About Authors:
Mubarak Patel*, Suresh. V. Kulkarni,
Department of Pharmaceutics,
Sree Siddaganga College of Pharmacy,
B. H. Road, Tumkur-572102, Karnataka, India.
*patel.mubarak2@gmail.com
ABSTRACT:
The purpose of the research work was to prepare and evaluate the microspheres of metoclopramide hydrochloride as a model drug by solvent evaporation method with carbopol and HPMC polymers in various proportions. A total of six formulations were prepared i.e. F1, F2, F3, F4, F5 and F6. The microspheres were evaluated for micromeritic properties, particle size, % yield, Drug content and Drug release. The size or average diameter of prepared microspheres were recognized and characterized by scanning electron microscopic methods. Microspheres were found discrete, spherical and free flowing. They ranged in particle size from 45.6- 52.2 μm. Metoclopramide hydrochloride release from these microspheres was slowed, extended and depended on the type of polymer used. The formulation F2 and F5 showed consistent drug release for up to 12 h time period. Among all the formulations, F2 contains carbopol 934 and F5 containing HPMC showed the reproducible results with best release profile and good surface morphology. Release data were analyzed based on Highuchi kinetics and Korsmeyer/Peppa’s equation and all the selected formulations showed good fit to Peppa’s equation. The work has demonstrated that among all the formulations of microspheres, particularly those of formulation F2 are promising candidates for the sustained release of metoclopramide hydrochloride in the gastrointestinal tract.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1321
INTRODUCTION:
Despite of tremendous advancements in drug delivery, Oral routes of drug administration have wide acceptance up to 50-60% of total dosage forms due to ease of ingestion, pain avoidance, versatility (to accommodate various types of drug candidates), and most importantly patient compliance. Also, solid oral delivery systems do not require sterile conditions and are, therefore, less expensive to manufacture1,2. Microspheres in general, have the potential to be used for targeted and controlled release drug delivery. Microencapsulation for oral use has been employed to sustain the drug release and to reduce or eliminate gastrointestinal tract irritation. In addition, multiparticulate delivery systems spread out more uniformly in the gastrointestinal tract. This result in more reproducible drug absorption and reduces local irritation when compared to single-unit dosage forms such as no disintegrating, polymeric matrix tablets. Microencapsulation is used to modify and retard drug release. Due to its small particle size, are widely distributed throughout the gastrointestinal tract which improves drug absorption and reduces side effects due to localized build-up of irritating drugs against the gastrointestinal mucosa.3,4. The conventional dosage forms of metoclopramide hydrochloride (MCP) contains drawbacks like dose related side effects like chills, dizziness, convulsions, irregular heartbeat, headache, abdominal pain and loss of appetite. It’s higher solubility in water results in burst effect with sudden peak levels of drug in blood. Though it is well absorbed undergoes a significant first pass metabolism which may reduce the systemic bioavailability up to 30%. It needs 3-4 times daily dosing which may leads to non-compliance. It is a commonly prescribed drug used for the management of gastrointestinal disorders such as gastric stasis, gastroesophageal reflux and for the prevention of cancer chemotherapy-induced emesis. It is a potent antiemetic and prokinetic, used in the treatment of certain disorders of the digestive tract. The drug has a short biological half-life (4±1h) and is usually administered in a dose of 10-15 mg given up to 4 timesdaily in order to maintain effective concentration throughout the day. Therefore the development of sustained release microspheres would clearly be advantageous. Moreover the site of absorption of MCP is stomach and dosage form that is retained in the stomach would increase the absorption. A sustained-release formulation that makes twice daily administration of MCP possible might be an advantageous dosage form, especially in long term therapy5, 6, 7. Carbopol 934 has been selected as a polymer in the preparation of microspheres because of its good mucoadhesive properties and is not absorbed by body tissues and being totally safe for human oral consumption. Hydroxy propyl methyl cellulose (HPMC) as a release retarding material. The aim of present study was to develop and evaluate sustained release microspheres of metoclopramide hydrochloride using Carbopol 934 and HPMC as polymer and emulsion-solvent evaporation as a method of preparation and to evaluate the effect of various formulation variables on the physical characteristics, drug content as well as the in Vitrorelease profiles of the microspheres. Metoclopramide hydrochloride whose physicochemical properties and short half life (4±1 hrs) make it suitable candidate for sustained release drug delivery system.
[adsense:468x15:2204050025]
MATERIALS AND METHODS:
Materials
Metoclopramide hydrochloride was received as gift sample from Vaikunth Chemicals, Ankleshwar, Carbopol-934 and HPMC was purchased from Loba Chemie Pvt. Ltd. Mumbai., Ethanol, Tween 80, dichloromethane and Heavy Liquid Paraffin was obtained from SD fine chemicals Ltd., Mumbai (India). All other chemical and reagent used in this study were of analytical grade.
Method of preparation4:-
Six batches of microspheres were prepared by taking drug: polymer ratio as 1:1, 1:1.5 and 1:2 with MCP as drug and two different polymers. The formulation batches were designated as F1, F2, F3 for Carbopol 934 (1:1, 1:1.5,1:2 respectively) and F4, F5, F6 for HPMC (1:1, 1:1.5,1:2 respectively). Drug and polymer in different proportions were weighed and co?dissolved at room temperature into a mixture of ethanol and dichloromethane (1:1% v/v) with vigorous agitation to form uniform drug-polymer dispersion. This was slowly poured into the dispersion medium consisting of heavy liquid paraffin (50ml) containing 1.5% Tween 80. The system was stirred using over head propeller agitator at a speed of 400 rpm at room temperature over a period of 2-3 hrs, to ensure complete evaporation of the solvent. Liquid paraffin was decanted and the microspheres were separated by filtration through a whatmann filter paper, washed thrice with 180 ml of acetone and air dried for 24 hrs.
|
Formulation Code |
Drug Polymer Ratio |
Drug (mg) |
Carbopol 934 (mg) |
HPMC (mg) |
Dichloromethane (ml) |
Methanol (ml) |
|
F1 |
1:1 |
500 |
500 |
- |
10 |
10 |
|
F2 |
1:1.5 |
500 |
750 |
- |
10 |
10 |
|
F3 |
1:2 |
500 |
1000 |
- |
10 |
10 |
|
F4 |
1:1 |
500 |
- |
500 |
10 |
10 |
|
F5 |
1:1.5 |
500 |
- |
750 |
10 |
10 |
|
F6 |
1:2 |
500 |
- |
1000 |
10 |
10 |
Table -1:- Composition of various MCP microspheres formulations
Dose Calculation8:
The dose of MCP is 10-15 mg four times a day. But the dose is reduced to 40 mg for formatting sustained release microspheres.
Dt = Di (1+ 0.693× tm / t1/2)
Where, Dt = total dose;
Di = initial dose;
tm = time to which the drug is sustained;
t1/2 = half life of the drug.
Dt = 15 (1+ 0.693×12/5)
Dt = 39.948≈40 mg
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
MICROSPHERE CHARACTERIZATION:
Particle size analysis9, 10:
Microsphere size was determined by using an optical microscope under regular polarized light, and the mean microsphere size was calculated by measuring 100 particles with the help of a calibrated ocular micrometer. The average particle size was determined by using the Edmondson’s equation
Dmean= Σ nd/ Σ n
Where, n = number of microspheres observed,
d = mean size range.
Microspheres yield2, 11:
Microspheres were sieved to remove polymeric sheets and microsphere aggregates. The yield was calculated as a percentage of the original amount of polymer and drug. Data are the average of three batches of each formulation.
Actual weight of product
% yield = ---------------------------- X 100
Total weight of excipient and drug
MICROMERETIC PROPERTIES
Determination of bulk density:
Bulk density was determined by the following formula.
Bulk density = Sample weight/Sample volume
The bulk density of Micro spheres was given in table 2.
Tapped Bulk density:
Tapped Bulk densitywas determined by transferring known quantity of microspheres to 50ml measuring cylinder and tapping 100 times from 1 inch at 2 sec interval. The tapped density was calculated by the following equation:
Tapped density (Pp) = M/Vo
Compressibility index (CI), Haussner’s ratio:
Carr’s index (% compressibility index), Hausner ratio were determined to predict flowability and these can be determined by following equations.
(Tapped density- Bulk density)
Compressibility index = ------------------------------ X 100
Tapped density
Haussner’s ratio = Tapped density/Bulk density
Determination of angle of repose12:
The angle of repose was determined by funnel method. Flow property of microspheres is usually assessed by determining angle of repose of microspheres. The angle of repose was determined according to the following formula.
θ= tan - 1(h/r)
Where, h = height of pile
r = radius of the pile formed by the microspheres
The angle of repose of microspheres was given in table 2.
Drug content:
The average drug content was measured by extracting a sample of about 20 mg accurately weighed of the microspheres using absolute ethanol. After filtration and appropriate dilution with ethanol, the concentration of MCP was determined using spectrophotometrically at spectrophotometrically at 272 nm using 0.1N hydrochloric acid as blank. Polymers did not interfere with the absorbance of the drug at the specified wavelength.
Actual drug content
Drug incorporation efficiency = ---------------- X 100
Theoretical drug content
Scanning Electron Microscopy:
A scanning electron photomicrograph of drug-loaded microspheres was taken. A small amount of microspheres was spread on glass stub. Afterwards, the stub containing the sample was placed in the scanning electron microscope chamber (Lisc, material engineering, Mathigere. Bangalore). The scanning electron photomicrograph was taken at the acceleration voltage of 5.0 kV, original magnifications ×350 and 750. The photomicrographs are depicted in Figure1.
Fourier Transform Infra-Red spectroscopy (FT-IR) analysis:
The Fourier transform infra–red analysis was conducted for the analysis of drug polymer interaction and stability of drug during microencapsulation process. Fourier transform infra-red spectrum of Metoclopramide hydrochloride and microspheres containing drug and polymers were recorded (fig. 2).
In-vitro release study13,14:
The dissolution studies were carried out using basket type apparatus at 50 rpm and 37±0.5°C. The microspheres equivalent to 40 mg of drug were filled in to colorless hard gelatin capsules and placed in basket separately. The dissolution medium was 0.1 N HCl having pH 1.2 as simulated gastric fluid (SGF) for the first 2 hour, followed by phosphate buffer pH 7.4 as simulated intestinal fluid (SIF) for the next 10 hrs. Five ml of sample solution was withdrawn at predetermined time intervals, filtered through a whatmann filter paper, diluted suitably and analyzed spectrophotometrically. Equal amount of fresh dissolution medium was replaced immediately after withdrawal of the test sample. Samples were analyzed at 272 nm. The In-Vitro drug release studies results were mentioned in the (Fig 3) Percentage drug dissolved at different time intervals was calculated using the Lambert-Beer’s equation (y=0.03194x + 0.01523, R2=0.9989) described above.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RESULTS AND DISCUSSION
The present study was an attempt to develop and characterize microspheres of metoclopramide hydrochloride. Emulsification solvent evaporation method was used for preparation of microspheres.
Scanning electron microscopy (SEM):
Morphology of microspheres was examined by scanning electron microscopy. The view of the microspheres showed hollow structure with a smooth surface morphology (fig. 1)exhibited range of sizes within each batch (shown in table 3). The outer surface of microspheres was smooth and dense.
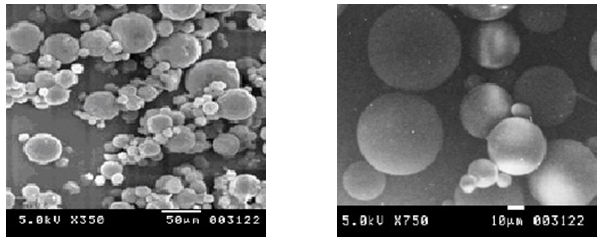
Fig. 1: Scanning electron microphotographs of metoclopramide hydrochloride microsphere (F-2).
Infrared spectroscopy:
The FT?IR spectra study showed no change in the finger print of pure drug spectra, thus confirming absence of drug and polymer interaction.
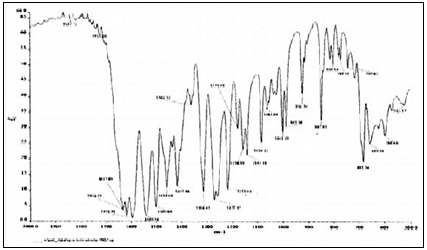
FTIR spectrum of metoclopramidehydrochloride
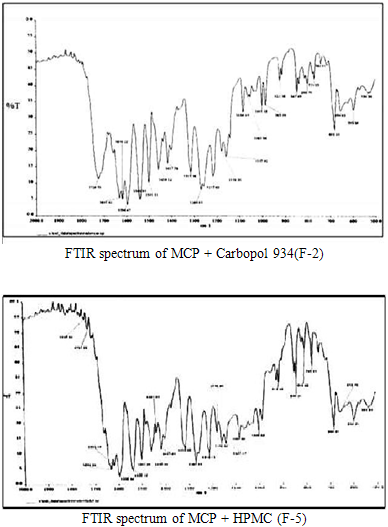
Fig. 2: Image showing drug polymer interaction study by FTIR
Particle size analysis:
Particle size analysis of different formulations was done by optical microscopy. The average particle size was found to be in the range of 45.6 to 52.2 μm. The mean particle size was significantly increases with increasing polymer concentration this may be due to high viscosity of polymer solution. Since high viscosity of polymer solution requires high shearing energy for breaking of droplets of the emulsion.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Drug content
The Drug contentof formulation F1 to F6 was carried out and found to be in a range 65.87 ± 0.52 to 78.84 ± 0.75 (as shown in table 3.).
Table- 2:- The Angle of repose, Bulk density, Tapped density and Compressibility index of MCP microspheres.
|
Parameters |
Formulation code |
|||||
|
|
F-1 |
F-2 |
F-3 |
F-4 |
F-5 |
F-6 |
|
Angle of repose |
24° 37’ |
25° 24’ |
26° 51’ |
23° 48’ |
25° 40’ |
23° 17’ |
|
Bulk density (gm/ml) |
0.46 |
0.57 |
0.49 |
0.44 |
0.53 |
0.52 |
|
Tapped density (gm/ml) |
0.52 |
0.64 |
0.55 |
0.50 |
0.61 |
0.60 |
|
Compressibility index (%) |
11.53 |
10.93 |
10.90 |
12.00 |
13.11 |
13.33 |
Table- 3:- The particle size, Drug content and Percentage Yieldof MCP microspheres.
|
Parameters |
Formulation code |
|||||
|
|
F-1 |
F-2 |
F-3 |
F-4 |
F-5 |
F-6 |
|
% Yield |
75.52 ± 2.8 |
73.45 ± 3.20 |
72.12 ± 3.10 |
70.24 ± 1.19 |
74.44 ± 2.60 |
74.35 ± 2.87 |
|
Average particle size (μm) |
48.5 |
51.5 |
52.2 |
45.6 |
46.9 |
50.8 |
|
Drug content(%) |
78.84 ± 0.75 |
76.11 ± 0.84 |
71.70 ± 0.23 |
74.29 ± 0.58 |
69.25 ± 0.47 |
65.87 ± 0.52 |
Drug release study:
The drug release from formulation F-1 to F-6 (as shown in fig 3) was as follows. F-1, F-3, F-4 and F-6 show percentage drug release 83.21 ± 1.55 to 89.24 ± 2.41 at end of 12 hour and formulation F-2 and F-5 show percent drug release 92.34 ± 1.06 and 89.45 ± 2.41 at end of 12 hr. Among all formulation F-2 was found to be the best formulation as it release metoclopramide hydrochloride in a sustained manner with constant fashion over extended period of time (after 12 hr). It was observed as the concentration of carbopol 934 and HPMC was increased percent release of metoclopramide hydrochloride decreases. The increase in carbopol 934 and HPMC concentration leads to the increased density of polymer matrix into the microspheres which result in an increased diffusional path length. This may decrease the overall drug release from polymer matrix. Furthermore smaller microspheres are formed at lower polymer concentration and have larger surface area exposed to dissolution medium.
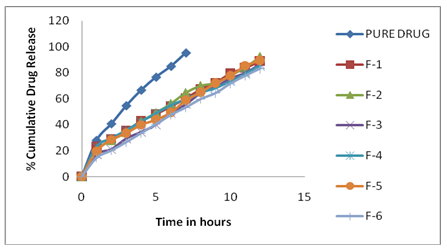
Fig. 3: In vitro drug release profile of MCP microspheres formulation F-1 to F-6
DISCUSSION:
The particle size of microspheres were ranged between 45.6μm-52.2μm.It was mentioned in table3.The shape of the microspheres shows hollow structure with a smooth surface and presence of drug particles even on this surface. It was evidenced from Scanning Electron micrographs (SEM). It was shown in fig 1.The prepared metoclopramide hydrochloride microspheres were subjected to the measurement of flow properties by determining angle of repose and the results indicate good flow property. The results were shown in table 2. The in-vitro dissolution profile of carbopol and HPMC containing MCP microspheres in Phosphate buffer pH 1.2 and 7.4 showed that microspheres with low amount of polymers released MCP faster (as shown in figure 3) and the drug release was compared with pure drug release.
CONCLUSION:
Sustained-release MCP microspheres have been successfully prepared using carbopol 934 and HPMC polymers and employing the solvent evaporation technique. The formulation F-2 containing carbopol 934 with drug: polymer ratio (1:1.5) was found to be satisfactory in terms of excellent micromeritic properties, yield of microspheres (73.45 ± 3.20%), incorporation efficiency (76.11 ± 0.84 %) and highest in vitro drug release of 92.34 % in sustained manner with constant fashion over extended period of time for 12 hrs. Release data were analyzed based on Highuchi kinetics and Korsmeyer/Peppa’s equation and all the selected formulations showed good fit to Peppa’s equation. From the results it was observed that Drug Polymer ratio influences the particle size as well as drug release pattern of MCP microspheres.
ACKNOWLEDGEMENTS:
The authors are thankful to the management, Sree Siddaganga College of Pharmacy, for providing necessary facilities to carryout this work.
REFERENCES:
1. S Raju, P Sandeep Reddy, V Anirudh Kumar, A Deepthi, K Sreeramulu Reddy, PV Madhava Reddy. Flash release oral films of metoclopramide hydrochloride for pediatric use: Formulation and in-vitro evaluation. Journal of Chemical and Pharmaceutical Research 2011; 3(4):636-646.
2. Yuveraj Singh Tanwar, Pushpendra Singh Naruka, Garima Rani Ojha. Development and evaluation of floating microspheres of verapamil hydrochloride 2007; 43(4):529-534.
3. Ram Chand Dhakar, Sunil K Prajapati, Sheo Datta Maurya, Anish K Gupta, Girish K Yadav, Girija Dangi. Rosiglitazone Maleate Microspheres for Extending Drug Release: Formulation and Evaluation. International Journal of Pharma Research and Development 2010; 2(10):56-65.
4. Prasanth VV, Akash Chakraborthy Moy, Sam T Mathew, Rinku Mathapan. Microspheres - An Overview. International Journal of Research in Pharmaceutical and Biomedical Sciences 2011; 2 (2): 332-338.
5. S H Khidr, E M Niazyt, Y M El-Sayed. Preparation and In-Vitro Evaluation of Sustained-Release Metoclopramide Hydrochloride Microspheres. J. Microencapsulation 1995; 12(6): 651-660.
6. JK Patel, MS Bodar, AF Amin, MM Patel. Formulation and optimization of mucoadhesive microspheres of metoclopramide. Indian J. Pharm. Sci 2004; 66(3): 300-305.
7. Hemalatha K, Lathaeswari.R , Suganeswari.M, Senthil Kumar V, Anto Shering M . Formulation And Evaluation Of Metoclopramide Hydrochloride Microbeads By Ionotropic Gelation Method. International Journal of Pharmaceutical & Biological Archives 2011; 2(3):921-925.
8. E.A.Rawlins, Bentley’s Text Book of Pharmaceutics, Bailliere Tindall, London, 8th Edition, 663.
9. Anand Gadad, Chirag Naval, Krunal Patel, Panchaxari Dandagi, Vinayak Mastiholimath. Formulation and Evaluation of Floating Microspheres of Captopril for Prolonged Gastric Residence Time. Indian Journal of Novel Drug Delivery 2011; 3(1): 17-23.
10. Sapna Desai1, Gali Vidyasagar, Anil Bhandhari. Mucoadhesive Microspheres of Midazolam: Nose to Brain Delivery. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2011; 2(4): 382-391.
11. Asha Patel, Subhabrata Ray, Ram Sharangat Thakur. In vitro evaluation and optimization of controlled release floating drug delivery system of metformin hydrochloride. DARU 2006; 14(2): 57–64.
12. Sengodan Tamizharasi, T Sivakumar, Jagdish Chandra Rathi. Preparation and evaluation of Aceclofenac floating oral delivery system. Pelagia Research Library 2011; 2 (5):43-53.
13. The United States Pharmacopoeia, XXVI. Rockville, MD: The United States Pharmacopoeial Convention, Inc; 2003: 859.
14. Nelson KG, Wang LY. Determination of time course of tablet disintegration II: Method using continuous functions. J. Pharm. Sci 1961; 67(1): 86-89.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









