About Authors:
*Thoriya J. G, Patel S. D, Tank H. M
Matushree V. B. Manvar College of Pharmacy- Dumiyani,
Rajkot
*thoriya.jignesh@gmail.com
ABSTRACT
Pioglitazone HCl is used for the management of type-2 diabetes. It is an absorption window limited drug, whose solubility decreases with increase in the pH and has a short half life of 3-7 h. Here an attempt is made to developed the floating matrix tablets, which design in way that after oral administration the GI resistant time is prolonged and thus to give sustained action with increase in the bioavailability of the drug. Pioglitazone HCl showed maximum absorption at wavelength 269 nm in 0.1N HCl. various formulations were developed by using release rate controlling and gel forming polymers like HPMC, and Carbopol-934 in single and combinations by direct compression method with the incorporation of sodium bicarbonate as gas generating agent. The prepared tablets were characteristics by drug content, floating property, swelling and in vitro dissolution test using USP dissolution test apparatus Type – II (paddle method) in dissolution medium of 0.1 N HCl. The in vitro dissolution results of all tablets were computed by using dissolution software. The prepared tablets were found to be good hardness, diameter, weight variation, thickness, friability drug content, floating property and in vitro drug release. Drug-polymer compatibility studies by FTIR gave conformation about drug purity and showed no interaction between drug and selected polymers. All the formulations had floating lag time below 3 minutes and constantly floated on dissolution medium for more than 12 h. Swelling studies indicated significant water uptake and contributed in drug release. From among all the developed formulations, as F7 prolonged the drug release (95.45 %) for longer period of time (12 hrs.); they were selected as best formulations. The best formulations were found to be stable during stability studies for two months. Thus, selected formulations satisfied floating time, swelling index and in vitro drug release profile requirements for a floating drug delivery system.Tablets of Pioglitazone HCl prepared with HPMC K4M, HPMC K100M and Carbopol 934P were found to be acceptable floating property, water uptake and in vitro drug release.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1376
1. INTRODUCTION
Development of newer drugs and medicines will be the goal of scientists across the world. In order to achieve satisfying results, a drug has to be properly formulated in proper dosage form. It is an established fact that the conventional immediate release drug delivery systems when taken frequently in a day can maintain drug concentration levels in therapeutically effective range. However, this result in significant fluctuations in plasma drug levels recently, several technical advancements have led to the development of various Novel Drug Delivery Systems (NDDS) that could revolutionize method of drug delivery and hence could provide definite therapeutic benefits.1
Oral route of administration is the most important and convenient route for drug delivery. Due to differential absorption from various regions of GI, the benefits of long-term delivery technology have not been fully realized for dosage forms designed for oral administration. Only recently drug delivery systems have been designed to target drugs to differential regions of GIT. These include gastro retentive systems, delayed release systems and colon targeting. The real issue in the development of oral controlled release dosage form is not just to prolong the delivery of drugs for more than 12 h but also to prolong the presence of dosage forms in the stomach or somewhere in the upper small intestine. Dosage forms with prolonged gastric residence time (GRT), i.e. gastro remaining or GRDF will bring about new and important therapeutic options.2, 3
Various approaches have been developed for gastric retention which includes Low density systems or floating delivery, High density (sinking) systems or non floating delivery, Swelling and expandable systems and Mucoadhesive/bioadhesive systems.Floatingsystems are also known as hydro dynamically balanced systems. (HBS/FDDS) They have a bulk density lower than gastric fluid (i.e. < 1.004 gm/ml)the specific gravity of gastric fluid is approximately 1.004-1.010 g/cm3 according to the “Documenta Geigy” and thus the FDDS remains buoyant in the stomach without affecting the gastric emptying rate for a prolonged period of time. It is an oral dosage form that is designed to prolong the residence time of the dosage form within the GIT. Based on the mechanism of buoyancy, two distinctly different technologies have been utilized in development of FDDS that are: Effervescent System, and Non- Effervescent System.
The Non effervescent floating drug delivery system is based on mechanism of swelling of polymer or bioadhesion to mucosal layer in GIT. The most commonly used excipients in non-effervescent floating drug delivery system are gel forming or highly swellable cellulose type hydrocolloids, polysaccharides and matrix forming material such as polycarbonate, polyacrylate, polymethacrylate, polystyrene as well as bioadhesive polymer such as chitosan and carbopol.4, 5
[adsense:468x15:2204050025]
2. MATERIALS AND METHODS
2.1 Materials
Pioglitazone HCl was received from Taj pharmaceutical, Hyderabad, Methocel® K4M, K15MandK100M which are commercially available grades of hydroxypropyl methylcellulose from SEVA Fine Chemicals, Ahmadabad, carbopol 934P and sodium bicarbonate from Oxford Lab. Reagent, Mumbai Microcrystalline Cellulose (MCC), magnesium stearate and talc from SEVA Fine Chemicals, Ahmadabad.
2.2 Methods
2.2.1 Preparation of floating tablets
Pioglitazone HCl, selected polymers, sodium bicarbonate and lactose were taken in required quantities and passed through 60 # separately. In dry state, the drug with other ingredients was mixed for the period of 10 min in mortar to get uniform mixture power. The mixture was blended with Magnesium Stearate for 2-3 min. to improve flow property. The powder was compressed into tablets using a Rotary tablet press.6, 7
2.2.2 Experimental design
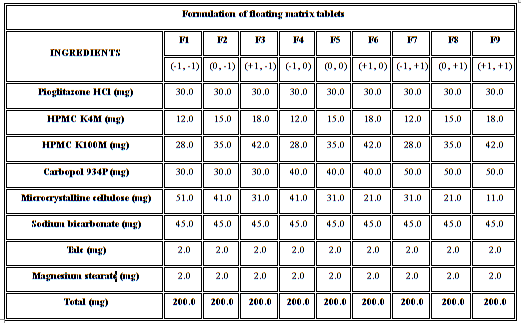
The formulations were fabricated according to a 32 full factorial design, allowing a simultaneous evaluation of two formulation variables and their interactions. The experimental design with corresponding formulations was given in Table 1.8
X1: HPMC K4M: HPMC K100M (mg)
X2: Concentration of Carbopol (mg)
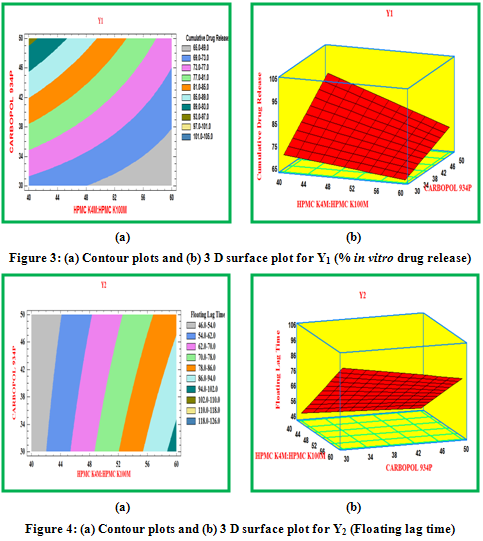
Y1: % cumulative drug release at 12 hr.
Y2: Floating lag time
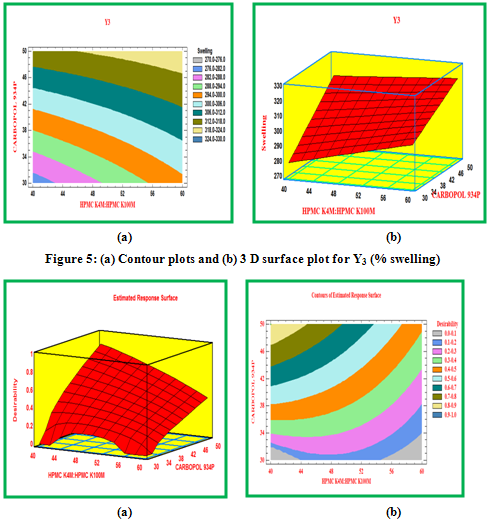
Y3: % Swelling
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
2.2.3 Floating lag time
The lag time was carried out in beaker containing 100 ml of 0.1 N HCl as a testing medium maintained at 37 °C. The time required for the tablet to rise to the surface and float was determined as floating lag time.9
2.2.4 Total floating time
Floating time was the time, during which the tablet floats in 0.1 N HCl dissolution medium (including floating lag time).9
2.2.5 Swelling characteristics
The swelling properties of matrix tablet containing drug were determined by placing the tablet matrices in the USP Dissolution Testing Apparatus II, in 900 ml of 0.1 N HCl at 37 ± 0.5 °C, rotated at 50 rpm. The tablets were removed periodically from dissolution medium, blotted to remove excess water and weighed. Swelling characteristics were expressed in terms of percentage water uptake (WU %) according to the equation.10
Wt. of swollen tablet – Initial wt. of the tablet
WU % =---------------------------------------------------- x 100
Initial wt. of the table
2.2.6 Dissolution studies
The release rate of Pioglitazone HCl from floating tablets was determined using USP Dissolution Testing Apparatus II (Paddle type). The dissolution test was performed using 900 ml of 0.1 N HCl, at 37 ± 0.5 °C and 50 rpm. Aliquot volume was withdrawn from the dissolution apparatus hourly for 12 h, and the samples were replaced with fresh dissolution medium. After filtration and suitable dilution the amount of drug release was determined from the calibration curve.11
Details of Dissolution Test:
1.Apparatus : USP Type II
2.Volume of medium : 900 ml
3.Temperature : 37 °C
4.Paddle Speed : 50 rpm
5.Dissolution medium used : 0.1 N HCl
6.Aliquot taken at each time interval : 5 ml
2.2.7 Stability study
Stability testing of drug products begins as a part of drug discovery and ends with the demise of the compound or commercial product. To assess the drug and formulation stability, stability studies were done according to ICH guidelines Q1C.The stability studies were carried out on the most satisfactory formulations as per ICH guidelines Q1C. The most satisfactory formulation sealed in aluminum packaging and kept in humidity chamber maintained at 30 ± 2 °C / 65 ± 5 %RH and 40 ± 2 °C / 75 ± 5 %RH for 1 months. At the end of studies, samples were analyzed for the drug content, in vitro dissolution, floating behavior and other physicochemical parameters.12
Table 1: Formulation of floating matrix tablets
3. RESULTS AND DISCUSSION
3.1 Floating lag time
On contact with the dissolution medium, medium reacted with sodium bicarbonate in the floating tablet, inducing CO2 formation in the floating tablet. Because the gas generated is trapped in and protected by the gel formed by the hydration of HPMC, the expansion of the floating section keeps the whole tablet buoyant on the surface of the test medium. Floating lag time of the all 32 formulations was found between 47-100 second.
3.2 Total floating time
From the results of total floating time it can be concluded that all 32 formulations showed good duration of floating i.e. floating time more than 12 hours.
Table 2: Lag time and Floating time of formulation F1 to F9
|
Batch |
Lag Time (sec.) (n=3) |
Total Floating Time (hr.) (n=3) |
|
F1 |
50 |
> 12 |
|
F2 |
68 |
> 12 |
|
F3 |
100 |
> 12 |
|
F4 |
54 |
> 12 |
|
F5 |
63 |
> 12 |
|
F6 |
93 |
> 12 |
|
F7 |
47 |
> 12 |
|
F8 |
60 |
> 12 |
|
F9 |
87 |
> 12 |
3.3 Swelling characteristics
Swelling study was performed on all the formulation for 12 hr. The results of swelling index were given in Table 3, while the plot of %swelling index against time (hr) was depicted in Figure 5.6. From the results it was concluded that swelling increases as the time passes because the polymer gradually absorb water due to hydrophilicity of polymer. The outer most hydrophilic polymer hydrates and swells and a gel barrier are formed at the outer surface. In the present study, the higher swelling index was found for tablets of batch F9 containing higher concentration of HPMC K100M and Carbopol 934P.
Table 3: Swelling data offormulation F1 – F9
|
Batch No. |
1 hr |
4 hr |
8 hr |
12 hr |
|
F1 (%) |
50.45±0.75 |
109.18±2.46 |
165.76±1.27 |
282.39±2.01 |
|
F2 (%) |
43.31±2.03 |
99.38±0.35 |
161.43±1.05 |
291.78±0.04 |
|
F3 (%) |
39.38±1.55 |
97.10±0.87 |
180.13±0.08 |
305.65±0.10 |
|
F4 (%) |
48.11±1.36 |
103.46±1.17 |
178.08±0.9 |
290.19±1.41 |
|
F5 (%) |
50.13±0.68 |
106.36±2.65 |
180.33±2.09 |
299.15±1.09 |
|
F6 (%) |
41.76±0.53 |
100.67±1.07 |
188.89±1.04 |
295.03±3.06 |
|
F7 (%) |
39.45±2.95 |
95.93±0.61 |
169.08±3.26 |
319.23±2.40 |
|
F8 (%) |
51.89±3.03 |
105.25±2.41 |
171.65±1.83 |
323.28±2.01 |
|
F9 (%) |
55.41±0.49 |
110.08±0.84 |
174.49±0.48 |
328.71±0.57 |
Mean (3) ± SD
Figure 1: Swelling data of formulation F1 to F9
3.4 Dissolution studies
The progressive decrease in the amount of drug release from formulations attributed to gradual decrease in HPMC content as well as increase in Carbopol-934P content. It has been concluded that a combination of anionic polymer Carbopol-934P with non ionic HPMC produce a synergistic increase in the viscosity which could be due to the stronger hydrogen bonding between the carboxyl group of Carbopol and hydroxyl group of HPMC leading to stronger cross linking between two polymers and diminished the release.Drug release was between 66.58 to 94.45 %.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
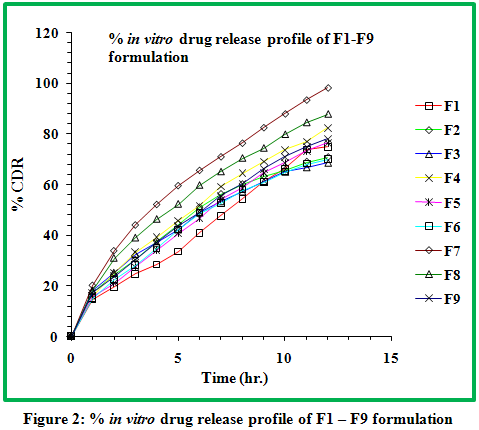
Table 5: Kinetic values obtained from in-vitro release profile
|
Batch No. |
Zero order |
First order |
Higuchi |
Hixon Crowell |
||||
|
R2 |
k0 |
R2 |
k1 |
R2 |
kH |
R2 |
kHC |
|
|
F1 |
0.9735 |
6.574 |
0.9747 |
0.099 |
0.9170 |
18.853 |
0.9838 |
0.029 |
|
F2 |
0.8258 |
6.874 |
0.9768 |
0.111 |
0.9878 |
20.131 |
0.9486 |
0.032 |
|
F3 |
0.8012 |
6.661 |
0.9620 |
0.106 |
0.9948 |
19.549 |
0.9289 |
0.030 |
|
F4 |
0.9054 |
7.547 |
0.9948 |
0.128 |
0.9765 |
21.939 |
0.9871 |
0.036 |
|
F5 |
0.9375 |
6.960 |
0.9928 |
0.110 |
0.9596 |
20.137 |
0.9902 |
0.032 |
|
F6 |
0.8771 |
6.667 |
0.9881 |
0.105 |
0.9802 |
19.435 |
0.9691 |
0.030 |
|
F7 |
0.8433 |
9.149 |
0.9905 |
0.189 |
0.9918 |
26.764 |
0.9848 |
0.051 |
|
F8 |
0.8473 |
8.271 |
0.9933 |
0.154 |
0.9909 |
24.186 |
0.9783 |
0.042 |
|
F9 |
0.9086 |
7.182 |
0.9910 |
0.117 |
0.9752 |
20.867 |
0.9825 |
0.033 |
NOTE:R2= coefficient of determination, k0=Zero-order release constant, k1= First-order release constant, kH= Higuchi release constant, kHC= Hixon Crowell release constant.
Table 6: Kinetic values obtained from in-vitro release profile
|
Batch No. |
Korsmeyer Peppas |
Mechanism of drug release |
||
|
R2 |
n |
kKP |
||
|
F1 |
0.9909 |
0.816 |
9.821 |
Non-Fickian |
|
F2 |
0.9932 |
0.564 |
17.655 |
Non-Fickian |
|
F3 |
0.9969 |
0.540 |
18.036 |
Non-Fickian |
|
F4 |
0.9989 |
0.644 |
16.349 |
Non-Fickian |
|
F5 |
0.9973 |
0.698 |
13.404 |
Non-Fickian |
|
F6 |
0.9953 |
0.614 |
15.405 |
Non-Fickian |
|
F7 |
0.9987 |
0.574 |
23.037 |
Non-Fickian |
|
F8 |
0.9986 |
0.578 |
20.628 |
Non-Fickian |
|
F9 |
0.9985 |
0.648 |
15.419 |
Non-Fickian |
NOTE:R2= coefficient of determination, n= diffusional exponent, kKP= Korsmeyer Peppas release constant
From the Korsmeyer Peppas equation, the diffusion exponent ranges from 0.540 to 0.816. From the results, all formulations showed non-Fickian release. Coefficients of correlation (R2) were used to evaluate the accuracy of the fit. The R2 values were given in Table 5 and 6.
3.5 Statistical Analysis
Statistical analysis was done using Microsoft Excel 2007. Predicted and residual values of all formulation were obtained from Microsoft Excel 2007.
Table 7: Summary of results of multiple regression analysis for Y1, Y2 and Y3
|
Dependent variables |
Response Y1 |
Response Y2 |
Response Y3 |
|||
|
P value |
Coefficients |
P value |
Coefficients |
P value |
Coefficients |
|
|
Intercept |
0.0110 |
69.6833 |
0.1499 |
105.8889 |
0.0643 |
323.8256 |
|
X1 |
0.0049 |
-0.3065 |
0.0064 |
5.01667 |
0.4043 |
3.2077 |
|
X2 |
0.6394 |
0.1744 |
0.0289 |
-1.916667 |
0.0464 |
7.7275 |
|
X12 |
0.0012 |
-0.032 |
0.1298 |
-0.025 |
0.2584 |
-0.034 |
|
X12 |
0.0034 |
0.0035 |
0.0173 |
0.081667 |
0.7538 |
-0.012 |
|
X22 |
0.4225 |
0.033 |
0.4913 |
-0.01333 |
0.0296 |
0.14 |
Table 8: Statistical regression analysis data
|
Batch No. |
Response Y1 (In vitro drug release at 12 hr.) |
Response Y2 (Floating lag time) |
Response Y3 (% Swelling) |
||||||
|
Y1 |
Predicted |
Residual |
Y2 |
Predicted |
Residual |
Y3 |
Predicted |
Residual |
|
|
F1 |
73.08 |
72.72 |
0.36 |
50 |
51.39 |
-1.39 |
282.39 |
283.16 |
-0.77 |
|
F2 |
68.75 |
69.23 |
-0.48 |
68 |
67.22 |
0.78 |
291.78 |
294.08 |
-2.3 |
|
F3 |
66.58 |
66.46 |
0.12 |
100 |
99.39 |
0.61 |
305.65 |
302.58 |
3.07 |
|
F4 |
80.28 |
80.77 |
-0.49 |
54 |
51.22 |
2.78 |
290.19 |
288.13 |
2.06 |
|
F5 |
74.52 |
74.04 |
0.48 |
63 |
64.56 |
-1.56 |
299.15 |
295.59 |
3.56 |
|
F6 |
68.02 |
68.02 |
0.00 |
93 |
94.22 |
-1.22 |
295.03 |
300.65 |
-5.62 |
|
F7 |
95.45 |
95.33 |
0.12 |
47 |
48.39 |
-1.39 |
319.23 |
320.52 |
-1.29 |
|
F8 |
85.35 |
85.35 |
0.00 |
60 |
59.22 |
0.78 |
323.28 |
324.54 |
-1.26 |
|
F9 |
75.96 |
76.08 |
-0.12 |
87 |
86.39 |
0.61 |
328.71 |
326.16 |
2.55 |
3.6 Contour plot and surface plot of the design
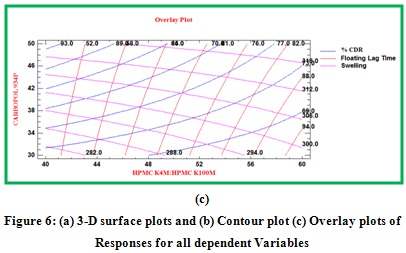
Contour plots and surface plots were drawn using the Statgraphic 16.1.17. These types of plots were useful in study of the effects of two factors on the response at one time.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table 9: ANOVA for dependent variables
|
Source |
Sum of Squares |
Degrees of Freedom |
Mean Square |
F Value |
Significance |
|
For Y1 = % Cumulative drug release |
|||||
|
Regression |
697.0913 |
5 |
139.4183 |
474.7994 |
0.000152 |
|
Residual |
0.880908 |
3 |
0.293636 |
--- |
--- |
|
Total |
697.9722 |
8 |
--- |
--- |
--- |
|
For Y2 = Floating lag time |
|||||
|
Regression |
3031.444 |
5 |
606.2889 |
104.2662 |
0.001462 |
|
Residual |
17.44444 |
3 |
5.814815 |
--- |
--- |
|
Total |
3048.889 |
8 |
--- |
--- |
--- |
|
For Y3 = % Swelling |
|||||
|
Regression |
2054.365 |
5 |
410.8729 |
16.7488 |
0.021162 |
|
Residual |
73.59448 |
3 |
24.53149 |
--- |
--- |
|
Total |
2127.959 |
8 |
--- |
--- |
--- |
All variables show significant F value, less than 0.05. All three variables showed significant change in the responses.
3.7 Stability Study of Optimized Batch F7
Stability study was done to see the effect of temperature and humidity on tablets during the storage time. Tablets were evaluated periodically (0 and 1 months) for hardness, swelling index, floating test, drug content and in vitro drug release. Results of stability study were given in Table 10 and 11.
Table 10: Evaluation of formulation F7 for Stability
|
Tested after days |
Hardness (kg/cm2) |
Swelling index (%) |
Floating test |
Drug Content (%) |
|
|
FLT (sec) |
TFT (hrs) |
||||
|
At 30 ± 2 °C / 65 ± 5 % RH |
|||||
|
0 |
4.70 |
311.25 |
48 |
>12 |
99.62 |
|
30 |
4.80 |
317.72 |
47 |
>12 |
98.90 |
|
At 40 ± 2 °C / 75 ± 5 % RH |
|||||
|
0 |
4.70 |
311.25 |
48 |
>12 |
99.62 |
|
30 |
4.70 |
313.04 |
50 |
>12 |
99.07 |
Table 11: % in vitro drug release profile of formulation F7 for Stability
|
Time (hr.) |
Tested after time (day) |
||
|
Initial |
At 30 ± 2 °C / 65 ± 5 % RH |
At 40 ± 2 °C / 75 ± 5 % RH |
|
|
30 day |
30 day |
||
|
1 |
20.25 |
20.97 |
20.11 |
|
2 |
34.01 |
35.17 |
33.67 |
|
3 |
44.14 |
45.09 |
43.84 |
|
4 |
52.10 |
52.54 |
52.07 |
|
5 |
59.33 |
58.89 |
58.94 |
|
6 |
65.11 |
64.73 |
65.41 |
|
7 |
70.17 |
70.59 |
71.02 |
|
8 |
75.23 |
76.04 |
76.59 |
|
9 |
81.00 |
81.87 |
81.62 |
|
10 |
86.06 |
87.21 |
87.55 |
|
11 |
91.12 |
92.01 |
92.39 |
|
12 |
95.45 |
96.37 |
96.83 |
No significant changes were observed in any of study parameter during study period. So, batch F7 was stable.
4. CONCLUSION
Floating tablets of pioglitazone HCl was satisfactorily formed with appropriate physical characteristic. The Floating tablets of pioglitazone HCl was found appropriate for the sustained release of pioglitazone HCl with acceptable floating property. The pioglitazone HCl release from the floating tablets was sustained up to 12 hours by optimizing amount of (HPMC K4M: HPMC K100M) and Carbopol 934P. So pioglitazone HCl could be successfully administered through sustains release floating tablets for treatment of diabetis.
5. REFERENCES
1. Chain Y. W, Novel Drug Delivery Systems, 2nd Edn, Marcel Dekker, New York, 1992, 50.
2. Shweta A, Javed A, Alka A, Roopb K. K and Sanjula B, “Floating drug delivery systems: a review”, AAPS Pharma. Sci. Tech. 2005, 06(3), 267-271.
3. Mayavanshi V. S. and Gajjar S. “Floating drug delivery systems to increase gastric retention of drugs: A review”, Res. J. Pharma. Tech., 2008, 1(4), 1-9.
4. Talukder R. and Fassihi R. “Gastroretentive delivery system: A mini review”, Drug Dev. Ind. Pharm., 2004, 30(10), 1019-1028.
5. Manmohan C, Shukla T. P, Mathur A, Upadhyay N and Sharma S, “A review on gastroretentive drug delivery system: an emerging approach to improve the gastric residence time of solid dosage forms”, Int. J. Pharm. Sci. Review and Res., 2011, 8(2), 176-182.
6. Streubel A, Siepmann J and Bodmeier R, Floating matrix tablets based on low density foam powder: effect of formulation and processing parameters on drug release”, Eur. J. Pharm. Sci., 2003, 18, 37-45.
7. Shoufeng L, Senshang L, Bruce P. Daggyc B and Haresh L. M, “Effect of HPMC and Carbopol on the release and floating properties of Gastric Floating Drug Delivery System using factorial design”, Int. J. Pharma., 2003, 253, 13–22.
8. Garse H, Vij M, Yamgar M, Kadam V and Hirlekar R, “Formulation and evaluation of a gastroretentive dosage form of Labetalol hydrochloride”, Arc Pharma. Res., 2010, 33, 405-410.
9. Singh B. N and Kim K. H, “Floating drug delivery systems: An approach to oral controlled drug delivery via gastric retention”, J. Con Rel., 2000, 63, 235-59.
10. Chavanpatil M. D, Jain P, Chaudhari S, Shear R and Vavia P. R, “Novel sustain release, swellable and bioadhesive gastroretentive drug delivery system for ofloxacin”, Int. J. Pharm., 2006, 316(1-2), 86-92.
11. Rahman Z, Ali M and Khar R. K, “Design and evaluation of bilayer floating tablets of captopril, Acta Pharm., 2006, 56, 49-57.
12. Note for guidance on stability testing. Stability testing of new drug substances and products 2003. [online] [Cited 2012 Jan. 7]; Available from URL: ich.org/cache/compo/363-272-1.html.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









