 About Authors:
About Authors:
1Sahu Deepak*, 2Ketawat Santosh
1Ass.Professor, Geetanjali Institute of Pharmacy,
2Lecturer, Geetanjali Institute of Pharmacy,
Dabok, Udaipur [Rajasthan] – 313022
*deepak.sahu.bhl@gmail.com
Abstract
Tablet is the most preferred oral dosage form, due to many advantages it offers to formulators as well as physicians and patients. However, the process of manufacturing tablets is complex. Hence, careful consideration has to be given to select right process, and right excipients to ultimately give a robust, high productivity and regulatory compliant product of good quality.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1436
Introduction:
Tablets may be defined as solid pharmaceutical dosage forms containing drug substances with or without suitable diluents and prepared either by compression or molding methods. They have been in widespread use since the latter part of the 19th century and their popularity continues. The term compressed tablet is believed to have been first used by 'John Wyeth and Brother of Philadelphinn’ During the same period molded tablets were introduced to be used as Hypodermic tablets for injections. Tablets remain popular as a dosage form because of the advantages, afforded both to the manufacturer [e.g.: simplicity & economy of preparation, stability and convenience in packing, shipping, and dispensing] and the patient [e.g.: accuracy of dosage, compactness, post ability, blandness of taste and ease of administration. Although tablets are more frequently discoid in shape, they also may be round, oval, oblong, cylindrical or triangular. They may differ greatly in size and weight depending on the amount of drug substance present and the intended method of administration.
The objective of this article is to provide a comprehensive overview of the tablet core manufacturing process with emphasis on oral immediate release formulations, along with common excipients used.
Properties of Tablets :
The attributes of an acceptable tablet are as follows:
1) The tablet must be sufficiently strong and resistance to shock and abrasion and to with stand handling during manufacturing, packing, shipping, and use. Hardness and friability tests measure this property.
2) Tablet must be uniform in weight and in drug content of the individual tablet. This is me assured by the weight variation and content uniformity tests.
3) The drug content of the tablet must be bioavailable. This property is measured by the dissolution test. Accurate bioavailability can be obtained from the drug levels of the drug after its administration.
4) Tablets must be elegant in appearance and must have characteristic shape, color, and other markings necessary to identify the product.
5) Tablets must retain all these functional attributes, which include drug stability and efficacy.
Advantages of Tablet :
1) It is easy to be administered.
2 It is a unit dosage form, and they offer the greater capabilities of all oral dosage forms for the greatest dose precision and the least content variability.
3) Cost is lowest of all oral dosage forms.
4) It is the lightest and most compact of all oral dosage forms.
5) Product identification is potentially the simplest and cheapest, requiring no additional processing steps when employing an embossed or monogrammed punch face.
Disadvantages of Tablets:
1) Some drugs resist compression into dense compacts, owing to their amorphous nature or flocculent, low-density character.
2) Drugs with poor wetting, slow dissolution properties, intermediate to large dosages, optimum absorption high in the gastrointestinal tract, or any combination of these features may be difficult or impossible to formulate and manufacture as a tablet that will still provide adequate or full drug bioavailability.
3) Bitter drugs, drugs with objectionable odor or drugs that are sensitive to oxygen or atmosphere moisture may require encapsulation or a special type of coating which may increase the stability of the finished tablets.
Types of Tablets:2-3
Tablets are classified according to their route of administration or function. The following are the 5 main classification groups.
1. Tablets ingested orally
1.1. Compressed tablets
1.2. Multiple compressed tablets
i) Multilayered tablets
ii) Inlay tablets
1.3. Sustained action tablets
1.4. Enteric coated tablets
1.5. Sugar coated tablets
1.6. Film coated tablets
1.7. Chewable tablets
2. Tablets used in the oral cavity
2.1. Buccal tablets
2.2. Sublingual tablets
2.3. Lozenge tablets and torches
2.4. Dental cones
3. Tablets administered by other routes
3.1. Implantation tablets
3.2. Vaginal tablets
4. Tablets used to prepare solutions
4.1. Effervescent tablets
5. Molded tablets or tablet triturates (TT)
5.1. Dispensing tablets (DT)
5.2. Hypodermic tablets (HT)
* Compressed tablets1,2,3:
These tablets are uncoated and made by compression of granules. These tablets are usually intended to provide rapid disintegration and drug release. These tablets after swallowing get disintegrated in the stomach, and its drug contents are absorbed in the gastrointestinal tract and distribute in the whole body.
* Multiple compressed tablets2,3:
These tablets are prepared to separate physically or chemically incompatible ingredients or to produce repeat action prolonged action products. To avoid incompatibility, the ingredients of the formulation except the incompatible materials are compressed into a tablet then incompatible substances along with necessary excipients are compressed tablet.
* Multilayered tablets3:
These tablets consist of two or more layer of materials compressed successively in the same tablets. The color of each layer may be the same or different. The tablets having layers of different colors are known as "multicolored tablets".
* Inlay Tablet3,4:
A type of layered tablet in which instead the core tablet being completely surrounded by coating, top surface is completely exposed. While preparation, only the bottom of the die cavity is filled with coating material and core is placed upon it. When compression force is applied, some coating material is displaced to form the sides and compress the whole tablet. It has some advantages over compression coated tablets:
i) Less coating material is required.
ii) Core is visible, so coreless tablets can be easily detected.
iii) Reduction in coating forms a thinner tablet and thus freedom from capping of top coating.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
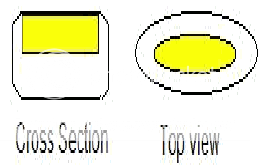
Cross section and top view of inlay tablets
* Sustained action tablets4,5:
These tablets are used to get a sustained action of medicament. These tablets when taken orally release the medicament in a sufficient quantity as and when required maintaining the maximum effective concentration of the drug in the blood throughout the period of treatment.
* Enteric-coated tablets4,5:
These are compressed tablets meant for administration by swallowing and are designed to bypass the stomach and get disintegrated in the intestine only. These tablets are coated with enteric coated polymer to have release within the intestine.eg: tablets containing anthelmentics and amoebicides.
* Immediate release tablets2,3,5:
The mechanisms for release of drug from modified-release dosage forms are more complex and variable than those associated with immediate release dosage forms. According to BCS (Bio pharmaceutics classification system), there are three major factors that govern the rate and extent of drug absorption of immediate release (IR) solid oral dosage forms: dissolution rate, solubility and intestinal permeability. For IR dosage forms containing active pharmaceutical ingredients (APIs) showing high solubility, high intestinal permeability and rapid dissolution, a waiver from performing bioequivalence studies (bio waiver) can be scientifically justified.
Tablet Ingredients4 :
In addition to active ingredients, tablet contains a number of inert material known as additives or excipients. Different excipients are:4
* Diluents
* Binder and adhesive
* Disintegrants
* Lubricants and glidants
* Colouring agents
* Flavouring agents
* Sweetening agents
Excipients present in a formulation must meet certain criteria; few such criteria are listed below.
* They must be nontoxic & acceptable by all countries.
* They must be physiologically inert.
* Cost must be low.
* They must be physically & chemically stable.
* They must be free from any unacceptable microbiological load.
* They must be color compatible.
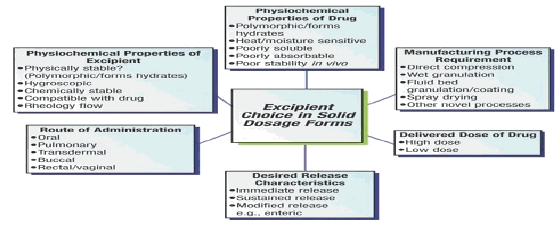
1.2 Discussion on excipients and their properties which makes it desirable for different modified release formulations
Role of each excipients listed above are explained briefly in the following discussion.
Diluents & Fillers4,5:
Diluents are added where the quantity of active ingredient is small or difficult to compress. Where the amount of active ingredient is small, the overall tableting properties are in large measure determined by the filler. Because of problems encountered with bioavailability of hydrophobic drugs of low water solubility, water-soluble diluents are used as fillers for these tablets. Diluents are also used to provide better tablet properties such as cohesion & permit direct compression.5
Diluents used are:-
Lactose, Spray Dried Lactose, Starch, Dextrose, Mannitol, Sucrose, Microcrystalline Cellulose etc.
Diluents form a major portion of most of the tablet formulations due to newer high potency API's. The moisture content, more specifically water activity coefficient of such diluents, may influence API stability since many API are prone to degradation by hydrolysis. In general, moisture content may indicate hydrate form or tightly bound water molecule of crystallization or surface-bound or surface absorbed water on the excipient. The bound water may not cause hydrolysis of sensitive API, but free- or surface-absorbed water may be responsible for hydrolysis of sensitive API. This free water is termed as water activity coefficient. Figure 1 gives loss on drying (LOD) and water activity coefficient of some commonly used filler. Lactose, for instance, has low LOD, but very high water activity coefficient. On the other hand, Starch 1500 has very high LOD, but very low water activity coefficient. Hence, for API such as Aspirin or Ranitidine that undergo hydrolysis, Starch 1500 provides stable formulation, whereas lactose or plain microcrystalline cellulose leads to higher impurity levels on stability (Cunnigham CR 2001).
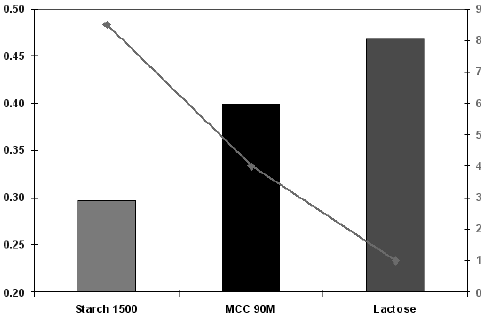
Figure 1: Water activity Vs Moisture content of some commonly used fillers.
Binder & Adhesive4,5,6:
Binder is added during granulation step to an API-filler mixture to ensure that granules and tablets can be formed with the required mechanical strength.
Binders are classified according to their applications:
1. Wet binders: these are dissolved in a solvent (for ex: water or alcohol) and used in wet granulation process.
Ex: gelatin, cellulose, cellulose derivatives, PVP, starch, sucrose, PEG
2. Dry binders: these are added to the powder blend, either after a wet granulation Step, or as part of a direct powder compression formula.
Ex: cellulose, Methylcellulose, PVP.
Table:-1 Characteristics of commonly used binder
|
BINDER |
SPECIFIED CONCENTRATION |
COMMENTS |
|
Starch Paste |
5-25%w/w |
* Freshly prepared starch paste is used as a binder. * Its method of preparation is very crucial. |
|
Pregelatinized Starch (PGS) [Partially and Fully PGS]
|
5-10%w/w (Direct Compression) 5-75%w/w (Wet Granulation ) |
* It is starch that have been processed chemically and/or mechanically to rupture all or part of the granules in the presence of water and subsequently dried. * It contains 5% free amylose, 15% free amylopectin and 80%unmodifiedstarch. * Obtained from maize, potato or rice starch. * It is multifunctional excipient used as a tablet binder, diluent, disintegrant. * Compressibility and can be used as binders in Direct Compression as well as Wet Granulation. * High purity PGS allow simplified processing as they swell in cold water and therefore reduce time/costs compared with traditional starch paste preparation. |
|
Hydroxypropyl Methyl Cellulose (HPMC) |
2-5%w/w |
* Comparable to Methyl Cellulose. * Used as a binder in either wet or dry granulation processes. |
|
Polyvinyl Pyrrolidone (PVP) |
0.5-5%w/w |
* Soluble in both water and alcohol. * Used in wet granulation process. * It is also added to powder blends in the dry form and granulated in situ by the addition of water, alcohol or hydroalcoholic solution. * Valuable binder for chewable tablets. |
|
Polyethylene Glycol (PEG) 6000 |
10-15%w/w |
* Used as a meltable binder. * Anhydrous granulating agent where water or alcohol cannot be used. * It may prolong disintegration time when concentration is 5% or higher * It improves the plasticity of other binders. |
Disintegrants5,6:
Disintegrant is included in the formulation to ensure that the tablet breaks up into small fragments in contact with liquid. Figure 2 shows influence of disintegrating agent on disintegration time of tablets. Tablets must have sufficient strength to withstand the stresses of subsequent manufacturing operations, such as the coating, packaging, and distribution process. However, once the tablet is taken by the patient, it must break up rapidly to ensure rapid dissolution of the active ingredient in immediate release preparations. To overcome the cohesive strength produced by the compression process, and to break down the tablet into the primary particles as rapidly as possible, disintegrants are added to the tablet formulations. The positioning of disintegrants within the intra- and extragranular portions of granulated formulations can affect their water uptake and disintegration time.
Table:-2 Commonly Used disintegrates along with concentration.
|
S.NO |
Disintegrants |
Concentration In Granules (%W/W) |
Special Comments |
|
1. |
Starch USP |
5-20 |
Higher amount is required, poorly compressible |
|
2. |
Starch 1500 |
5-15 |
- |
|
3. |
Microcrystalline cellulose®(PH 101, PH 102) |
10-20 |
Lubricant properties and directly compressible |
|
4. |
Solkafloc® |
5-15 |
Purified wood cellulose |
|
5. |
Alginic acid |
1-5 |
Acts by swelling |
|
6. |
Na alginate |
2.5-10 |
Acts by swelling |
|
7. |
Explotab® |
2-8 |
Sodium starch glycolate, superdisintegrant. |
|
8. |
Polyplasdone®(XL) |
0.5-5 |
Crosslinked PVP |
|
9. |
Amberlite® (IPR 88) |
0.5-5 |
Ion exchange resin |
|
10. |
Methyl cellulose, Na CMC, HPMC |
5-10 |
- |
|
11. |
AC-Di-Sol® |
1-3 |
Direct compression |
Mechanism of Tablet Disintegration5:-
The tablet breaks to primary particles by one or more of the mechanisms listed below:
* By capillary action
* By swelling
* Because of heat of wetting
* Due to disintegrating particle/particle repulsive force
* Due to deformation
* Due to release of gases
* By enzymatic action
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
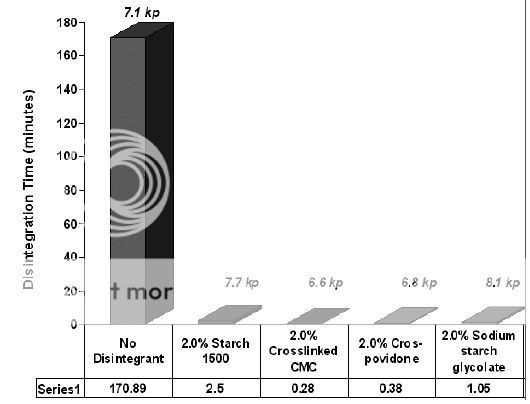
Figure 2: Effect of different disintegrants on the physical properties of Hydrochlorthiazide tablets
Lubricants, Antiadherants, & Glidants2,3,5,6:-
Lubricants:- They are intended to reduce the friction during tablet ejection between wall of the tablet & die cavity. E.g. Mineral oil,, Stearic Acid, Talc.
Classification of Lubricants:
Lubricant are classified according to their water solubility i.e. water insoluble and water soluble. Selection of lubricant is depends partly on mode of administration, type of tablet, desired disintegration and dissolution properties, physicochemical properties of granules or powder and cost.
a) Water Soluble lubricants
b) Water Insoluble lubricants
Table:-3 List of water Soluble and water insoluble Lubricants
|
Water Soluble Lubricants |
Concentration Range (%w/w) |
|
Boric acid |
1 |
|
Sodium benzoate |
5 |
|
Sodium oleate |
5 |
|
Sodium acetate |
5 |
|
Sodium Lauryl sulfate |
1 – 5 |
|
Magnesium lauryl sulfate |
1 – 2 |
|
Water Insoluble Lubricants |
Concentration Range (%w/w) |
|
Metal(Mg.,cal.,Na.)Stearate |
¼-2% |
|
Stearic acid |
¼-2% |
|
Sterofex |
¼-2% |
|
Talc |
1-5% |
|
Waxes |
1 – 5% |
|
Stearowet |
1 – 5% |
Anti-adherents5:- They reduce sticking or adhesion of any of tablet granulation or powder material by reducing friction between particles. They are:-
Talc, Starch & Starch derivatives, various colloidal silica such as Cab- o- Sil, Sylod, Aerosil. Some material have strong adhesive properties towards the metal of punches and dies or the tablet formulation containing excessive moisture which has tendency to result in picking and sticking problem. Therefore Anti-adherents are added, which prevent sticking to punches and die walls. Talc, magnesium stearate and corn starch have excellent Anti-adherents properties.
Table:- 4 List of Antiadherants
|
Antiadherants |
Range (%W/W) |
Comment |
|
Talc |
1 – 5 |
Lubricant with excellent Antiadherants properties |
|
Cornstarch |
3 – 10 |
Lubricant with excellent Antiadherants properties |
|
Colloidal silica |
0.1 – 0.5 |
Does not give satisfactory results due to small surface area. Cab-O-Sil® and Syloid® |
|
DL-Leucine |
3 – 10 |
Water soluble lubricant; excellent Antiadherants properties |
|
Sodium lauryl sulfate |
< 1 |
Antiadherants with water soluble lubricant |
|
Stearates |
< 1 |
Antiadherants with water insoluble lubricant |
Glidant5:- It is a substance that is added to a powder to improve its flow ability. A glidant will only work at a certain range of concentrations. Above a certain concentration, the glidant will in fact function to inhibit flow ability (Which means that there's a critical concentration to be used if increasing powder's flow ability is intended with respect to the glidant and the powder properties). In tablet manufacture, Glidants are usually added just prior to compression.Examples of Glidants include magnesium stearate, Aerosil (colloidal silicon dioxide), starch and talc.
Mechanism of Action:-
A Glidants effect is due to a counter-action to factors resulting in poor flow ability of powders. For instance, correcting surface irregularity, reducing inter particular friction & decreasing surface charge. The result is a decrease in the angle of repose which is an indication of an enhanced powder's flow ability.
Colors, Flavors & Sweeteners6:-
Colors are used for
Ø Distinguishing of off color drug
Ø Product identification
Ø Production of more elegant product.
Colors used are
Ø Natural color:- unstable
Ø FD &C and D&C dyes:-applied as a solution form
Ø Lakes:- Employed as a dried powder.
Flavors are used in chewable tablets, in mouth dissolving tablets.
Flavors are water soluble flavors:-
Ø Little acceptable,
Ø Oil flavors,
Ø Dry flavors
Sweeteners are used inchewable tablets
Ø Fructose
Ø xylitol
Various sugars are
* Mannitol-72% sweet as sucrose
* Saccharin- 500 times sweeter than sucrose
* Artificial sweetener
Tablet Manufacturing5,6
Tablets are compressed powders and their manufacturing is a complex, multistep process. The ultimate aim of these compressed solids is to easily disperse in gastrointestinal fluid, aid in complete absorption of API and, at the same time, offer stability to the formulation. The tablet manufacturing process can be broadly classified as granulation (wet granulation or dry granulation) and direct compression. Granulation is an agglomeration process to improve the flow, density and compressibility of particulate material by size enlargement and densification. Granulation can be achieved by the use of binder solution (wet granulation) or dry binder (dry granulation).
Granulation Technology : 6-8
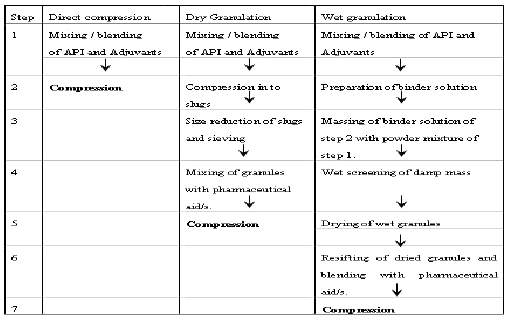
Table-:5 Different compression strategies of tablet
A. Direct Compression5,6,7:
Processing steps are:
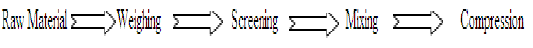
Direct compression consists of compressing tablets directly from powdered materials without modifying physical nature of materials. This method is applicable for crystalline chemicals having good compressible characteristic and flow properties such as: Potassium salt (chlorate, chloride and bromide), Sodium chloride, Ammonium chloride, Methylamine etc. If necessary, direct compression vehicles can be used which are having good flow and compressible characteristics. Commonly used directly compression diluents are: MCC (Microcrystalline Cellulose), Spray dried lactose, Starch (Sta Rx 1500, Embdex, Celutab), Sugar (Sugar tab, Nutab), Dicalcium phosphate dehydrate (Di-Tab) and Mannitol.
Advantages:
Ø Low labour input
Ø A dry process
Ø Fewest processing steps and less time consuming
Disadvantages:
* Stratification may occur due to differences in particle size and bulk density which results poor content uniformity.
* A large dose drug may cause problem in direct compression. It requires diluents. The tablet becomes large in size which is difficult to swallow and also costly.
* During handling of dry materials static charge may form which may present uniform distribution of drug.
* Direct compression diluent may interact with the drug. For example, amine drug with lactose produce discoloration of tablet.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
B. Wet granulation5,7,8:
Processing steps are:
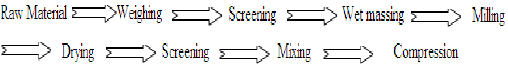
The most widely used and most general method of tablet preparation is the wet granulation method. The active ingredient, diluents and disintegrates are mixed or blended well. For large-scale production twin shell blender, double cone blender, planatory mixer, sigma blade mixer, ribbon mixer etc. are commonly used. Solutions of the binding agent are added to the mixed powder with stirring. The powder mass is wetted with the binding solution until the mass has the consistency of damp snow. If the granulation is over wetted the granules will be hard, if not wetted sufficiently, the resulting granules will be too soft, breaking down during lubrication.
The wet mass is forced through a 6 or 8 mesh (Mesh number is the number of wires passing through an inch) screen or several mills can be used. Moist materials from wet milling steps is placed on large trays and placed in drying chambers with a circulating air current and thermo stable heat controller. Commonly used dryers are tray dryer and fluidized bed dryer. After drying, the granulation is reduced in particle size by passing smaller mesh screen. After size reduction the lubricant or glidants is added as fine powder to promote flow of granules. These granules then compressed to get tablet.
C. Dry granulation4,5,8:
Processing steps are:
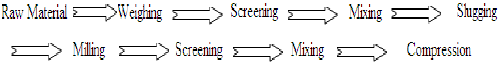
When tablet ingredients are sensitive to moisture and/or unable to withstand elevated temperature during drying and when the tablet ingredient have insufficient cohesive properties, slugging may be used to form granules. This method is referred to as dry granulation. This technique is used in preparation of aspirin, aspirin combination, acetophenetidin, thiamine hydrochloride, ascorbic acid and magnesium hydroxide. Compression granulation involves the compaction of the components of a tablet formulation by means of flat punch. These compact masses are called slug and the process is called slugging. Slugs are then milled and screened to produce a granular form. On large scale operation compression granulation can be performed on specially designed machine called Roller Compactor (Chilsonator Roller Compactor).
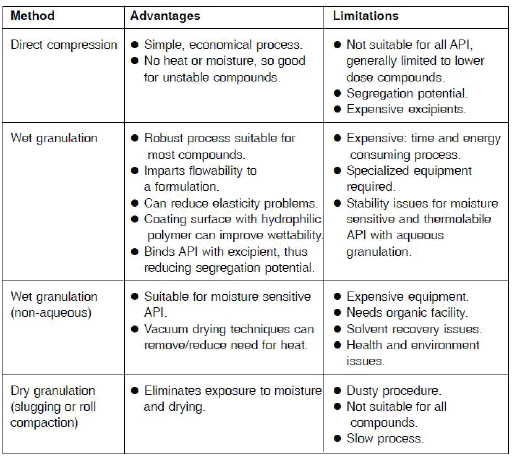
Table-6: Provides the advantages and limitations of different tablet manufacturing methods.
References
1. Kottke KM, Rudnic ME. Tablet dosge form. In: Banker GS, Rhodes CT, editors. Modern Pharmaceutics, 4th ed. New York: Marcel Dekker, Inc.; 2002. p. 280
2. Rudnic ME, Joseph D. Oral solid dosage form. In: Gennaro AR, editor. Remington: the science and practice of pharmacy. 20th ed. Philadelphia: Lippincott Williams & Wilkins; 2000. vol. 1 p. 858-62.
3. Lachman L, Lieberman HA, Kanig JL. The Theory and Practice of Industrial Pharmacy, 3rd ed. Bombay: Varghesh Publishing House; 1987. p. 293-5.
4. Ghosh TK, Jasti BR. Theory and Practice of Contemporary Pharmaceutics. 1st ed. New York: CRC Press; 2005. p. 294-8.
5. Cunnigham C. R. and Scattergood L. K.,The effect of Starch 1500® on the stability of aspirin tablets stored under accelerated conditions, AAPS, October 2001.
6. Siling W., Guanhao Y., Paul W.S.H., Mingxin M. Investigation of high shear wet granulation processes using different parameters and formulations. Chem. Pharm. Bull., 2008; 56(1): 22-27.
7. Parikh DM. Handbook of Pharmaceutical Granulation Technology. 2nd ed. London: Taylor & Francis Ltd. 2005. 1-6.
8. Summers M, Aulton M. Granulation. In: Aulton ME, editor. Pharmaceutics: The Science of dosage Form Design. 3rd ed. Churchill Livingstone; p. 364-78.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









