral Formulation Using Ion Exchange Resin as Drug Carrier; AAPS PharmSciTech, Vol. 9, No. 2, June 2008 (# 2008) DOI: 10.1208/s12249-008-9056-6.
34. Sharma S, Lewis S: Taste Masking Technologies: A Review. International Journal of Pharmacy and Pharmaceutical Sciences 2010; Vol 2, Issue 2:25-33.
35. Shirai Y, Sogo KA: Novel Fine Granules System For Masking Bitter Taste. Biol. Pharm. Bull. 1993; 16: 172?177.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









