About Authors:
Mistry G. S*, Patel S. D, Tank H. M
Matushree V. B. Manvar College of Pharmacy
Dumiyani, Rajkot.
*Gaurav_mistry123@yahoo.com
ABSTRACT
Acyclovir is an Anti-viral drug, widely used in the treatment of Ocular herpes simplex. Ophthalmic insert of acyclovir formulated using Methyl cellulose (MC A4CP), polyvinylpyrrolidone (PVP K30) and polyvinyl alcohol as polymers and glycerin use as plasticizer by solvent casting method with aim of increasing the contact time, achieving sustained release drug. The prepared ophthalmic insert were evaluated for uniformity of thickness, weight uniformity, drug content, % moisture absorption, % moisture loss, folding endurance and surface pH. In vitro drug release of formulated batches was performed using Modified Franz Diffusion cell. A 32 full factorial design was applied to systematically optimize the ocular insert. FTIR spectroscopy was performed to study the drug interaction effect in formulation using KBr disc method. On the basis of all physicochemical parameters and in vitro drug release studies, and overall Desirability, the formulation (F8) was found to vary significantly depending on the type of polymers used and their combinations and it was selected for sterility, stability, ocular irritancy study. The result of invitro diffusion study of formulation exhibited non-fickian in nature. From stability studies inserts were remained stable both physically and chemically. The formulation was found to be practically nonirritant in ocular irritation studies using hen's egg chorioallantoic membrane.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1432
INTRODUCTION
Amongst the various routes of drug delivery, the field of ocular drug delivery is one of the most interesting and challenging endeavors facing the pharmaceutical scientist. The usefulness of this route of drug administration can be easily appreciated because the drug enters the systemic circulation circumventing the hepatic first pass effect.(1-5)
Ocular manifestations of HSV are varied and include blepharitis (inflammation of the eyelids), canalicular obstruction, conjunctivitis, corneal complications, iritis, and retinitisanging from a simple infection to a condition that can possibly cause blindness.6,7Acyclovir is an Anti-viral drug, widely used in the treatment of Ocular herpes simplex Ocular manifestations of HSV. Acyclovir which has less oral bioavailability and elimination half-lifeof acyclovir is approximately 2-3 hours.(8, 9, 10)
In the present study, an attempt has been made to formulate ocular insert of Acyclovir using polymers like PVP K30, methylcellulose (MC) and Glycerin use as plasticizer, by solvent casting method with aim of increasing the residence time, achieving controlled release, reduction in frequency of administration and greater therapeutic efficacy.
[adsense:468x15:2204050025]
MATERIALS AND METHODS
Materials
Acyclovir (%Purity-99.6%) was obtained as gift sample from Cadila pharmaceuticals Pvt. Ltd., Ahmedabad. PVP K90, PVP K30, PVA were obtained from SD Fine chemicals, Mumbai. All other reagent and solvent used were ofanalytical grade.
Methods
Preparation of the Ocular Insert(11, 12, 13)
The calculated amount of powdered polymers PVP/MC/PVA was dispersed in cold water with stirring and the prepared dispersion was then left in a refrigerator overnight.
The clear solution obtained which gelled at room temperature. The calculated amount of drug was mixed with glycerol in a small beaker. The final formulation contained acyclovir in polymeric matrix. The gel mass was then transferred to a Petri dish, and allowed to dry in an oven maintained at 50?C until a constant weight was reached. A flexible film was obtained. The dried film was cut into small circular inserts (10 mm diameter)
Experimental design
The formulations were fabricated according to a 32 full factorial design, allowing a simultaneous evaluation of two formulation variables and their interactions. The experimental design with corresponding formulations was given in Table 1.
X1: (%w/v) PVP K30, X2: Glycerin (% of polymer)
Y1: % Cumulative Drug Release at 10 hr.
Y2: Folding endurance
Y3: Thickness
Table 1: Formulation of Ocular Insert
|
|
Batch |
||||||||
|
INGREDIENT |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
F9 |
|
(X1,X2) |
(-1,-1) |
(0-1) |
(+1-1) |
(-1,0) |
(0,0) |
(+1,0) |
(-1+1) |
(0+1) |
(+1+1) |
|
Acyclovir (mg) |
162 |
162 |
162 |
162 |
162 |
162 |
162 |
162 |
162 |
|
MCA4CP (%w/v) |
2.50 |
2.50 |
2.50 |
2.50 |
2.50 |
2.50 |
2.50 |
2.50 |
2.50 |
|
PVPK30 (%w/v) |
2.25 |
2.50 |
2.75 |
2.25 |
2.50 |
2.75 |
2.25 |
2.50 |
2.75 |
|
GLYCERN (% of polymer) |
70 |
70 |
70 |
90 |
90 |
90 |
110 |
110 |
110 |
Drug- excipients compatibility study
Fourier-transformed infrared spectra were obtained on Shimadzu FTIR-8400 spectrophotometer using the KBr disk method (2 mg sample in 200 mg KBr).
Thickness Uniformity(14, 15)
The thickness of randomly selected ocuserts (n=3) was measured using Vernier calipers and mean thickness and standard deviation (SD) were calculated.
Weight Uniformity(14, 15)
The weight Uniformity test was carried out by weighing three inserts individually using digital balance.
Folding Endurance(11, 14)
This was determined by repeatedly folding the film at the same place until it broke. The number of times the film could be folded at the same place without breaking/cracking gave the value of folding endurance.
Surface pH (11, 14)
Surface pH was determined by allowing Ocular insert to swell in a closed petridish at room temperature for 30 minutes in 0.1ml of distilled water. pH paper was kept on surface and after one minutes the colour developed was compared with the standard colour scale.
Drug Content Uniformity(11, 15)
The optimized ocular insert of the drug was powdered in the mortar and dissolved in isotonic phosphate buffer (pH 7.4) and volume was made up to 100 ml. The solution was filtered and analyzed spectrophotometrically at 254 nm.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
(A) Moisture uptake(11, 15)
The percentage moisture uptake test was carried out to check physical stability or integrity of the film at humid condition. The films were weighed and placed in desiccatorscontaining 100 ml of saturated solution of aluminum chloride and 80% humidity was maintained. After three days, the films were taken out and reweighed.The % moisture uptake was calculated using the formulae
% Moisture uptake= final weight-initial weight
----------------------------
initial weight
(B) Moisture loss:
The percentage moisture loss was carried out to check the integrity of the film at dry condition. The films were weighted and kept in dessicator containing anhydrous calcium chloride. After three days, the films were taken out and reweighed.
The percentage moisture loss was calculated using the formula:
% Moisture loss = initial weight-final weight
--------------------------- x 100
initial weight
in vitro diffusion studies( 11, 16, 17)
The in-vitro diffusion studies were carried out by using bi-chambered donor receiver compartment model designed by using commercial semipermeable membrane of cellophane paper. The insert was placed inside the donor compartment. In order to simulate the tear volume, phosphate buffer pH 7.4 was placed and maintained at the same level throughout the study in the donor compartment. The semipermeable membrane was used to mimic in-vivo conditions like corneal epithelial barrier. The entire surface of the membrane was in contact with reservoir compartment which contains 30 ml phosphate buffer pH 7.4 and stirred continuously using a magnetic stirrer at 20 rpm to simulate blinking action. 2 ml of sample was withdrawn from the sampling port at periodic intervals and replaced with equal volume of phosphate buffer pH7.4. The drug content was analyzed at 254 nm against reference standard using phosphate buffer pH 7.4 as a blank on a UV–visible spectrophotometer.
Desirability Function(8)
The method was adopted to calculate the desirability of individual dependent variable and overall desirability by taking geometric mean. The batch having highest overall desirability (near to 1) value should be considered as an optimum batch. The combination of the responses in one desirability function requires the calculation of the individual functions. The individual desirability for each response was calculated as described below. %Cumulative Drug Release (d1), Folding Endurance (d2) and thickness (d3) are desirable.
The desirability function can be calculated using following equations:
d1 or d2 or d3= (Yi-Ymin)
------------------ ,for Yi ?Y target
(Ytarget-Ymin)
d1 or d2 or d3 = 1 , for Yi ? Y target
The overall desirability values were calculated from the individual values by using the following equation
D = (d1d2d3)1/3
Test of sterility(21)
In the present study, two media namely, fluid Thioglycollate Medium and Soya bean-Casein Digest (SBCD) medium were used to investigate the presence or absence of aerobic, anaerobic bacteria and fungi in the formulated sterilized delivery systems. The test for sterility of formulation was carried out according to the method prescribed in Indian Pharmacopoeia.
Comparison of in vitro drug release profile Ocular Insert with market acyclovir ointment
In Vitro Ocular Irritancy Test using chorioallantoic membrane (19.20)
Modified hen's egg chorioallantoic membrane (HET-CAM) test was carried out. Fertilized hen's eggs were obtained from poultry farm. Two eggs for formulation weighing between 50 and 60 g were selected. These eggs were incubated in humidified incubator at a temperature of 37ºC ± 0.5ºC for 3 days. The trays containing eggs were rotated manually in a gentle manner after every 12 h. On day 3, egg albumin was removed by using aseptic techniques from the pointed end of the egg. The eggs were kept in the equatorial position for the development of chorioallantoic membrane (CAM) away from the shell. The fifth day of incubation and every day, thereafter, nonviable embryos were removed. On the tenth day, a window (2 Χ 2 cm) was made on the eggs through which formulations) were instilled. A placebo film (0.6% carbopol and 1.4% HPMC) was used as a control as it is reported to be practically nonirritant and surface structure determination carried out.
Stability study(22, 23)
All characteristics were evaluated during the course of stability assessment. Physically stable ocular insert should retain its transparency, smoothness and drug content throughout its shelf life. To perform stability study the selected formulations were packed in aluminum foil and kept in dessicator containing saturated solution of aluminum chloride to maintain relative humidity up to 75% as per ICH guidelines for 30 days. The formulations were observed for transparency, smoothness and drug content at the interval of days.
RESULTS AND DISCUSSION
Drug- excipients compatibility stud
All the above characteristic peaks of drug appear in the spectra of ocular insert at the same wave number, indicating no modification or interaction between the drug and the excipients.
Thickness Uniformity
The insert thickness was varying from 0.14 ± 0.003 mm to 0.017 ± 0.008 mm. The dimensions of first ever commercially available ocular insert, OCUSERT® system by ALZA Corporation, Pilo-20 system is 0.30 mm thick, the Pilo-40 system is 0.50 mm thick. The prepared formulations were rather thinner than the commercially available ones, indicating their physiologic suitability
Table 2 ThicknessUniformity of Formulation F1-F9
|
BATCH |
Mean(mm)±SD |
BATCH |
Mean(mm)±SD |
|
F1 |
0.14±0.007 |
F6 |
0.17±0.002 |
|
F2 |
0.15±0.001 |
F7 |
0.15±0.002 |
|
F3 |
0.17±0.008 |
F8 |
0.16±0.009 |
|
F4 |
0.14±0.003 |
F9 |
0.17±0.001 |
|
F5 |
0.16±0.008 |
|
|
(Mean±SD, n=3)
WeightUniformity
The weight Uniformity studies as shown in table 3 suggested that weight of the ocular insert measured in range of 0.44±0.13 to 0.51±0.12 mg.
Table 3 WeightUniformity of Formulation F1-F9
|
BATCH |
Mean(mg)±SD |
BATCH |
Mean(mg)±SD |
|
F1 |
0.46±0.12 |
F6 |
0.52±0.14 |
|
F2 |
0.48±0.14 |
F7 |
0.44±0.13 |
|
F3 |
0.51±.0.12 |
F8 |
0.48±0.17 |
|
F4 |
0.45±0.31 |
F9 |
0.51±0.01 |
|
F5 |
0.50±0.22 |
|
|
(Mean±SD, n=3, 2×2 cm2)
Folding Endurance
Folding Endurance of Ocular insert found to be range of 378±2.12 to 405±1.09..
Table 4 Folding Endurance of Formulation F1-F9
|
BATCH |
Mean±SD |
BATCH |
Mean±SD |
|
F1 |
378±2.12 |
F6 |
399±4.76 |
|
F2 |
385±5.43 |
F7 |
400±1.65 |
|
F3 |
390±6.16 |
F8 |
402±1.54 |
|
F4 |
386±3.22 |
F9 |
405±1.09 |
|
F5 |
388±1.02 |
|
|
(a) Moisture Absorption (%)
Moisture absorption studies as shown in the table 4. Entire ocular insert Moisture absorption were found to be in the range of 2.21±0.45 (%) to 4.78±0.23 (%). The study suggested that ocular insert have high moisture uptake.
Table 5Moisture absorption (%) of Formulation F1-F9
|
BATCH |
Mean±SD |
BATCH |
Mean±SD |
|
F1 |
2.21±0.45 |
F6 |
4.36±0.07 |
|
F2 |
2.25±0.12 |
F7 |
2.12±0.18 |
|
F3 |
4.23±0.19 |
F8 |
3.74±0.25 |
|
F4 |
4.78±0.23 |
F9 |
2.31±0.15 |
|
F5 |
4.56±0.08 |
|
|
(Mean±SD,n=3)
(b)MOISTURE LOSS (%)
Moisture loss studies as shown in the table 6. suggested that the entire ocular insert were found to be in the range of 0.32±0.018 (%) to 0.80±0.059 (%). The study suggested that ocular insert have moisture loss very less as ocular insert were dried completely.
Table 6Moisture Loss(%) of Formulation F1-F9
|
BATCH |
Mean±SD |
BATCH |
Mean±SD |
|
F1 |
0.44±0.021 |
F6 |
0.76±0.043 |
|
F2 |
0.32±0.018 |
F7 |
080±0.059 |
|
F3 |
0.71±0.061 |
F8 |
0.61±0.082 |
|
F4 |
0.66±0.023 |
F9 |
0.65±0.016 |
|
F5 |
0.51±0.065 |
|
|
(Mean±SD, n=3)
Surface pH
pH was all formulation found to be the range between of 6.8-7.4.which was good for ocular formulation.
Table 7 Surface pH of Formulation F1-F9
|
BATCH |
Surface pH |
BATCH |
Surface pH |
|
F1 |
7.2 |
F6 |
7.3 |
|
F2 |
6.8 |
F7 |
7.0 |
|
F3 |
6.9 |
F8 |
7.1 |
|
F4 |
7.4 |
F9 |
7.2 |
|
F5 |
6.8 |
|
|
CONTENT UNIFORMITY (%)
Drug contentuniformityof the ocular inserts range from 94.56±0.02 (%) to 100.53±0.02(%). It suggested that the drug dispersed throughout all the formulation.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table 8 ContentUniformity (%) of Formulation F1-F9
|
BATCH |
Mean±SD |
BATCH |
Mean±SD |
|
F1 |
98.07±0.01 |
F6 |
98.52±0.02 |
|
F2 |
97.13±0.05 |
F7 |
99.54±0.02 |
|
F3 |
94.56±0.03 |
F8 |
96.62±0.04 |
|
F4 |
97.89±0.01 |
F9 |
100.53±0.02 |
|
F5 |
94.84±0.01 |
|
|
(Mean±SD, n=3)
In vitro Diffusion study

Figure 1 Comparison of %drug release from F1 to F9
Table 9 Release kinetics for batches F1 – F9
|
Batch no. |
Zero order kinetics |
Higuchi kinetics |
First order kinetic |
Korsmeyer Peppas |
Mechanism of drug release |
|
|
|
R2 |
R2 |
R2 |
R2 |
n |
|
|
F1 |
0.9810 |
0.7827 |
0.8630 |
0.9820 |
0.63 |
Non-Fickian |
|
F2 |
0.9880 |
0.8668 |
0.9223 |
0.9980 |
0.87 |
Non-Fickian |
|
F3 |
0.9442 |
0.9060 |
0.9482 |
0.9899 |
0.65 |
Non-Fickian |
|
F4 |
0.9723 |
0.8208 |
0.9018 |
0.9745 |
0.63 |
Non-Fickian |
|
F5 |
0.9408 |
0.9155 |
0.9389 |
0.9924 |
0.75 |
Non-Fickian |
|
F6 |
0.9110 |
0.9390 |
0.9672 |
0.9963 |
0.70 |
Non-Fickian |
|
F7 |
0.9199 |
0.9145 |
0.9550 |
0.9948 |
0.73 |
Non-Fickian |
|
F8 |
0.9323 |
0.9123 |
0.9492 |
0.9635 |
0.74 |
Non-Fickian |
|
F9 |
0.9246 |
0.8930 |
0.9467 |
0.9873 |
0.75 |
Non-Fickian |
The release constants were calculated from the slope of the respective plots. It indicates that the release of drug from the films might have followed first order kinetics. For planer geometry, the value of n=0.5 indicates a Fickian diffusion mechanism, for 0.5<n<1.0, indicates anomalous (non-fickian) transport, and n=1 implies (relaxation controlled) transport.
In the present systems, the value for n was found to be in the range of 0.63 to 0.87 indicating that the release mechanisms followed fickian diffusion and anomalous (non-fickian) transport. The optimized formulation (F8) was having n=0.74, indicating that the release mechanism followed is non-fickian diffusion controlled mechanism.
Statistical Analysis
Statistical analysis was done using Microsoft Excel 2007. Predicted and residual values of all formulation were obtained from Microsoft Excel 2007.
Contour plot and surface plot of the design
Contour plots and surface plots were drawn using the Statgraphic 16.1.17. These types of plots were useful in study of the effects of two factors on the response at one time.

SELECTION OF OPTIMIZED FORMULA
Table 10 Overall Desirability
|
|
Response value |
|||
|
Batch |
% Drug Release |
Folding Endurance |
Thickness (mm) |
Overall Desirability |
|
F1 |
99.64 |
378 |
0.15 |
0.00 |
|
F2 |
90.12 |
385 |
0.15 |
0.29 |
|
F3 |
89.25 |
390 |
0.17 |
0.45 |
|
F4 |
88.11 |
386 |
0.14 |
0.00 |
|
F5 |
86.34 |
388 |
0.16 |
0.00 |
|
F6 |
87.65 |
399 |
0.17 |
0.42 |
|
F7 |
89.45 |
400 |
0.15 |
0.39 |
|
F8 |
91.22 |
402 |
0.16 |
0.66 |
|
F9 |
87.77 |
405 |
0.17 |
0.47 |
From the polynomial equation, the contour plots, and Overall Desirability the optimized batch was found batch F8.
The selected optimized batch F8 was subjected for stability testing and sterility testing study as per Pharmacopoeia. Formulation of F8 batch is given in table 10
Comparison of in vitro drug release profile of acyclovir ocular insert with market acyclovir ointment
The percentage drug release was in ointment and acyclovir ocular insert determined. From the results it was found that, drug release of market acyclovir Ointment formulation after 5th hour was found to be more than 90% and. Drug released from the optimized formulation from 95-99% within 8-12h.
The results are depicted in Figure 5 as per comparison study sustained release of acyclovir obtained ophthalmic ocular insert.
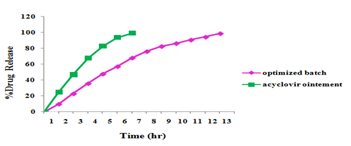
Figure 5 Comparison of in vitro drug release profile between marketed acyclovir Ointment & optimized Ocular Insert
Stability Study of Optimized Batch F8
Short term Stability study was carried at 35?C-40?C and 75% RH for one month storage. After one month ocular insert were analyzed by various parameters to check whether the formulation was stable or not. The drug content, folding endurance, thickness of ocular insert before one month 99.54±0.02, 400±1.65,0.15±0.002and after one month stability study it was found 98.11 ± 0.014 , 390± 5.12, 0.14± 0.001. ((Mean±SD, n=3). The change in the drug release i.e. in vitro release profile was not significantly different from the one month’s stability study. It was confirmed by ‘t’ test. In t-test calculated t was 0.0012 and t table 2.10. t-statistical value is less than t- table hence formulation showed insignificant difference. Unpaired ‘t’ test between before and after 30 days of batch F8 showed insignificant difference (tcal .< ttab.). So, conclude that drug was stable at accelerated condition.
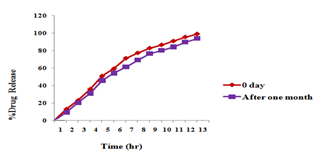
Figure 6 Comparison of in vitro drug release profile for StabilityStudy
After evaluation conclude That Selected batch F8 showed negligible change in drug content, folding endurance, thickness & % drug release and can be inferred that ocular insert do not show any effect at room temperature.
Sterility Testing
The ocular inserts were sterilized by UV radiation. After sterilization ocular insert transferred into polyethylene bag with help of forcep. Sterility test of ocular insert carried out using fluid thioglycollate media and soybene casin media for detect aerobes, anaerobes and fungi. End of sterility testing period examine the media for microscopic evidence of microbial growth. It was found visually that the fluid thioglycollate media containing sterilized ocular inserts and soybene casin media containing sterilized ocular inserts were free from turbidity. This confirms the absence of aerobic bacteria and anaerobic bacteria and fungi.
in vitro Ocular Irritancy Test
Hen's egg chorioallantoic membrane is, complete tissue, including veins, arteries, and capillaries, and is technically very easy to study. It responds to injury with a complete inflammatory process, which similar to that induced in the conjunctival tissue of the rabbit eyes.
The formulation developed was tested by this method and the result was compared with those obtained using normal saline film, which was used as the control that supposed to be practically nonirritant. Because of product not produce any injury like structure in the part of chorioallantoic membrane so formulated ocular insert practically non irritant.
CONCLUSION
Ocular insert of acyclovir was satisfactorily formed with appropriate physical characteristic. The Ocular insert of acyclovir was found appropriate for the sustained release of acyclovir, with ideal sterility and stability. The acyclovir release from the ocular insert was sustained up to 12 hours by optimizing amount of PVPK30 and Glycerin.
So acyclovir could be successfully administered through sustained release ocular inserts for treatment of ocular Herpes Simplex infection.
REFERENCES
1. Rajasekaran A, Gaul K, Kumaran J, Preetha P, “A comparative review on conventional and advanced ocular drug delivery formulations” Int. J. Pharmtech. Res. , 2(1), 668-674. 2010.
2. Rajasekaran A, Gaul K, Kumaran J, Preetha P, “A comparative review on conventional and advanced ocular drug delivery formulations” Int. J. Pharmtech. Res., 2(1), 668-674, 2010
3. Daniel J. H, “New Controlled Release Technologies Broaden Opportunities for Ophthalmic Therapies” Drug Deli. Techno, 8(7) 2008
4. Arto Urtti, “Challenges and obstacles of ocular pharmacokinetics” Adv. Drug Deli. Reviews, 58, 1131–1135. 2006,
5. Yasukawa T, Ogura Y, Sakurai E, Tabata Y, Kimura H, “Intraocular sustained drug delivery using implantable polymeric devices” Adv. Drug Deliv. Reviews 2005, 57, 2033– 2204.
6. Nigel. H. Barker, Ocular Herpes Simplex, clinicalevidence.
7. Cabezas A, “Treatment of mild keratitis with Acyclovir eye drops a new pharmaceutical formulation” Eur. J. Clin. Pharmacol. 40, 533-534, 1991,
8. allaboutvision.com/conditions/ocular-herpes.htm
9. John M, James M, Barrie R. Jones “Summary code for ocular herpes simplex” Brit. Y. Ophth. 59, 539, 1975,
10. umetrics.com/pdfs/books/DOEBook.pdf
11. Rajasekaran A, Sivakumar V, Karthika K, Padma Preetha J, Abirami T, “Design and evaluation of Polymeric Controlled release Natamycin Ocular Insert.” Kathmandu university J. Science, Engineering and Technology, 108-115. 2010,
12. Kumaresan C, “Development of novel ocusert contains Norfloxacin and in-vitro evaluation” J. Pharmacy Res. 4(2),393-395, 2011,
13. Patel U. L, Chotai N. P, Nagda C. D, Patel M. P, Patel K.N. “Formulation and in vitro evaluation of moxifloxacin hydrochloride ophthalmic inserts” Int. J. of Pharma Res, 2009, 1(1), 23-30
14. Renu K. “An ocular insert: overview” Int. J. Pharm. 2011, 3(6), 48-152
15. SarathChandran C, Arun S, Sarala A, “Development and Evaluation of Chitosan Ocuserts Containing Ciprofloxacin - b CD Complex” Int. J. Pharm Tech. Res. 2010 , 1, 246-252
16. Dipiti P, Patel M, Patel N. “Formulation and Optimization Of Two Days Ophthalmic Inserts of Brimonidine Tartrate” 12, 123-133, 2001,
17. Di Colo G, Burgalassi S, Chetoni P, Zambito Y, “Gel-forming erodible inserts for ocular controlled delivery of Ofloxacin.” Int. J. Pharma251,101–111, . 2001,
18. Ganesh R, Falguni M, Jayvadan P, “Formulation and Evaluation of Mucoadhesive Glipizide Films” Acta Pharm. 61, 203–216, 2011,
19. Ritu M, Kalpana N, Dinanath M, “Azithromycin novel drug delivery system for ocular application” Original Research Article, 1(1), 22-28, 2011
20. Bagley M, Water D, Kong M B, “ Development of A 10-Day Chorioallantoic Membrane Vascular Assay as an Alternative to the Dranize Rabbit Eye Irritation Test” fd. Chem. Toxic 32 (12), 1160-1994, 1994,
21. Ganesh R, Falguni M, Jayvadan P, “Formulation and Evaluation of Mucoadhesive Glipizide Films” Acta Pharm. 61, 203–216, 2011
22. Note for guidance on stability testing. “Stability testing of new drug substances www.ich.org/cache/compo/363-272-1.html.and products”. [online] [Cited 2012 Jan. 7] Available from URL,2003
23. Remington: The Science and Practice of Pharmacy, Lippincott Williams & Wilkins, New York 1(1) Edn: 2006
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









