About Authors:
Yamini Pendyala*, Sudha Talasila, M.sulthana
Department of Pharmaceutics,
Padmavathi College of Pharmacy and Research Institute,
Periyanahalli, Tamilnadu.
*Yamini.pendyala45@gmail.com
ABSTRACT
The purpose of this research was to formulate and systemically evaluate in-vitro and in-vivo performances of mucoadhesive Ramipril microspheres for its potential use in the treatment of hypertension, myocardial infraction. Ramipril mucoadhesive microspheres, containing chitosan as mucoadhesive polymer and ethyl cellulose as carrier polymer, were prepared by an emulsion-solvent evaporation technique. Preformulation studies were carried out before formulation design. Total seven formulations were prepared.Microspheres were discrete, spherical, free-flowing and showed a good percentage of drug entrapment efficiency. An in-vitro wash off test showed that Ramipril mucoadhesive microspheres adhered more strongly to the gastric mucous layer and could be retained in the gastrointestinal tract for an extended period of time. In-vitro dissolution test was carried out by using phosphate buffer pH 6.8. All the formulations showed good dissolution profiles. Among all the formulation F5 showed good dissolution profile with 81.0% of drug release in 12 hours. In-vitro release kinetic data of Ramipril microspheres showed that the drug release mechanism was diffusion controlled as the plots of Higuichi model was linear. All formulations exhibited Non-Fickian diffusion (n value is in between 0.5 to 1) mechanism. Stability studies were done for the selected formulation indicates that there is no change in drug content of the formulation.The results showed a sustained anti-hypertensive effect over a longer period of time in case of mucoadhesive microspheres, compared to the powder. In conclusion, the prolonged gastrointestinal residence time and slow release of Ramipril resulting from the mucoadhesive microspheres, could contribute to the provision of a sustained anti-hypertensive effect.
Reference Id: PHARMATUTOR-ART-1304
INTRODUCTION
For many drugs a well-designed drug delivery system is an important as pharmacological activities of the drug. A well designed drug delivery system can accurately deliver the drug to the site of action at desired rate and minimize its side effects by reducing exposure of drug to other tissues.1 Microencapsulation is a process by which very tiny droplets or particles of liquid or solid material are surrounded or coated with a continuous film of polymeric material. In microencapsulation particle size is ranging from several 10 microns to 5000 microns.
Microencapsulation provides the means of converting liquids to solids, of altering colloidal and surface properties, of providing environmental protection and of controlling the release characteristics or availability of coated materials. Several of these properties can be attained by macro packaging techniques; however, the uniqueness of microencapsulation is the smallness of the coated particles and their subsequent use and adaptation to a wide variety of dosage forms2.Mucoadhesive drug delivery system is delivery system which utilizes the property of bioadhesion of certain polymers which become adhesive on hydration and can be used for targeting a drug to a particular region of the body for extended periods of time. Mucoadhesive drug delivery system could be designed for buccal, oral, vaginal, rectal, nasal and ocular route of administration.3
Ramiprilisa potent ACE inhibitor used in the treatment of hypertensive disease. It is a highly lipophilic (log p octanol/water, 3.32) and poorly water soluble drug, with absolute bioavailability of 28-35% and half-life 2-4 hours4. It undergoes significant 'first pass' metabolism. Ramipril is a prodrug and is converted into an active metabolite ramiprilat by liver esterase enzymes. Ramiprilat is mostly excreted by the kidneys. The half-life of ramiprilate is variable, and is prolonged by heart and liver failure, as well as kidney failure.
Ramipril is marketed in India under the brand names of Cardace, Zigpril and Zorem. Single doses of Ramipril of 2.5 - 20 mg produce approximately 60 - 80 % inhibition of ACE activity 4hours after dosing with approximately 40 - 60% inhibition after 24 hours.5
MATERIALS AND METHODS
Ramipril obtained as a gift sample from Watson Pharma Pvt.Ltd., Thane. Chitosan was also obtained from Watson Pharma Pvt. Ltd., Thane. Ethyl cellulose, Span 80, Liquid paraffin(light), Ethanol,Pottassiumdihydrogen phosphate, Petroleum ether, Sodium hydroxide were obtained from Supra Pharmaceutical Pvt. Ltd., Hyderabad.
Method of Preparation
Emulsion Solvent Evaporaion Method
Accurately weighed quantity of polymers ( chitosan and ethyl cellulose) were dissolved in 20ml of ethanol. Weighed quantity of drug is dispersed in the above polymer phase and emulsified with 100ml of liquid paraffin containing 2ml of span 80 with continuous stirring at 800 rpm using mechanical stirrer. The stirring was continued for 3.5 hrs. to ensure complete evaporation of ethanol. The microspheres were separated from liquid paraffin by filtration through watmann filter paper No.44 washed three times with petroleum ether and air dried for 12 hours.
Table No.1 Formulation Design
|
Formulation |
Drug(mg) |
Chitosan(mg) |
Ethyl cellulose(mg) |
|
F1 |
20 |
460 |
340 |
|
F2 |
20 |
480 |
320 |
|
F3 |
20 |
530 |
270 |
|
F4 |
20 |
400 |
400 |
|
F5 |
20 |
270 |
530 |
|
F6 |
20 |
320 |
480 |
|
F7 |
20 |
340 |
460 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
EVALUATION
Flow Properties:
Angle of Repose
The flow characteristics are measured by angle of repose. Improper flow is due to frictional forces between the particles. Angle of repose is defined as maximum angle possible between the surface of the pile of the powder and the horizontal plane. Lower the angle of repose the better the flow property
Tan? = h/r
? = tan‾ 1 (h/r)
Where, h= height of pile
r= radius of base of pile
?= angle of repose
Bulk Density
Bulk density is defined as the weight per unit volume of material. Bulk density is primarily used for powders or pellets. The test can provide a gross measure of particle size and dispersion which can affect material flow consistency and reflect packaging quantity. Each experiment was conducted in triplicate.
Particle Size Determination
Sieving Method
Particles having size range between 50 and 1500 µm are estimated by sieving method. Standard sieves of different mesh numbers are available commercially as per the specifications of IP and USP. Sieves are arranged in a nest with the coarsest at the top. A sample (50gms) of the powder is placed on the top sieves. This sieve set is fixed to the mechanical shaker apparatus and shaken for a certain period of time (20 min).
The powder retained on each sieve is weighed. Frequently the powder is assigned the mesh number of the screen through which it passes or on which it is retained. It is expressed in terms of arithmetic or geometric mean of the two sieves.
Percentage Yelid
The total amount of Microspheres obtained was weighed and the percentage yeild was calculatedtaking into consideration the weight of drug and polymer6
%Yield=(Practical yield / Theoretical yield) x100
Entrapment Efficiency
Drug entrapment efficiency of Ramipril was performed by accurately weighing 100 mg of micro particles and suspended in 100ml of simulated intestinal fluid of pH 7.4±0.1 and it was kept for 12hrs. Next day it was stirred for 15mins, and subjected for filtration. After suitable dilution, Ramiprilcontent in the filtrate was analyzed spectrophotometrically at 210nm using Shimadzu UV-visible Spectrophotometer.
The absorbance found from the UV-spectrophotometer was plotted on the standard curve to get the concentration of the entrapped drug. Calculating this concentration with the dilution factor we get the percentage drug encapsulated in microparticles.7
Encapsulation efficiency = Estimated drug content/theoretical drug content × 100
In- vitroWash-off Test
The mucoadhesive property of microspheres was evaluated by an In vitro adhesion testing method known as wash-off method. Freshly excised piece of intestinal mucosa (2 x 2 cm) from goat were mounted on to glass slides (3 x 1 inch) with cyanoacrylate glue. Two glass slides were connected with a suitable support, about 100 microspheres were spread on to each wet rinsed tissue specimen and immediately thereafter the support was hung on to the arm of a USP tablets disintegrating test machine. When the disintegrating test machine was operated, the tissue specimen was given slow, regular up-and-downmoment in the test fluid (900ml of 0.1N HCl) at 37± 0.5?C. At the end of 30 min, at the end of one hour, and at the hourly intervals up to 5 hours, the machine was stopped and number of microspheres still adhering to tissue was calculated. The studies were carried out in triplicate.8
In- vitroDissolution Studies
Dissolution studies were carried out for all the formulation, employing USP XXIII apparatus (Basket method) at 37± 0.5?C rotated at constant speed of 50 rpm using 0.1N HCl as the dissolution medium. A sample of microspheres equivalent to 100mg of Ramipril was used in each test. An aliquot of the sample was periodically with drawn at suitable time interval and the volumes were replaced with fresh dissolution medium in order to maintain the sink condition. The sample was analyzed spectrophotometrically.
Kinetics of Drug Release
In order to understand the mechanism and kinetic of drug release, the drug release data of the in vitrodissolution study were analyzed with various kinetic model like zero order, first order, Higuchi’s, Peppa`s and Coefficient of correlation (r) values were calculated for the liner curves by regression analysis of the above plots.
Stability Studies
The formulations were stored in an oven at 30 ± 2?C and 65% RH for a period of six weeks. The samples were analyzed for drug content every week by spectrophotometer at 210nm. Microspheres were individually wrapped using aluminium foil and packed in ambered color screw cap bottle and put at above specified condition in incubator for one month. After one month microspheres were evaluated for in-vitro drug release.
Scanning Electron Microscopy
The samples were dried thoroughly in vacuum desiccator before mounting on brass specimen studies. The samples were mounted on specimen studies using double sided adhesive type, and gold palladium alloy of 120A? knees was coated on the sample using sputter coating unit (Model E5 100 Polaron U.K) in Argon ambient of 8-10 Pascal with plasma voltage about 20MA. The sputtering was done for nearly 3mins to obtain uniform coating on the sample to enable good quality SEM images. The SEM was operated at low accelerating voltage of about 15KV with load current of about 80MA. The condenser lens position was maintained between 4.4-5.1.The objective lens aperture has a diameter of 240 microns and the working distance WD=39mm.
RESULTS AND DISCUSSION
In this present work efforts have been made to develop mucoadhesive microspheres of Ramipril by emulsion solvent evaporation method using chitosan and ethyl cellulose. Total seven formulations were prepared and the detailed composition is shown in Table No. 1. The prepared microspheres were subjected to determine angle of repose, bulk density, particle size, drug entrapment efficiency, In-vitro dissolution and stability studies.
Flow properties of the prepared microspheres were determined by conducting Angle of repose, Bulk density determinations shown inTable No. 2. All the formulations showed angle of repose within the range of 22?24 to 26?51. Results indicating that they are having good flow properties. Particle size was determined by sieving method results were showed in Table No. 3. The particle size of formulations was mostly in the range of 297- 590 µm.
The Percentage yield of microspheres of all the formulations was in the range of 78.90% to 90.95% shown in the Table No. 4. The Entrapment efficiency was in the range of 62.68 to 73.82 shown in Table No. 4. The test results of wash-off test were showed in the Table No. 5, 6. Microspheres showed good mucoadhesive properties in the In-vitro wash-off test. Formulation F5 has more mucoadhesive strength than others.
The results of the in-vitro dissolution studies of formulations F1 to F7 are shown in Table No.7. Among all the formulations F5 showed good dissolution profile with 81.0% of drug release in 12 hours.
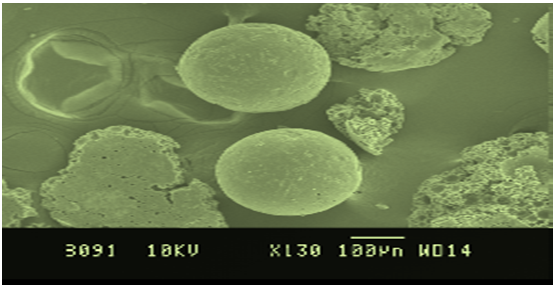
Drug release kinetic datafor microspheres was shown in Table No. 8. All the formulations exhibited anomalous (Non-Fickian) diffusion (n value is in between 0.5 to 1.0) mechanism. The drug release mechanism was diffusion controlled as the plot of Higuichi model is linear.Morphology of the microspheres was investigated by scanning electron microscopy. The photograph of formulations were taken by scanning electron microscope shown in the Figure No.2. The microspheres prepared by this method were found to be spherical, free flowing and it was observed by Scanning electron microscopy
Table No.2 Flow Properties of Ramipil Microspheres
|
S.No |
Formulation |
Angle of Repose |
Bulk density |
|
1 |
F1 |
24º 15’ |
0.592 |
|
2 |
F2 |
25º 39’ |
0.624 |
|
3 |
F3 |
25 º23’ |
0.624 |
|
4 |
F4 |
26 º51’ |
0.596 |
|
5 |
F5 |
22º 56’ |
0.622 |
|
6 |
F6 |
22º 24’ |
0.610 |
|
7 |
F7 |
25º 10’ |
0.590 |
Table No.3 Particle size determination of Ramipril microspheres
|
Batch No.
|
Sieve No.16(1.19mm) 840-1190µm |
Sieve No.20(0.84mm) 590-840µm |
Sieve No.25(0.600mm) 600µm |
Sieve No.30(0.59mm) 297-590µm |
|
F1 |
0.07 |
0.08 |
0.20 |
0.65 |
|
F2 |
0.03 |
0.06 |
0.31 |
0.55 |
|
F3 |
0.02 |
0.1 |
0.12 |
0.78 |
|
F4 |
0.06 |
0.21 |
0.11 |
0.68 |
|
F5 |
0.10 |
0.15 |
0.52 |
0.24 |
|
F6 |
0.04 |
0.23 |
0.18 |
0.48 |
|
F7 |
0.16 |
0.09 |
0.27 |
0.57 |
Table No.4 Percentage Yield and Entrapment Efficiency of Ramipril Microspheres
|
Formulation |
Percentage Yield |
Entrapment efficiency |
|
F1 |
82.64 |
67.43 |
|
F2 |
78.90 |
63.10 |
|
F3 |
80.50 |
62.68 |
|
F4 |
86.53 |
69.55 |
|
F5 |
90.95 |
72.82 |
|
F6 |
83.16 |
69.40 |
|
F7 |
80.93 |
73.82 |
Table No.5 In–vitroWash-off Test for Ramipril Microspheres in 0.1N HCL
|
Formulation |
Mean Percentage of Microspheres Adhering to Tissue in 0.1N HCL
|
||||
|
1hr |
2hr |
4hr |
6hr |
8hr
|
|
|
F1 |
78.3 |
70.2 |
57.6 |
34.8 |
20.4 |
|
F2 |
80.7 |
71.4 |
58.6 |
36.4 |
21.8 |
|
F3 |
81.6 |
73.5 |
59.7 |
37.1 |
23.7 |
|
F4 |
82.8 |
74.6 |
62.3 |
39.6 |
25.6 |
|
F5 |
84.1 |
75.2 |
64.1 |
40.8 |
27.1 |
|
F6 |
77.9 |
69.6 |
56.8 |
33.4 |
19.6 |
|
F7 |
79.6 |
71.8 |
57.9 |
34.6 |
20.2 |
Table No.6 In- vitro Wash off Test for Ramipril Microspheres in PBS pH 6.8
|
Formulation |
Mean percentage of Microspheres Adhering to Tissue in PBS pH 6.8 |
|||
|
1hr |
2hr |
4hr |
6hr |
|
|
F1 |
68.4 |
58.3 |
29.2 |
14.2 |
|
F2 |
70.3 |
60.1 |
31.1 |
16.1 |
|
F3 |
71.6 |
61.3 |
32.4 |
17.3 |
|
F4 |
73.8 |
63.4 |
34.3 |
19.6 |
|
F5 |
74.2 |
64.9 |
35.8 |
20.5 |
|
F6 |
67.1 |
57.5 |
28.6 |
13.8 |
|
F7 |
69.6 |
59.8 |
30.9 |
15.7 |
Table No.7 Cumulative Percentage Release Profile of FormulationsF1-F7
|
Time (hrs) |
Cumulative percentage drug release |
||||||
|
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
|
|
1 |
10.50 |
12.75 |
14.62 |
17.25 |
16.50 |
17.62 |
19.87 |
|
2 |
13.87 |
16.12 |
18.37 |
19.87 |
21.75 |
22.87 |
24.75 |
|
4 |
28.88 |
32.62 |
38.17 |
38.24 |
40.87 |
34.12 |
37.87 |
|
6 |
33.71 |
38.24 |
44.99 |
41.99 |
52.50 |
44.62 |
48.75 |
|
8 |
42.37 |
42.74 |
53.62 |
47.99 |
61.50 |
50.24 |
57.75 |
|
10 |
49.12 |
48.37 |
62.20 |
55.87 |
75.75 |
57.37 |
61.12 |
|
12 |
53.17 |
55.49 |
66.37 |
58.49 |
81.00 |
63.37 |
67.87 |
Figure No.1 Dissolution Profile For Formulations F1-F7
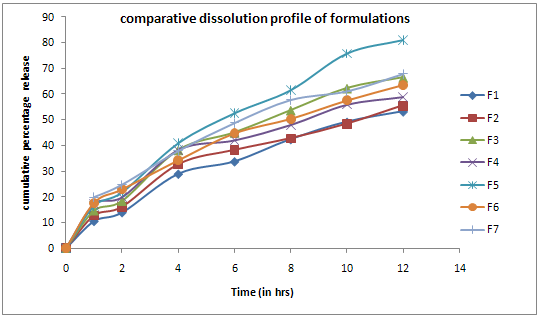
Table No.8In- Vitro Release Kinetic Data For Ramipril Microspheres
|
Formulation |
Zero order plots |
First order plots |
Higuichi plots |
Korsemeyerpeppa`s plot |
Possible drug release mechanism |
|
|
R2 |
R2 |
R2 |
R2 |
n |
||
|
F1 |
0.9718 |
0.9899 |
0.9908 |
0.9836 |
0.6892 |
Non-Fickian, Higuichi |
|
F2 |
0.9543 |
0.976 |
0.983 |
0.974 |
0.6194 |
Non-Fickian, Higuichi |
|
F3 |
0.9604 |
0.9913 |
0.9882 |
0.9782 |
0.6547 |
Non-Fickian,First order |
|
F4 |
0.9414 |
0.9774 |
0.9768 |
0.9649 |
0.534 |
Non-Fickian, First order |
|
F5 |
0.9821 |
0.9861 |
0.9916 |
0.9886 |
0.6773 |
Non-Fickian, Higuichi |
|
F6 |
0.9824 |
0.9987 |
0.9774 |
0.9941 |
0.5354 |
Non-Fickian, First order |
|
F7 |
0.969 |
0.9933 |
0.9924 |
0.9903 |
0.5253 |
Non-Fickian, First order |
Table No.9 Stability Studies of Percentage Drug Content of Formulation F1
|
Formulation |
Drug content before storage |
Drug content after 30 days |
||||||
|
Percent drug content |
Percent drug content at 4?C |
Percent drug content at room temperature |
Percent drug content at humidity 30?C ± 2?C/ 65% RH |
|||||
|
Drug content |
Percent drug |
Drug content |
Percent content |
Drug content |
Percent content |
Drug content |
Percent content |
|
|
F1 |
67.8 |
67.8 |
66.98 |
66.98 |
64.32 |
64.32 |
61.76 |
61.76 |
Figure No. 2 SEM Of Microspheres
CONCLUSION
Ramipril mucoadhesive microspheres were prepared successfully using chitosan as a mucoadhesive polymer and ethylcellulose as a release retardant in different proportions. Preformulation studies of ramipril were done initially and results directed for the further course of formulation. Based on the preformulation studies different batches were prepared using selected excipients. Prepared microspheres were evaluated for the percentage yield, drug content, entrapment efficiency, particle size determination, in-vitro wash- off test, in-vitro dissolution test. The drug content and entrapment efficiency were good. Among all the formulations F5 showed better results. Mucoadhesive property of F5 was better than the other formulations.
Dissolution was carried out in phosphate buffer pH 6.8 at 210nm. All the formulations were evaluated using different kinetic models i.e. Zero order kinetics, First order kinetics, Krosmeyerpeppa`s model and Higuichi kinetics. All the formulations exhibited Non-Fickian diffusion mechanism. The drug release was diffusion controlled as the plot of Higuichi model was found to be linear. The formulation F5 was selected as an optimized formulation with 81.00 % of drug release in 12 hours.Data for the stability study indicate that there was no change in residual drug content for the selected formulation F1
REFERENCES
1. Gilbert. S. Banker, Christopher. T. Rhodes “Modern pharmaceutics” third edition (P. No: 470,598,603,638,834).
2. Microencapsulation, Wikipedia, a free encyclopedia, “en.Wikipedia.Org/wiki/ microencapsulation”.
3. HarshadParmaret.al., “Different methods of formulation and evaluation of mucoadhesive microspheres” JABPT Vol 1, Issue 3, Nov 2010.
4. P.ekambaramet.al .,“formulation and evaluation of solid lipid nano particles of ramipril’’Journal of young pharmacists, Volume 3, year 2011, page No. 216-220.
5. Ramipril DP capsules product information VI 080606 Page no. 2-24.
6. S.K. Singh “ pharmaceutical characterization of Amoxicillin trihydrade as mucoadhesive microspheres in mamagement of H. Pylori” International journal of pharma tech research Jan 2010, Vol.2, page No.348-358.
7. Ahuja A., Khar R.K., Ali J.,Mucoadhesive drug delivery system,Drug. Dev.Ind. Pharm., 1997; 23(5), 489-515.
8. Chowdary K.P.R., Srinivas L.,Mucoadhesive drug delivery systems:A review of current status. IndianDrugs. 2000, 37(9), 400-406.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









