 About Authors:
About Authors:
Shaik .Shabbeer*, V.Sruthi, P.Usha, K.Mahesh
Department of Pharmaceutical sciences, Swami Ramananda Tirtha Institute of Pharmaceutical Sciences,
Nalgonda, Andhra Pradesh, India-508001
*shkshabbeer@yahoo.com
Abstract
Metformin11,12 hydrochloride is recommended globally as first line therapy due to its favorable profile on morbidity and mortality associated with type-2 Diabetes mellitus. However, limitations of multiple dosing and risk of triggering GIT symptoms make its dose optimization difficult. And so it is reasonable to assume the requirement of sustained release Metformin hydrochloride 5,6,7 formulations to prolong its duration of action and bioavailability.
Metformin Hcl is anti hyperglycemic agent, witch is absorbed mainly from the small intestine. Guar gum being a natural biodegradable polymer acts as a disintegrating agent along with its binding activity and thus produces a matrix tablet with good hardness, which it finally enables for increased disintegration time so that the tablet formulation attains the sustained release nature.
Different batches of (F1-F4) Sustained release matrix tablets of Metformin Hcl were prepared by using various concentrations of guar gum and HPMC as polymers. Evaluation parameters like thickness, hardness, friability, weight variation and disintegration tests of the formulations were found to be satisfactory. Among all prepared formulations F3 was shown desired release pattern than others. Formulations F1 and F2 did not show the optimum drug release which might be due to low level of the polymers. Where F4 shows drug retardation due to more concentration of polymer witch could affect the drug release.
And thus the F3 formulation was found to be the desired sustained release matrix tablet for the treatment of type-2 diabetes mellitus.
Reference Id: PHARMATUTOR-ART-1459
Introduction
Diabetes mellitus (DM): 9,14, 15, 16,17
Diabetes mellitus defined as a heterogeneous metabolic syndrome characterized by chronic hyperglycemia i.e. increase in blood glucose (sugar) levels with carbohydrate, fat and protein metabolism disturbances. It is endocrinological metabolic disorders which occur due to deficiency in production of insulin by the pancreas or by the ineffectiveness of the insulin produced. It is a very chronic or complex disease which effects each and every part cell.
Classification of DM:
It is broadly classified into three types. They are as follows:
1. Type-1 DM/Insulin dependent DM
a) Type-1A
b) Type-1B/Idiopathic
2. Type-2 DM/Non insulin dependent DM
3. Others
a. Gestational DM
b. Latent auto immune DM in adults
c. Maturity onset DM of the young
Type - 1 DM:
It is also called as insulin dependant diabetes mellitus (IDDM), juvenile onset diabetes mellitus. In this type there is beta cell destruction in pancreatic islets; majority of cases are auto immune (type -1 A) antibodies that destroy beta cells are detectable in blood, but some are idiopathic (type-1 B) where no beta cell antibody is found. In all type -1 cases circulating insulin levels are low or very low, and patients are more prone to ketosis. This type is less common and has a low degree of genetic predisposition.
Type-2 DM:
It is also called as Non insulin dependent diabetes mellitus (NIDDM), maturity onset diabetes mellitus. In this type there is no loss or moderate reduction in beta cell mass; insulin in circulation is low, normal or even high, no anti beta cell antibody is demonstrable; Has a high degree of genetic predisposition; generally has a late onset (past middle age) over 90%cases are type-2 DM.
Causes of type -2 DM:
· Abnormality in gluco-receptor of beta cells so that they respond at higher glucose concentration or relative beta cell deficiency.
· Reduced sensitivity of peripheral tissues to insulin: reduction in number of insulin receptors, down regulation of insulin receptors.
· Excess of hypoglycemic hormones (glucagons, etc.)/obesity cause relative insulin deficiency with the beta cells lagging behind.
This type of DM occurs due to relative insulin deficiency or resistance or both. The onsets of symptoms are slower and are less marked than type-1 DM it requires external supply of insulin in order to maintain blood glucose levels.
Insulin Resistance:
The basic cause for the occurrence of type-2 DM insulin (peripheral tissues do not respond to insulin).this occurs to obesity (excess of abdominal fat).
Obesity has the high levels of free fatty acids due to insulin’s , anti action is inhibited it will has the development of insulin resistance in liver and muscle it forms increase in gluconeogenesis in liver &increase in glucose uptake by muscle it forms increase levels of blood glucose.
Further most qty of fat itself will contribute to insulin resistance. This is due to the fat that when adiposities become large they cannot store excess amount of fat. Thus fat is stored in muscle and liver pancreas leading to insulin resistance in these regions.
Sometime obesity leads to CVS disease like atherosclerosis leads to hyper coagulabiliy, impaired fibrinolysis, endothelial damage, oxidative stress and finally increases blood glucose levels.
Impaired Secretion of insulin:
Glucose toxicity around islets of langerhans may impair functioning of beta cells. Lipotoxicity-increase free fatty acid levels. Deposition of fibrillary proteins around islets of langerhans of pancreas by amylin (on islets amyloid polypeptide) which may impair the functioning of beta cells.
Genitical factors:
Occurrence of type-2 DM is linked with the presence of metabolic syndrome also individuals with paternal history at a high risk of developing type 2 DM.
Experimental work:
Materials:
Metformin hydrochloride – Sd-fine chemicals Ltd, Mumbai, India.
Hydroxy propyl methyl cellulose – Qualikems fine chem pvt Ltd, vadodara, India.
Guar gum – Jyothi chemicals, modhi nagar, India.
Micro crystalline cellulose - Otto pharmaceuticals pvt Ltd, Mumbai, India.
Poly vinyl pyrolidine - Molychem, Mumbai, India.
Pre formulation tests: 1,2, 3, 4
The general preformulation tests for the tablet formulations include the following:
Bulk density:
Bulk density is defined as the mass of the powder divided by the bulk volume. The bulk density (gm/cm3) of powder depends mainly on particle-size distribution, particle shape, and then tendency of the particles to adhere to one another.
It is given by following equation
D = M / V
Where,
D – Bulk density
M- Mass of the particles
V – Bulk volume
Compressibility Index:
The initial & final density’s can be used to determine the percentage compressibility also called as compressibility index.
It is given by the equation:
I = (D1-D0/D1)*100
Where,
I – Compressibility index
D1 – Final density
D0 – Initial density
The compressibility of a powder dictates its flow properties. A highly compressible powder exhibits less flow properties and vice-versa.
Angle of repose:
Angle of repose is defined as the maximum angle formed between the sides of the powder heap with the horizontal surface. It is a very simple technique which determines the flow properties of the powder. The equation is given as:
Tan θ = H/R
Where,
θ - Angle of repose
R - Radius of powder bed
H -Height
3. Tablet formulation design:
The different batches of tablets are designed mainly to observe the relative changes in disintegration and the dissolution of the tablets so formed. It is mainly achieved by altering the polymers concentrations.
Table 1: Formulation design of Guar gum and HPMC matrix tablets of Metformin Hcl.
|
S.no |
Drug |
HPMC |
Guar gum |
Poly vinyl pyrolidine |
Micro crystalline cellulose |
Magnesium stearate
|
Talc |
|
(mg) |
(mg) |
(mg) |
(mg) |
(mg) |
(mg) |
(mg) |
|
|
F1 |
200 |
30 |
80 |
60 |
120 |
5 |
5 |
|
F2 |
200 |
40 |
100 |
60 |
90 |
5 |
5 |
|
F3 |
200 |
50 |
120 |
60 |
60 |
5 |
5 |
|
F4 |
200 |
60 |
140 |
60 |
30 |
5 |
5 |
Preparation of Tablets:
Matrix tablets were prepared by non aqueous wet granulation method2 reported earlier with slight modification. The composition of various formulations including HPMC, guar gum9,10,12, MCC were mixed in a polybag, and the mixture was passed through mesh(no.40).Granulation was done using a solution of PVP in sufficient isopropyl alcohol. Thereafter the drug was added to the wet granules, the granules were dried for about 2 hrs. The dried granules were then mixed with magnesium stearate and talc then subjected to punching.
Evaluation test for the formulations: 1, 2, 3, 4
Hardness:
The hardness of the tablet is also termed as its crushing strength. It may be defined as the compression force required break/fracture the tablet when such force is applied diametrically. The hardness of the tablet is related to its disintegration and has more pivotal role in controlling the rate of drug release from the tablet.
The hardness of the tablet formulations (F1-F4) are tested by using the Monsanto hardness tester method.
Friability:
Friability in addition to hardness gives the measure of tablet strength. The friability of a tablet may be defined as its resistance to shock and abrasion encountered during the process of manufacture etc. The friability of the tablet formulations (F1-F4) are determined by using the Roche friabilator.
Weight variation:
The weight variation test is determined by individually weighing 20 tablets. The average weight of these tablets is determined. The weight variation of the individual tablets is determined with respect to average weight and %weight variation.
Table 2: Permissible % weight variation for uncoated tablets as per I.P
|
Tablet weight(average mg) |
% weight variation (maximum limit) |
|
<130 |
10 |
|
130-324 |
7.5 |
|
>324 |
5 |
If two tablets fall out of the permissible %weight variation and none of the tablet was twice the %weight variation value, then the batch passes the weight variation test.
Disintegration:
It is a method to evaluate the rate of disintegration of solid dosage forms (tablets).Disintegration is defined as the break down of tablet into small particles after it is ingested. The disintegration test for the formulations (F1-F4) is performed in the SECOR (Scientific engineering corporation, Delhi) India disintegration test apparatus.
In Vitro Dissolution:
The dissolution test for the formulations (F1-F4) is performed in the SECOR (Scientific engineering corporation, Delhi) India dissolution test apparatus. The in-vitro release of Metformin hydrochloride from formulated tablets was carried out for 2 hours inpH 1.2 Hydro Chloric acid bufferand pH 6.8 phosphate buffer (19h). Samples were taken at 1hr, 2hr, 3hr, 5hr, 6hr, 8hr, 12hr & 19 hours and diluted to suitable concentration and analyzed for metformin hydrochloride content at 232 nm by using UV–visible spectrophotometer.
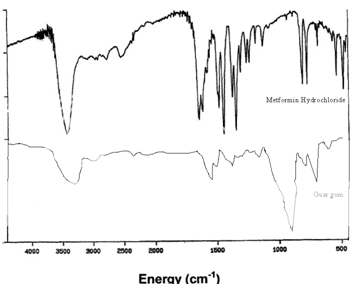
IR spectrum:
The IR spectrum for the drug (metformin hydrochloride), polymers (guar gum & HPMC) and the formulated tablet was performed to identify any incompatibility between the drug and the polymers.
Results and discussions:
Weight Variation:
To study weight variation, 20 tablets of each formulation were weighed using an electronic balance, average weights was calculated, individual tablet weights were compared with the average weight. and the results were shown in the table.3
Hardness:
For each formulation, the hardness of three tablets was checked using the Monsanto hardness tester, average values are shown in Table.3
Friability:
For each formulation, six tablets were selected randomly and weighed. Tablets were then placed in friability testing apparatus (Roche fribrilator), which was rotated at a speed of 25 rpm for 4 minutes Tablets were then weighed and friability values were determined and are reported in table.3
Disintegration:
The disintegration test for the formulations (F1-F4) is performed in the SECOR (Scientific engineering corporation, Delhi) India disintegration test apparatus and the disintegration times of the formulations are reported in table 3.
Table 3:
|
Formulation |
Weight variation |
Hardness |
Friability |
Disintegration time |
|
F1 |
Passes |
10.6 |
0.6 |
26min |
|
F2 |
Passes |
13 |
0.8 |
27min |
|
F3 |
Passes |
14.5 |
0.5 |
28 min |
|
F4 |
Passes |
12 |
0.6 |
27min |
Dissolution studies:
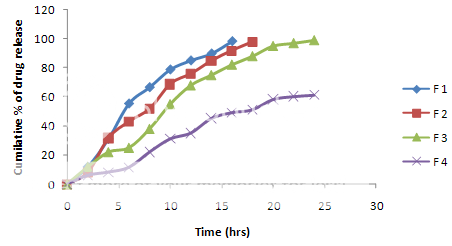
The in vitro drug release studies are performed in Hydro choric acid buffer PH 1.2 and phosphate buffer PH 7.2. F1 and F2 formulation do not show the sustained release and maximum amount of the drug released in first 2-3 hrs. The F3 formulations showed good sustained drug release and a very small amount of drug during the initial hours and about 80% of the drug releases sustained .The formulations and F4 mostly retards the drug release and thus possess the required sustained drug release matrix tablet properties.fig.1
Figure 1:Release profiles of metformin hydrochloride tablets with guar gum and HPMC.
IR spectrum of pure drug (metformin hydrochloride):
The infrared spectrum of metformin hydrochloride is shown in Figure 2.
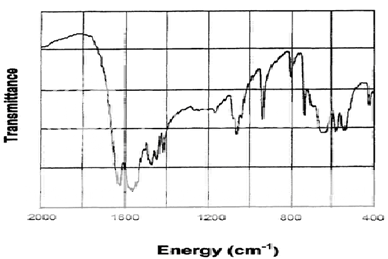
Table 4: Assignment of Major Infrared Absorption Bands
|
Energy (cm-1) |
Assignment |
|
1630, 1565 |
N-H deformation, and asymmetric NCN stretch |
|
1470, 1440, 1410 |
CH3 asymmetric and symmetric deformations |
|
1060,940 |
C-N stretch and CH3, rock |
Figure 3: IR spectrum of guar gum
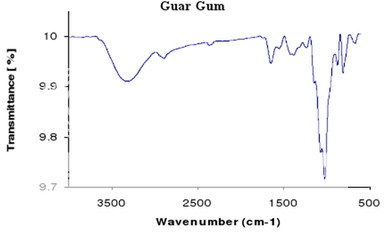
Figure 4:IR spectrum of polymer (HPMC):
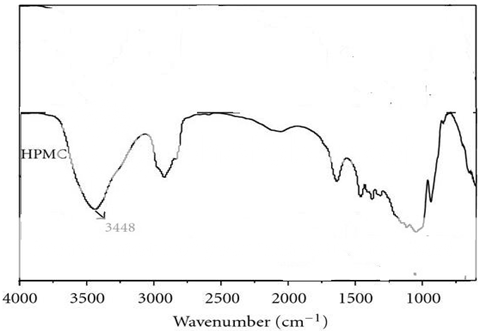
Figure 5: IR spectrum of formulated metformin hydrochloride tablet:
Conclusion:
Metformin Hcl is anti hyperglycemic agent, and is absorbed mainly from the small intestine. Sustained release tablets of Metformin Hcl were prepared using guar gum and HPMC as rate retardant polymers. Various evaluation parameters like thickness, hardness, friability, weight variation and disintegration tests of the formulations were found to be satisfactory. Among all formulations prepared and evaluated F3 appeared to have desired release pattern than others. Formulations F1 and F2 did not show the optimum drug release which might be due to low level of the polymer in the tablets. Where F4 shows high drug retarding due to more concentration of polymer witch could affect the drug release.
And thus the F3 formulation shows desired sustained release for the treatment of type-2 diabetes mellitus.
References:
1. Martin A.. “Physical Pharmacy.’’ . 2001:423-454.
2. Ansel HC, Loyyd VA. “Pharmaceutical dosage forms and Drug Delivery System’’1999; 8: 275-280.
3. Aulton ME. “Pharmaceutics: The Science of Dosage Form Design.’’2002.
4. Bankar J A. “The theory and practice of industrial pharmacy’’ (3rdedn), 1987;453-455.
5. “Indian Pharmacopoeia’’. Ministry of health. The controller of publications, New Delhi1996; 4ed; 432.
6. USP 30-NF25, “The United States Pharmacopoeial Convention’’ 2007.
7. Bailey CJ, Day C. “Metformin: its botanical background. Practical Diabetes International’’. 2004; 21(3):115–117.
8. Diabetes Prevention Program Research Group “Reduction in incidence of type 2 diabetes with lifestyle intervention or metformin’’. J Med 2002; 346:393
9. Srivastava Pranati*, “Preparation and evaluation of matrix based tablet using natural polymers as release modifiers “Int.J.Pha.Sci 2010; 2(1):411-4175-nfo
10. Ram Prasad Y V, Krishnaiah Y S, Satyanarayana S. “Studies of guar gum compression-coated 5-aminosalicylic acid tablets for colon-specific drug delivery’’ Drug development and industrial pharmacy. 1999;25 (5):651-657.
11. Hamman, “Drug Target Insights’’ 2, 2007, 80
12. Vandamme Th F. The use of polysaccharide to retard drug release 2002; 48: 219-231.
13. Williams R O. “Advanced drug formulation design to optimize therapeutic outcomes.’’ Informa healthcare; 2007; 172:239-241.
14. American Diabetes Association “Standards of medical care in diabetes’ 2006; (Suppl.1):S4?42.
15. Bolen S, Feldman L, Vassy J, ”Comparative Effectiveness and Safety of Oral Medications for Type 2 Diabetes Mellitus’’. Ann Intern Med. 2007;16.
16. Carroll MF, Izard A, Riboni K, et al. Control of postprandial hyperglycemia: optimal use of short?acting insulin secretagogues. Diabetes Care. 2002 Dec; 25(12):2147?52.
17. Herman WH, Hoerger TJ, Brandle M, et al. The cost?effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med 142:323?332, 2005.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









