{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Bhumi B. Patel1*, Chainesh N. Shah2, Rumit M. Shah3
1Department of Pharmaceutics, Shree Naranjibhai Lalbhai Patel College of Pharmacy, Umrakh, Gujarat, India.
2 Department of Pharmacy, Sumandeep Vidyapeeth, Piparia, Gujarat, India
3 Department of Pharmacognosy, Shree Naranjibhai Lalbhai Patel College of Pharmacy, Umrakh, Gujarat, India.
* patelbhumi198@gmail.com
ABSTRACT
Lamotrigine belongs to biopharmaceutical classification systems; BCS class II (Low solubility & High permeability). In addition, it requires immediate therapeutic action. Hence, the main objective of this study is to improve the solubility by solid dispersion technique and formulate it as Orodispersible tablets to avert the problems of swallowing and to provide rapid onset of action. Lamotrigine solid dispersion was prepared by using PEG 6000 as solubility enhancers and formulated it into ODT by direct compression technique using different concentrations of Sodium Starch Glycolate, Cross Carmalose Sodium, and Kyron T-314 as cross linked polymers. The tablets were evaluated for various parameters and the results were found to be satisfactory. The batches batch containing Kyron T-314 as super disintegrant in 7% concentration, as they showed 95.75% drug release in 15 minutes with a disintegration time of 11 seconds which shows improved dissolution rate in compare to marketed formulation of Lamotrigine.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2426
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 4, Issue 6 Received On: 10/03/2016; Accepted On: 22/03/2016; Published On: 01/06/2016 How to cite this article: Patel BB, Shah CN, Shah RM; Formulation and evaluation of Orodispersible tablets to enhance dissolution rate of Lamotrigine by using Solid Dispersion Technique; PharmaTutor; 2016; 4(6); 35-39 |
INTRODUCTION
The dissolution of drugs is a prime determinant in the absorption of poorly water-soluble drugs and also serves as a rate-limiting step [1,2]. Improvements in the apparent solubility and/or dissolution rate of a poorly water-soluble drug through the formation of an inclusion complex or solid dispersion technique may enhance its bioavailability. Various techniques have been used to enhance the solubility/dissolution rate of poorly water-soluble drugs, including the use of surfactants [3], inclusion complexation, use of polymorph, drug micronization into an amorphous form and solid dispersion [4-6]. Among them, inclusion complexation with cyclodextrins and the solid dispersion technique are most frequently used. Lamotrigine (6-(2,3-diclorophenyl)-1,2,4-triazine-3,5-diyldiamine) is used for the prevention of tonic–clonic epileptic seizures and is also effective against partial seizures, primary and secondary generalized seizures, absence seizures and other types of epileptic seizures. Its primary mechanism of action is blocking voltagedependent sodium channels, leading to inhibition of excitatory neurotransmitter release and stabilization of neuronal membranes. Although lamotrigine has gained widespread acceptance in the treatment of seizures, its poor aqueous solubility (0.17mg mL−1) limits its absorption and dissolution rate and thus delays onset of action. [1].
The aim of this work was to improve the aqueous solubility and dissolution rate of lamotrigine using solid dispersion techniques hydrophilic carriers PVP K30 and PEG 6000. Solid dispersion systems of lamotrigine were prepared with PEG 6000, using a solvent method. Different ratios of polymers were selected on a purely random basis. Powder X-ray diffractometry (XRD), Fourier transform infrared spectroscopy (FTIR) and differential scanning calorimetry (DSC) were used to characterize the solid-state properties of lamotrigine, the physical mixture of solid dispersions. The aqueous solubility and dissolution behaviour of pure lamotrigine and all its binary systems were evaluated further.
MATERIAL AND METHODOLOGY
Material
Lamotrigine was a gift from Lupin Ltd (Mumbai, India). PVP K30 was a gift from Signet Chem. Lab. (Mumbai, India). PEG 6000 was purchased from Loba Chemie (Mumbai,India). All reagents were of analytical grade. Double-distilled water was used throughout.
Preparation of solid dispersions of lamotrigine by solvent evaporation
Solid dispersions of lamotrigine were prepared by solvent evaporation. Lamotrigine and water-soluble polymers in different ratios were weighed accurately and transferred to a beaker containing methanol. The solvent was then evaporated in a vacuum evaporator and the resulting solid dispersions were collected and stored in desiccators until they attained constant weight. The solidified masses were crushed, pulverized and passed through size-40 mesh.
Solubility study of Lamotrigine [7]
High boiling point, inert, low viscous and water miscible organic solvents such as propylene glycol, tween 20, tween 80, polyethylene glycol 400 were selected and 1% solutions in water was prepared. From each of these solutions 10 ml was taken, excess quantity of drug was added and saturated solutions were prepared by shaking on a mechanical shaker. The saturated solutions were kept aside overnight, filtered and appropriate dilutions were made, and the absorbance was measured at 308nm using Systronics double beam UV visible spectrophotometer.
Saturation solubility studies
Saturation solubility studies were performed in triplicate according to the method reported by Higuchi and Connors (1965). Excess of pure drug, physical mixture, inclusion complex or solid dispersions were added to 20 mL distilled water in a screw-cap tube and shaken in a rotary flask shaker at room temperature (25°C) for 24 h. (Preliminary studies had shown that equilibrium solubility was achieved in 24 h.) Once equilibrium had been achieved, appropriate aliquots were withdrawn and filtered through Whatman filter paper no. 41 and the filtrate analysed spectrophotometrically at 308 nm.
FTIR studies
Infrared spectra were obtained using a Perkin-Elmer Spectrum-one FTIR spectrometer (Shelton, CT, USA) using KBr disks. The samples were previously ground and mixed thoroughly with KBr. The KBr disks were prepared by compressing the powder. The scanning range was kept from 4000 to 450cm−1 and the accumulations were 4 (Fig. 1, 2).
DSC studies
DSC measurements were performed on a TA SDT 2960 DSC differential scanning calorimeter. The accurately weighed sample was placed in an aluminium pan. An empty aluminium pan was used as a reference. The experiment was carried out in nitrogen atmosphere (flow rate 100mL min−1) at a scanning rate of 10°C min−1 in the range of 0–350°C.
[adsense:468x15:2204050025]
EVALUATION OF LAMOTRIGINE ORODISPERSIBLE TABLETS
Hardness [8]
The prepared tablets hardness was measured by using Monsanto hardness tester. The hardness was measured in terms of kg/cm2.
Thickness and diameter [8]
Thickness and diameter of prepared tablets were tested by Vernier callipers and the average was calculated.
Friability test : [8]
Ten tablets were weighed collectively and placed in the chamber of the friabilator. After 100 rotations (i.e. in 4 minutes), the tablets were taken out from the friabilator and intact tablets were again weighed collectively.
Weight variation test: [8]
The test will be performed as per USB by weighing 20 tablets individually on electronic balance, calculating the average weight, and comparing the individual tablet weights to the average.
In-vitro dispersion time:[9]
One tablet was placed in a beaker containing 10 ml of phosphate buffer of pH 6.8 at 37 ± 0.5oC and the time required for complete dispersion was determined.
In-vitro disintegration test: [10]
The disintegration time for all formulations was carried out using tablet disintegration test apparatus. Six tablets were placed individually in each tube of disintegration test apparatus and discs were placed. The water was maintained at a temperature of 37° ± 2°C and time taken for the entire tablet to disintegrate completely was noted.
Wetting time: [9]
A piece of tissue paper folded twice was placed in a small petridish (internal diameter of 5 cm) containing 6 ml of distilled water. A tablet was placed on the paper, and the time required for complete wetting of the tablet was measured.
Water absorption ratio (R): [9]
A piece of tissue paper folded twice was placed in a small Petri dish (internal diameter = 6.5cm) containing 5 ml of distilled water. A tablet was placed on the tissue paper. The wetted tablet was weighed. The test was done in triplicate. The water absorption ratio(R) was determined according to the following equation.
R=100 × (Wa-Wb)/Wb
Where, Wb and Wa are tablet weights before and after water absorption, respectively
In-vitro release studies: [1]
The prepared orodispersible tablets were subjected to in vitro dissolution studies using an 8 station USP (TYPE II) dissolution apparatus (Electro Lab, TDT-O8L, Mumbai). The dissolution studies were carried out in 900 ml of 0.1N HCl at 37 ± 0.5oC. The speed of the paddle was set at 50 rpm. Sampling was done every 2 minutes interval. For each sample, 5 ml of sample was withdrawn from the dissolution medium and replaced with equal volume of fresh medium. The samples withdrawn were analyzed in the UV spectrophotometer at 267 nm.
Stability studies:
Stability studies were carried out at 25°C ± 2°C / 60 % RH ± 5 % and 40°C ±2 °C / 75 % RH ± 5 % for a period of 30 days with selected formulations by storing the samples in stability chamber.
Table.1.Formulation of Lamotrigine Orodispersible tablets
|
Ingredients |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
F9 |
|
Lamotrigine |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
|
Mannitol |
52 |
50 |
48 |
52 |
50 |
48 |
52 |
50 |
48 |
|
PEG 6000 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
|
SSG |
3 |
5 |
7 |
|
|
|
|
|
|
|
CCS |
|
|
|
3 |
5 |
7 |
|
|
|
|
Kyron T-314 |
|
|
|
|
|
|
3 |
5 |
7 |
|
Arosile |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
|
Aspartame |
3 |
3 |
3 |
3 |
33 |
3 |
3 |
3 |
3 |
|
Magnesium Sterate |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
|
Total |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RESULT AND DISCUSSION
Table.2.Evaluation studies of tablets
|
Formulation code |
Disinteg-ration (sec) ±SD |
Water absorpti-on ratio ±SD |
Wetting time(sec) ±SD |
Drug content ±SD |
Drug release ( %) ±SD |
|
F1 |
29±2.78 |
62.9±1.20 |
57±2.15 |
97.54 ±0.75 |
82.19±0.50 |
|
F2 |
24 ±1.0 |
60.62±1.05 |
53±1.56 |
97.85 ±1.07 |
85.26±0.87 |
|
F3 |
18±1.0 |
55.04±1.73 |
50±2.01 |
98.45 ±0.50 |
90.75±0.74 |
|
F4 |
27 ±2.0 |
58.46 ±1.58 |
39.26±1.89 |
98.69 ±0.70 |
88.76±1.08 |
|
F5 |
22±1.7 |
54.16 ±1.21 |
36.18 ±2.20 |
98.75 ±0.87 |
91.89±0.88 |
|
F6 |
16 ±2.8 |
48.14±1.57 |
32.12±1.0 |
99.36 ±0.90 |
93.19±0.40 |
|
F7 |
21±1.45 |
49.32±1.47 |
28.21 ±2.51 |
96.74 ±1.07 |
92.19±0.50 |
|
F8 |
16 ±2.10 |
45.26±1.24 |
25.14±2.0 |
98.59 ±0.39 |
93.26±0.87 |
|
F9 |
11±2.0 |
39.15±1.34 |
20.12±2.40 |
98.87 ±0.77 |
95.75±0.74 |
Table.3.Accelerated stability studies
|
Parameters |
Time in months |
|||
|
0 |
1 |
2 |
3 |
|
|
Hardness (kg/cm2) |
3.27 |
3.27 |
3.27 |
3.26 |
|
Friability (%) |
0.33 |
0.33 |
0.34 |
0.35 |
|
disintegration time (sec.) |
11 |
11 |
12 |
12 |
|
Wetting time |
20.12 |
20.12 |
20.15 |
21.12 |
|
Drug Content (%) |
98.87 |
98.87 |
98.56 |
98.12 |
|
In-vitro drug release (%) |
95.75 |
95.76 |
95.21 |
95.12 |
All the developed Lamotrigine Orodispersible formulations were acceptable and their behavior in contact with water was very good as indicated by the short wetting time, rapid water sorption rate and a short disintegration time.
Wetting time is an indication of the hydrophilicity of the inner structure of the tablet and excepients used. Thus wetting time of a dosage form is related to contact angle. The lower the wetting time the quicker is the tablet disintegration. The disintegration time of orodispersible tablets ranges from 11 – 29 sec . The dissolution profiles of different Lamotrigine orodispersible tablet formulae indicate a higher, faster, and maximum drug release from formula F3 and F6 followed by F8 and F9 (Table. 1), where the percentage of drug release reached 90.75%, 93.19%, 93.26% and 95.75% respectively after 15 min. (Table 2, fig. 3-5) These results correlate with wetting and disintegration time data, and it reflects the effect of different formulation factors on tablet hydrophilicity. This can be attributed to the higher hydrophilic properties and stronger swelling power of Kyron T-314 in these formulations, while the presence of the very hydrophilic Super disintegrant in batches F8 and F9 resulted in a higher initial drug release. The release rate was also dependent on the Kyron T-314 proportion in both formulations.
Figure 1:Identification of Lamotrigine by IR Spectrum
Figure 2: Compatability of Lamotrigine with other excipients by IR Spectrum

Figure 3: In-vitro Disintegration Study

Figure 4: In-vitro Disolution Study
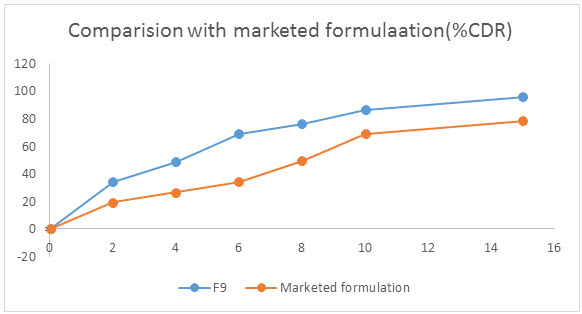
Figure 5: Comparision study with marketed formulation
CONCLUSION
An easy and economical method was implemented for the preparation of orodispersible tablets of lamotrigine solid dispersion using PEG 6000 as solubility enhancers and Kyron T-314, SSG and CCS as super disintegrant. The formula F8 and F9 was optimized as they showed satisfactory results in terms of disintegration time, wetting time and in vitro drug release (Table 2). These formula had shown better results in comparison with a marketed product. Optimized formulations showed physical stability when stored at 40°C under 75% RH for 3 months (Table. 3).
REFERENCES
1. Amrutkar P.; Design and evaluation of taste masked chewable dispersible tablet of lamotrigine by melt granulation; International Journal of Drug Delivery; 2010; 2; 183-191.
2. Horter D.; Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract; Adv. Drug Deliv. Rev; 25; 3–14.
3. Schott H.; The role of surfactant in the release of very slightly soluble drugs from tablets; J. Pharm.Sci; 71; 1038–45.
4. Chiou L.; and Riegelman S.; Pharmaceutical applications of solid dispersion systems; J. Pharm. Sci.; 60; 1281–1302.
5. Christian L.; and Dressman J.; Improving drug solubility for oral delivery using solid dispersions; Eur. J. Pharm. Biopharm; 50; 47–60.
6. Serajuddin A.; Solid dispersion of poorly water-soluble drugs: early promises; subsequent problems and recent breakthroughs; J. Pharm. Sci.; 88; 1058–66.
7. Kapure V.; Dissolution enhancement of rosuvastatin calcium by liquisolid compact technique; J Pharm; 1-9.
8. Lachman L; Lieberman A and Kanig L; The theory and practice of industrial pharmacy; Third Edition; Varghese Publication House; Bombay; India; 1987; 296-300.
9. Swamy P.; Orodispersible tablets of meloxicam using disintegrant blends for improved efficacy; Indian J Pharm Sci; 2007; 69(6); 836-840.
10. Mohsin A.; Formulation and evaluation of mouth dissolving tablets of amitriptyline hydrochloride by direct compression technique; Int J Pharm Pharm Sci.; 2010; 2(1); 204-210.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









