 About Authors:
About Authors:
S.Dinesh*, M.Senthil Kumar, Ashok kumar, Hariharan, jenish, Marshal joseph
Annai veilankanni’s pharmacy college
saidapet, chennai – 600 015.
Tamil nadu, pin:600015
*dinesh.pharmacy@gmail.com
ABSTRACT
Cinitapride1-2, chemically4-amino-N[3-(Cyclohexan-1-yl-methyl)-4-piperidinyl]-2-ethoxy-5-nitrobenzamide has the molecular formula C21H30N4O4 and molecular weight 402.49 g.Cinitapride is a drug that has against action to the serotoninergic 5-HT2 and D2 dopaminergic receptors that has been indicated in the gastro esophageal reflux and in the functional disorders of gastrointestinal motility treatment, The present study was aimed to formulate and evaluvate the tablets containing Cinitapride based on floating technique in order to increase gastric retention time, Total 9 formulation (F1-F9)were done using 3 different polymers like (HPMC k4m, HPMC e15m and HPMC k100m ). The formulations prepared were subjected to dissolution tests for 12 hrs, Among all the 9 formulation formulation (F5) were able to efficiently control Cinitapride release over a time period of 12 hrs. Thus the results of the current study clearly indicate, a promising potential of the floating tablet as an alternative to conventional dosage form.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1517
INTRODUCTION
Oral administration is the most convenient and preferred means of any drug delivery to the systemic circulation. Oral controlled release drug delivery have recently been of increasing interest in pharmaceutical field to achieve improved therapeutic advantages, such as ease of dosing administration, patient compliance and flexibility in formulation. Drugs that are easily absorbed from gastrointestinal tract (GIT) and have short half-lives are eliminated quickly from the systemic circulation. Frequent dosing of these drugs is required to achieve suitable therapeutic activity. To avoid this limitation, the development of oral sustained-controlled release formulations is an attempt to release the drug slowly into the gastrointestinal tract (GIT) and maintain an effective drug concentration in the systemic circulation for a long time.
[adsense:468x15:2204050025]
FLOATING DRUG DELIVERTY SYSTEMS (FDDS):[6]
Floating drug delivery systems also called as hydrodyanamically balanced systems float on the gastric contents to release the drug slowly from the dosage form. The density of the FDDS should be less than the density of gastric fluid.
Application of Floating Drug Delivery Systems:[7]
Floating drug delivery offers several applications for drugs having poor bioavailability because of the narrow absorption window in the upper part of the gastrointestinal tract. It retains the dosage form at the site of absorption and thus enhances the bioavailability. These are summarized as follows.
1. Sustained Drug Delivery: These systems can remain in the stomach for long periods and hence can release the drug over a prolonged period of time. The problem of short gastric residence time encountered with an oral CR formulation hence can be overcome with these systems.
2. Site-Specific Drug Delivery: These systems are particularly advantageous for drugs that are specifically absorbed from stomach or the proximal part of the small intestine.
3. Absorption Enhancement: Drugs that have poor bioavailability because of site specific absorption from the upper part of the gastrointestinal tract are potential candidates to be formulated as floating drug delivery systems, thereby maximizing their absorption.
The therapeutic effect of cinitapride lies on the capacity of increasing lower esophageal sphincter tone and has strong gastro kinetic activity, which generates significant increases in the gastric emptiness; besides, through the serotoninergic system it stimulates the intestinal activity. The use of cinitapride is efficient and safe in treatment of patients with disorders in the gastric emptiness related to gastro esophageal reflux and functional dyspepsia as well as in individuals that present irritable bowel syndrome with constipation and abdominal pain.
Literature survey reveals a polarographic3 method for its determination. Further, a fast, sensitive and selective method for measuring plasma cinitapride using LC-MS/MS with positive ion electrospray ionization using multiple reaction? monitoring (MRM) mode to quantify cinitapride in human plasma using respridone as the internal standard is also reported 4.To best of our knowledge, there is no work in the literature reported about the spectrophotometric method for the analysis of cinitapride in biological fluids or pharmaceutical formulations. Hence, the authors has made an attempt to develop few simple and rapid spectrophotometric methods 5-7 for the estimation of cinitapride.
REVIEW OF LITERATURE
· Roy et al.2008[9] described rapid, sensitive and specific method to quantitify cinitapride in human plasma using risperidone as the internal standard. Sample preparation involved simple solid phase extraction procedure. Plasma concentrations of cinitapride were determined by LC-MS/MS with a LOQ (limit of quantification) of 20.118 pg mL-1 that allowed an appropriate characterization of the pharmacokinetic profile of cinitapride at the therapeutic dose. The method was successfully applied to the bioequivalence study of cinitapride tablet (1.0 mg) administered as a single oral dose.
· Sangeetha et al [10] design and evaluate oral sustained release tablet of lamotrigine using polymer such as HPMC K 4M, HPMC K 100M and methocel E50 LV at 15%, 25%, and 35% Concentration range. In vitro release profile was studied using HPMC K4M, HPMC K 100M and methocel E50 LV at three different concentration. The percentage cumulative amount of drug release was found to be 82.72% in 12 hrs, 70.69% in 12hrs and 97.80% in 4hrs respectively for the entire batch at 15% polymer concentration. The study proves that fluctuation in the drug release is overwhelmed when lamotrigine is administered in the form of SR tablet.
MATERIALS AND METHODS
PRELIMINARY STUDIES
Determination of λmax:
Stock solution of 1mg/ml Cinitapride was prepared and the solution was found in the range from 200-400nm. The λ max of the solution was found to be 264nm.
FORMULATION OF FLOATING TABLETS:
Trial batches of floating tablets were prepared using different polymers [HPMC k4m, HPMC k100m,HPMC e15m ]; different drug: polymer ratio was used. Tablets prepared by direct compression technique
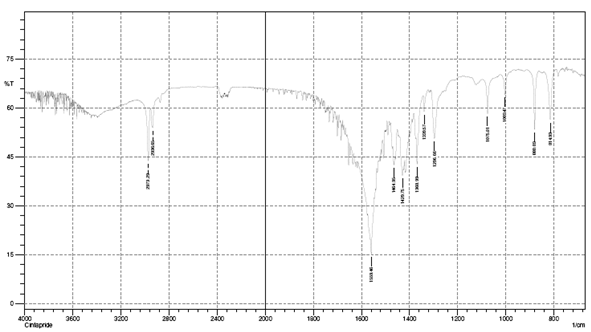
DRUG-EXCIPIENT COMPATABILITY STUDY BY FTIR:
IR spectra matching approach was used for detection of any possible chemical interaction between drug and polymers.
FORMULATION DESIGN:
· Polymers : HPMC k4m,HPMC k100m and HPMC e15m.
· Swelling agent : Carbapol.
· Filler : Micro crystalline cellulose.
EVALUATION OF POWDER BLEND:
Angle of repose
Bulk Density
Hausner’s Ratio
Compressibility Index
COMPRESSION OF FLOATING TABLET:
Weighed accurately about 100mg of the mixture blend and fed in 10 station compression machine and compressed at 1.5N compression force using 6mm concave punches.
EVALUATION OF FLOATING TABLET:
Weight variation test
Thickness and diameter:
Hardness:
Friability:
In-vitro Dissolution:
Cinitapride release from different formulations was determined using a USP XXIII paddle apparatus 2 under sink condition. To simulate in-vivo condition the dissolution medium was selected as 900 ml HCL buffer (pH 1.2) samples were withdrawn at 1st,2nd,3rd,6th,7th,9th and 12th hour time interval at 37 ± 0.2 °C
RESULTS
Compatibility studies by FTIR:
Drug polymer interaction was checked by comparing the IR spectra of pure drug with the IR spectra of physical mixtures of drug and excipients used.
COMPRESSION OF TABLETS:
The floating tablets were prepared by direct compression technique. The target weight of the prepared tablet was 100mg. The desired hardness is between 5 - 6 Kg/cm2. All the tablets were found to be uniform in size and shape and no processing problems were encountered during compression process.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
POST-COMPRESSION EVALUATION OF FLOATING TABLETS
Evaluation of physical parameters:
The compressed tablets were evaluated for their weight variation, hardness, thickness, friability and content uniformity as per Indian pharmacopoeia
Evaluation of Floating Behaviours:
The parameters determined were; Floating Lag Time- the time taken by the tablet to go upward and float on the surface, Floating Duration- the time at which the tablet remained buoyant (determined on the basis of visual inspection)
Evaluation of In-vitro Drug Release
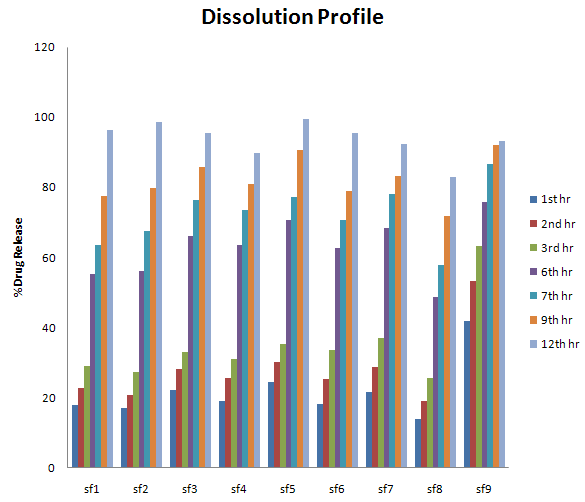
It was observed that the particular concentration of polymer HPMC e15m shows release of drug over an extended period of time. Hence formulation (F5) were able to efficiently control Cinitapride release over a time period of 12 hrs.
STABILITY STUDIES:
Accelerated stability studies were carried out according to ICH guidelines. Optimized formulations (F5) were sealed in aluminium packaging coated inside with polyethylene, and kept in a stability chamberat 40°C± 2°Cand 75%±5%RH for 3 months. Stability study of optimized formulations revealed no significant change.
TABLES AND GRAPHS:
Standard Calibration Data of Cinitapride
|
Concentration (µg/ml) |
Absorbance at 264nm |
|
5 |
0.102 |
|
10 |
0.205 |
|
15 |
0.309 |
|
20 |
0.412 |
|
25 |
0.520 |
|
30 |
0.630 |
Pre-compression parameters of powder blend
|
Formulation |
Bulk Density(gm\ml) |
Tapped Density |
Compressability Index |
Hausenr’s Ratio |
Angle Of Repose |
|
F1 |
0.3 |
0.35 |
19 |
1.16 |
32.1 |
|
F2 |
0.308 |
0.49 |
22.5 |
1.59 |
35.6 |
|
F3 |
0.31 |
0.5 |
24.3 |
1.61 |
35 |
|
F4 |
0.4 |
0.51 |
23.2 |
1.27 |
33.1 |
|
F5 |
0.416 |
0.34 |
21.7 |
0.817 |
39.8 |
|
F6 |
0.44 |
0.35 |
23.27 |
0.79 |
34 |
|
F7 |
0.38 |
0.48 |
24.1 |
1.26 |
36.5 |
|
F8 |
0.41 |
0.44 |
24.6 |
1.07 |
36 |
|
F9 |
0.39 |
0.43 |
22.5 |
0.90 |
37.4 |
Physical parameters of prepared tablets
|
Formulation |
Avg.Wt (mg) |
Thickness (mm) |
Diameter(mm) |
Hardness (Kg\cm2) |
Friability |
|
F1 |
100.2 |
2.8 |
6 |
5.5 |
0.11 |
|
F2 |
101.1 |
2.6 |
6 |
5.4 |
0.06 |
|
F3 |
100.6 |
2.5 |
6 |
5.6 |
0.14 |
|
F4 |
100.1 |
2.8 |
6 |
6 |
0.04 |
|
F5 |
100.8 |
2.7 |
6 |
5.1 |
0.14 |
|
F6 |
100.1 |
2.6 |
6 |
5.4 |
0.06 |
|
F7 |
100.4 |
2.6 |
6 |
5.5 |
0.16 |
|
F8 |
99.4 |
2.6 |
6 |
5.5 |
0.41 |
|
F9 |
101.6 |
2.65 |
6 |
5.4 |
0.02 |
* mean ± standard deviation (SD).
Results of in-vitro drug release study of Cinitapride floating tablets
|
Formulation code |
1st hr |
2nd hr |
3rd hr |
6th hr |
7th hr |
9th hr |
12th hr |
|
FI |
17.87 |
22.67 |
29.21 |
55.37 |
63.65 |
77.61 |
96.36 |
|
F2 |
17 |
20.92 |
27.46 |
56.24 |
67.58 |
79.79 |
98.54 |
|
F3 |
22.23 |
28.34 |
33.13 |
66.27 |
76.3 |
85.89 |
95.48 |
|
F4 |
19.18 |
25.72 |
30.95 |
63.65 |
73.68 |
81.09 |
89.82 |
|
F5 |
24.41 |
30.08 |
35.31 |
70.63 |
77.17 |
90.69 |
99.41 |
|
F6 |
18.31 |
25.28 |
33.57 |
62.78 |
70.63 |
78.91 |
95.48 |
|
F7 |
21.8 |
28.77 |
37.06 |
68.45 |
78.04 |
83.27 |
92.43 |
|
F8 |
13.95 |
19.18 |
25.72 |
48.83 |
57.99 |
71.94 |
82.84 |
|
F9 |
41.85 |
53.19 |
63.22 |
75.86 |
86.76 |
92 |
93.3 |
|
|
|
|
|
|
|
|
|
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
IR Spectra of Pure Drug
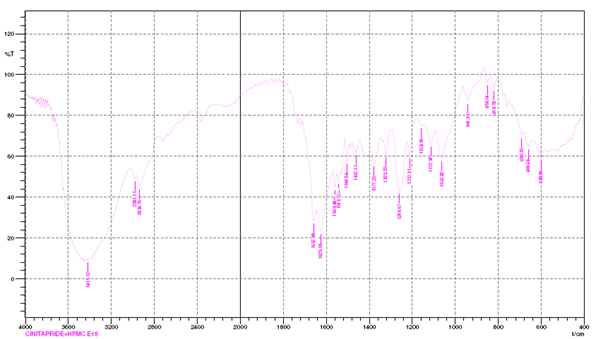
IR Spectraof Drug + HPMC e15
CONCLUSION
Drug absorption in the gastrointestinal tract is a highly variable process and prolonging gastric retention of the dosage form extends the time for drug absorption. Gastro-retentive floating drug delivery systems have emerged as an efficient means of enhancing the bioavailability and controlled delivery of drugs that exhibit absorption window, low bioavailability and extensive first pass metabolism.
In the present work floating tablets have been prepared incorporating a highly soluble anti-ulcer drug Cinitapride using polymers like (HPMC k4m, HPMC e15m and HPMC k100m). swelling agent (Carbapol 940) was used to keep the tablets floating over the simulated gastric fluid (pH 1.2) for more than 12 hrs. All the formulations showed floating lag time less than 5 min. Microcrystalline cellulose is used as a filler. The drug release mechanisms for these formulations were confirmed as zero-order release. The formulations (F5) were selected as an optimized formulations because it gave the best results in terms of the required in-vitro buoyancy as well as drug release in a sustained release manner and also were found to be stable under the stability conditions.
Thus the results of the current study clearly indicate, a promising potential of the floating tablet as an alternative to conventional dosage form, further clinical studies are needed to assess the utility of this system for patients suffering from Gastro-esophageal reflux disease, Non-ulcer dyspepsia.
BIBLIOGRAPHY
1) Amit Kumar Nayak, Ruma Maji, Biswarup Das. Gastroretentive drug delivery systems: a review. Asian Journal of Pharmaceutical and Clinical Research, (2010), 3(1):2-10.
2) Brahma N. Singh, Kwon H. Kim. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J Control Release, (2000), 63:235–259.
3) Manoj Goyal, Rajesh Prajapati, Kapil Kumar Purohit, Mehta SC. Floating drug delivery system. Journal of Current Pharmaceutical Research, (2011), 5(1):7-18.
4) Pooja Mathur, Kamal Saroha, Navneet Syan, Surender Verma and Vipin Kumar. Floating drug delivery system: An innovative acceptable approach in gastroretentive drug delivery. Archives of Applied Science Research, (2010), 2 (2):257-270.
5) Natasha Sharma, Dilip Agarwal, Gupta MK and Mahaveer PK. A comprehensive review on floating drug delivery system. IJRPBS, (2011), 2(2):428-441.
6) Ravichandiran V, Masilamani K, Senthilnathan B. Gastroretentive drug delivery systems: A review. Journal of Pharmacy Research, (2011), 4(9):3232-3236
7) Mayavanshi AV and Gajjar SS. Floating drug delivery systems to increase gastric retention of drugs: A review. Research J. Pharm. and Tech, (2008), 1(4):345-348.
8) Syeda humaira*1 Intl. Journal of Pharmacy and Pharmaceutical Sciences Vol 2, Suppl 1, 2010 Applications of colorimetric methods for the determination of Cinitapride hydrogen tartarate in Drug formulations
9) Roy MNS, Yetal SM, Chavan SV, Pradhan VR, Joshi SS, Determination of free levels of Cinitipride in human plasma by liquid chromatography-tandem mass spectrometry Journal of Chemistry 2008; 5:453-60.
10) Sangeetha et al., IJPSR, 2011; Vol. 2(2): 462-467 ISSN: 0975-823
11) Submitted: March 25, 2004; Accepted: May 30, 2005; Published: October 19, 2005Shweta Arora
12) Anuradha K. Salunkhe, Remeth J. Dias, Kailas K. Mali, Niranjan S. Mahajan and Vishwajeet S. Ghorpade. Formulation and evaluation of floating pulsatile drug delivery system of Metoprolol tartrate. Der Pharmacia Lettre, (2011), 3(3):147-160.
13) Vikrant K Nikam, Sachin B Somwanshi, Ramdas T Dolas, Vivekanand A Kashid, Kiran B Dhamak, Vinayak M Gaware, Atul N Khadse and Kiran B Kotade. A novel gastro retentive controlled release drug delivery system of Verapamil Hydrochloride: Formulation and evaluation. J. Chem. Pharm. Res., (2011), 3(1):932-939
14) Liandong Hu, Li Li, Xun Yang, Wei Liu, Jianxue Yang, Yanhong Jia, Chuang Shang, Hongxin Xu. Floating matrix dosage form for dextromethorphan hydrobromide based on gas forming technique: In vitro and in vivo evaluation in healthy volunteers. Eur. J. Pharm. Sci, (2011), 42: 99–105.
15) Nanjan Sockan Ganesh, Deecaraman. Formulation and evaluation of floating drug delivery system of ketoprofen. Journal of Pharmacy Research, (2011), 4(2): 424-428.
16) Pramod Patil, Someshwara Rao B, Suresh Kulkarni V, Basavaraj, Chetan Surpur, Anand Ammanage. Formulation and in vitro evaluation of floating matrix tablets of ofloxacin. Asian J. Res. Pharm. Sci., (2011), 1(1): 17-22.
17) Mina Ibrahim Tadros. Controlled-release effervescent floating matrix tablets of ciprofloxacin hydrochloride: Development, optimization and in vitro–in vivo evaluation in healthy human volunteers. Eur. J. Pharm. Biopharm., (2010),(74):332–339.
18) Kar RK, Mohapatra S, Mohapatra D, Dash RK, Barik BB. Preparation and in vitro characterization of cefuroxime axetil loaded gastro retentive floating tablets. Drug Invention Today, (2010), 2(10): 457-459.
19) Ramanathan G, Kavitha K, Archana TN, Nalini CN, Anandkumar MA. Formulation of floating tablets of mefenamic acid with different grades of hydroxy propyl methyl cellulose polymer and studying the release profiles. Int.J.Drug Dev. & Res., (2010),2(3): 599-604.
20) Margret Chandira R, Debjit Bhowmik, Chiranjib, Jayakar B. Formulation and evaluation of gastroretentive drug delivery system of gastroprokinetic drug Itopride Hydrochloride. IJPPS, (2010), 2(1): 53-65.
21) Pare A, Yadav SK, Patil UK. Formulation and evaluation of effervescent floating tablet of amlodipine besylate. Research J. Pharm. and Tech., (2008), 1(4):526-530
22) Sanjay Patel S, Ray S, Thakur RS. Formulation and evaluation of floating drug delivery system containing clarithromycin for HELICOBACTER PYLORI. Acta Poloniae Pharmaceutica-Drug Research, (2006), 63(1):53-61.
23) Alarcon-de-la-Lastra Romero C, Lopez A, Martin MJ, la Casa C, Motilva V: Cinitapride protects against ethanol-induced gastric mucosal injury in rats: role of 5-hydroxytryptamine, prostaglandins and sulfhydryl compounds. Pharmacology. 1997 Apr;54(4):193-202
24) Thangabalan B, Prabahar AE, Kalachelvi R, Kumar VR. UV spectrophotometric method for determination of Cinitapride in pure and its solid dosage form. E J Chem. 2009; 6(1): 21-4.
25) Raymond C Rowe, Paul J Sheskey and Marian E Quinn. Handbook of Pharmaceutical Excipients, 6th edn., London, Pharmaceutical Press, 2009, 129, 262, 326,404,438, 581, 728.
26) Raymond C. Rowe, Paul J. Sheskey and Sian C. Owen. Hand book of Pharmaceutical Excipients, 4 th edition, Pharmaceutical press, London, 2003,53-54,132,214-215,430,449-453,459-460,767-768.
27) Raymond Rowe C, Paul Sheskey J, Marian Quinn E. Handbook of Pharmaceutical Excipients. 6th edition. Pharmaceutical Press & American Pharmacists Association. (2009): 110-121.
28) Sudha T., Saminathan J. and Kondapalli Anusha. Simultaneous UV- Spectrophotometric Estimation of Lamivudine and Abacavir Sulphate in Bulk and in Tablet Dosage Form, J. Chem. Pharm. Res., 2010, 2(5), 45-51
29) Mina Ibrahim Tadros. Controlled-release effervescent floating matrix tablets of ciprofloxacin hydrochloride: Development, optimization and in vitro–in vivo evaluation in healthy human volunteers. Eur. J. Pharm. Biopharm., (2010), (74):332–339.
30) Kiran Chaturvedi, Umadevi S, Subhash Vaghani. Floating matrix dosage form for propranolol hydrochloride based on gas formation technique: Development and in vitro evaluation. Sci. Pharm., (2010), 78: 927–939.
31) Wagner JG. Interpretation of percent dissolved- time plots derived from in vitro testing of conventional tablets and capsules. J Pharm Sci., (1969), 58: 1253-1257
32) Indian pharmacopoeia 2007, Ministry of Health and Family Welfare, Govt. of India. The controller of publications, New Delhi, vol-3:995-997.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









