About Author: *Ghadage Ratnadeep V., Shah Vishal V., Bapat Bhushan A., Shirote Pramod J.
Department of Pharmaceutical Chemistry,
Appasaheb Birnale College of Pharmacy,
South Shivaji Nagar, Sangli, Maharashtra, India - 416 416
Abstract:
Quinoxaline and its derivatives are an important class of benzoheterocycles displaying a broad spectrum of biological activities which have made them privileged structures in pharmacologically active compounds.Quinoxaline also called benzopyrazine it has been considered as a wonder nucleus which posses almost all types of biological activities. This diversity in the biological response profile has attracted the attention of many researchers to explore this skeleton to its multiple potential against several activities. They are clinically effective as antibacterial, antifungal, anti-inflammatory, anticancer, anti-tubercular and antineoplastic agents. Interestingly, it also shows anti-HIV and anti-proliferative activity. Modification in their structure has offered a high degree of diversity that has proven useful for the development of new therapeutic agents having improved potency and lesser toxicity. Considering the extensive research on quinoxaline in the past, it was essential to review the wide spectrum of biological activity of quinoxalines. To conclude, this review will be beneficial for new drug discovery of quinoxaline moiety. Present article is sincere attempt to review wide range of biological activities of quinoxline moiety given by researcher.
[adsense:336x280:8701650588]
Reference ID: PHARMATUTOR-ART-1131
Introduction:
Heterocyclic compounds represent an important class of biological active molecules. Specifically those containing quinoxaline derivatives have evoked considerable attention in recent years as these are endowed. Quinoxalines are a versatile class of nitrogen containing heterocyclic compounds and they constitute useful intermediates in organic synthesis. Quinoxaline, also called a benzopyrazine, in organic chemistry, is a heterocyclic compound containing a ring complex made up of a benzene ring and a pyrazine ring and they are isomeric with cinnolenes, phthalazinesand quinazolines1. There are a number of processes available to generate quinoxaline but generally, they are synthesized by the condensation of 1, 2-dicarbonylswith 1, 2 diamines in presence of suitable catalyst using various solvent systems.
They possess well known biological activities including AMPA/GlyN receptor antagonis2,antihistaminic agents3, anti-trypanosomal activity4, anti-herps5, antiplasmodial activity6,Ca uptake/ Release inhibitor7, inhibit vascular smooth muscle cell proliferation8. Quinoxaline derivatives constitute the basis of many insecticides, fungicides, herbicides, as well as being important in human health and as receptor antagonists. Although rarely described in nature, syntheticquinoxaline moiety is a part of number of antibiotics such as echinomycin, levomycin and actinomycin which are known to inhibit the growth of Gram-positive bacteria and also active against various transplantable tumours.9,10 In addition, quinoxaline derivatives are reported for their application in dyes, efficient electroluminescent materials, organic semiconductors and DNA cleaving agents11. These are useful as intermediates for many target molecules in organic synthesis and also as synthons.
Numerous methods are available for the synthesis of quinoxaline derivatives whichExtensive researches have generated numerous synthetic approaches for the construction of the skeleton of such heterocycles. Among these methods, the most widely used one relies on the condensation of aryl-1,2- diamines with aryl ketones, usually α-dicarbonyl compounds or their equivalents12. Recent improvements on these conditions were reported via solid-phase13, oxidative coupling of epoxides with ene-1,2-diamines14. Improved methods have been reported via a condensation process catalyzed by CAN15, molecular iodine as a catalyst 16, manganese octahedral molecular sieves17, task-specific ionic liquid18, from PEG-40019, from IBX20, from PbO21, from ZrO222,from galactose23.Recently, a number of catalysts have been reported for the synthesis of quinoxalines. Considering the significant applications in the fields of medicinal, industrial andsynthetic organic chemistry, there has been tremendous interest in developing efficient methods for the synthesis of quinoxalines.
Our aim in this review is to focus on quinoxaline structure and to analyze how slight modification in quinoxaline nucleus can act as precursor for assembly of large number of quinoxaline derivatives and providing tremendous number of pharmacological active molecules having a wide variety of biological activity and also therapeutic applications and to highlight the importance of quinoxaline moiety as a novel drug template for discovery of new agents in various areas of medicines.
[adsense:468x15:2204050025]
BIOLOGICAL ACTIVITIES OF QUINOXALINE DERIVATIVES
A) Anti-tubercular activity:
1) Vicente E. et al evaluated for in vitro efficacies of the 1,4-di-N-oxide quinoxaline derivatives against Mycobacterium tuberculosis and has lead to the discovery of a derivative with in vivo efficacy in the mouse model of tuberculosis24. (Figure No.1)
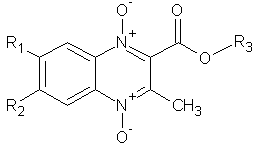
Where, R1/R2= H/CH3,H/OCH3, H/H, H/Cl, F/F, Cl/Cl,CH3/CH3, H/F, H/CF3
R3= CH2CH3, CH2Ph, CH3
Figure No.1: 1, 4-di-N-oxide quinoxaline
2) Carta A. et al synthesized 6-(7)-substituted-3-methyl- or 3-halogenomethyl-2 phenylthio–phenylsulphonyl–chloro-quinoxaline 1,4-dioxides derivatives were evaluated for in vitro antimycobacterial and Antitubercular screening showed a generally good activity of 3-methyl-2-phenylthioquinoxaline 1,4-dioxides against Mycobacterium tuberculosis25 (Figure No.2)
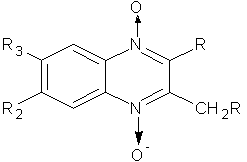
Where, R=Cl,S-Ph,SO2Ph, R1=H,Br and R2/R3=H, Cl,F,,CF3,CH3
Figure No.2: 3-halogenomethyl-2phenylthio–phenylsulphonyl–chloro-quinoxaline 1, 4-dioxides
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
3) Jaso A. et al synthesized A series of 2-acetyl and 2-benzoyl-6(7)-substituted quinoxaline 1, 4-di-N-oxide derivatives were evaluated for in vitro antituberculosis activity. The results show that 2-acetyl-3-methylquinoxaline 1,4-di-N-oxide derivatives with chlorine, methyl or methoxy group in position 7 of the benzene moiety and unsubstituted have good antitubercular activity.26 (Figure No.3)
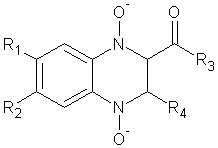
Where, R1=Cl, CH3, R2=Cl, H, R3 andR4=CH3
Figure No.3: 2-acetyl and 2-benzoyl-6(7)-substituted quinoxaline 1, 4-di-N-oxide derivatives
B) Antifungal activity:25
Carta A. et al synthesized (7)-substituted-3-methyl- or 3-halogenomethyl-2 phenylthio–phenylsulphonyl–chloro-quinoxaline 1, 4-dioxides.this derivatives were found to be good antimycobacterial and anticandida activity. (Figure No.4)
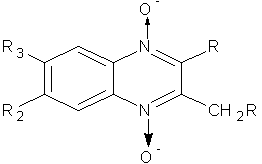
Where, R= Cl,S-Ph,SO2Ph, R1= H,Br and R2/R3= H, Cl,F,,CF3,CH3
Figure No.4: (7)-substituted-3-methyl- or 3-halogenomethyl-2 phenylthio–phenylsulphonyl -quinoxaline 1, 4-dioxides
C) Antimalerial activity:27
Vicente E. et al reported 3-phenylquinoxaline 1,4-di-N-oxide derivatives have been Antiplasmodial activity vitro against Plasmodium falciparum by the incorporation of [3H]-hypoxanthine. Some of them were shown to be more active than chloroquine in the resistant strain.( Figure No.5)
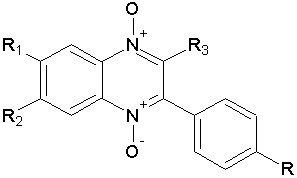
Figure No.5: 3-phenylquinoxaline 1,4-di-N-oxide derivatives
D) SRPK-1 kinase inhibitor:28
Szekelyhidi Z. et al synthesized novel tricyclic quinoxaline derivatives and synthesized as potential kinase inhibitory antiviral agents and were found to be active and selective for SRPK-1 kinase. (Figure No.6)
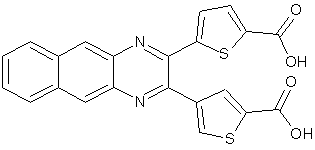
Figure No.6: Tricyclic quinoxaline derivatives
E) Adenosine A1 receptor inhibitory activity:29
Liu C. et al Synthesized 4-alkylamino-1-hydroxymethylimidazo [1,2-a]quinoxalines have been synthesized and evaluated for their adenosine A1 receptor inhibitory activity in the radioligand binding assays. The compounds were tested for the inhibition percent (IP) and the affinity toward A1AR (Ki) that IP were more than 90% in the nanomolar ranges. (Figure No.7)
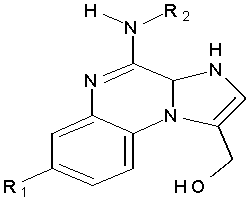
Where, R1=H and R2= (CH3)2CH2CH2-
Figure No.7: 4 -alkylamino-1-hydroxymethylimidazo [1,2-a]quinoxalines
F) Poly- (ADP-ribose) polymerase-1,2 inhibitor:3
Iwashita A. et al wereidentified as potent and selective poly- (ADP-ribose) polymerase-1 and 2 (PARP-1) and (PARP-2) inhibitors, respectively. In PARP enzyme assays using recombinant PARP-1 and PARP-2, quinazolinone derivatives displayed relatively high selectivity for PARP-1 and quinoxaline derivatives showed superior selectivity for PARP-2. SBDD analysis via a combination of X-ray structural study and homology modeling suggested distinct interactions of inhibitors with PARP-1 and PARP-2. These findings provide a new structural framework for the design of selective inhibitors for PARP-1 and PARP-2. (Figure No.8)
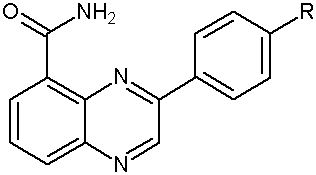
Where R= H, NH2, Cl, OMe
Figure No.8: Substituted 3-phenylquinoxaline-5-carboxamide
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
H) HIV-1 inhibitor:31
(S)-4-isopropoxycarbonyl-6-methoxy-3-(methylthiomethyl)-3,4dihydroquinoxaline-2(1H)-thione (HBY 097) was used to select for drug-resistant HIV-1 variants in vitro. The viruses first developed mutations affecting the NNRTI binding pocket, and five of six strains displayed the RT G190-E substitution, which is characteristic for HIV-1 resistance against quinoxalines. (Figure No.9)
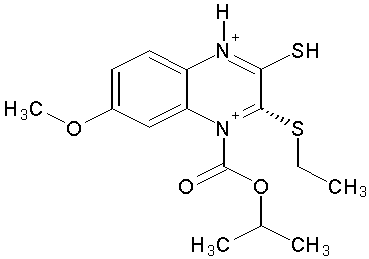
Structure of (HBY 097)
Figure No.9: Structure of (HBY 097)
I) Anti-convulsant activity:32
Wagle S. et al synthesized N-arylidenehydrazino quinoxalines. Further, the oxidative cyclizations of hydrazones by nitrobenzene yielded the synthesized compounds were showed anti-convulsant activity. (Figure No.10)
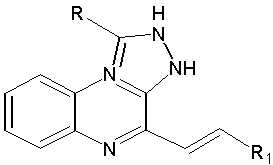
Where, R=H, CH3, CF3, (Un) substituted phenyl, R1= (UN) substituted phenyl
Figure No.10: 1-aryl-4-methyl [1,2,4] triazolo[4,3-a]quinoxalines.
J) Analgesic and anti-inflammatory activities:33
Hashem A. et al demonstrated analgesic and anti-inflammatory activities of 2-aminopyrimido [thiazolo[4,5-b]quinoxaline-4-one. Some of these compounds exhibited promising activities.(Figure No.11)
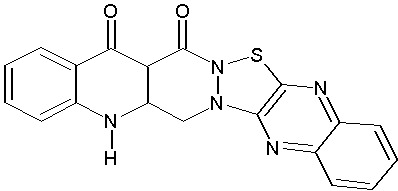
Where, R= F, H, CH3O
Figure No.11: 2-aminopyrimido [thiazolo[4,5-b]quinoxaline-4-one.
L) PDGF-R inhibitor:34
Myers M. et al Demonstrated activity novel substituted 2-anilino- and 2-cycloalkylaminoquinoxalines as inhibitors of PDGF-R autophosphorylation. The found that Replacement of an anilino-substituent with substituted cyclohexylamino- or norbornylamino substituents lead to significant improvements in the pharmacokinetic profile of these analogues. (Figure No.12)
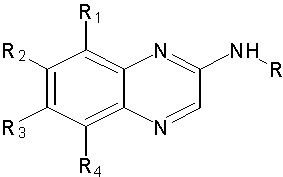
Where, R1=H, Me, R2= H, Me, R3 and R4= H, Me, MeO
Figure No.12: 2-anilino- and 2-cycloalkylaminoquinoxalines
N) Anti cancer activity:
1) Moarbess G. et al, were assessed In vitro cytotoxicity studies against melanoma (A375, M4Be, and RPMI-7591), colon (LS174T), breast (MCF7), and lymphoma (Raji) human cancer cell lines. In vivo studies were carried out in M4Be xenografted athymic mice. EAPB (I), EAPB (II), EAPB (III), showed significant in vitro activities against A375 compared to fotemustine and imiquimod used as references.35 (Figure No.13)
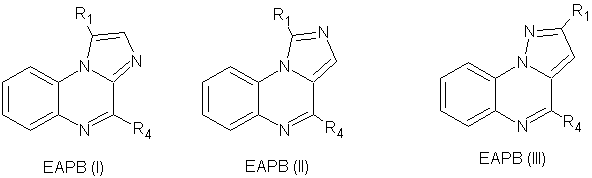
Where, R1= (CH3)2–CH–CH2–,C6H5–(CH2)2– andR4=CH3–NH–, NH2–
Figure No.13: Substituted pyrazolo[1,5-a]quinoxaline
2) Weng Q. et al Synthesized compounds a and showed that 3-(4-bromophenyl)-2-(ethylsulfonyl)-6-methylquinoxaline 1,4-dioxide (Q39), derived from Quinoxaline 1,4-Di-N-oxide, possessed high anti-cancer activity in hypoxia. Cytotoxicity assay demonstrated that Q39 is a potential and high efficient anti-cancer compound in all tested cell. In their work showing the mechanism of Q39 in hypoxia.36 (Figure No.14)
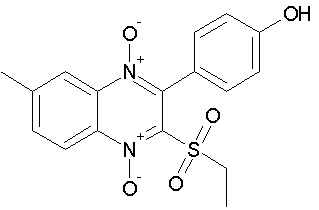
Figure No. 14: Chemical structure of Q39
3) Masquefa C. et al were synthesized New series of imidazo[1,2-a]quinoxaline analogues have been in good yields via a bimolecular condensation of 2-imidazole carboxylic acid, followed by a coupling with ortho-fluoroaniline and subsequent substitution on the imidazole ring by Suzuki Cross-coupling reaction using microwave assistance. Antitumor activities of these derivatives were evaluated by growth inhibition of A375 cells in vitro. It was proposed that all compounds exhibited high activities compared to imiquimod and fotemustine used as reference. 37(Figure No.15)
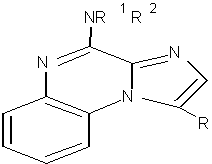
Where, R= (CH3)2CHCH, R= C6H5 (CH2)2
Figure No.15: Iimidazo [1, 2-a]quinoxaline analogues
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
O) Antimicrobial activity38:
Refaat H. et al were synthesized series of 2-[4-N-2-acylhydrazinocarbonyl) aniline]-3-methyl quinoxalines, as well as their cyclized oxadiazolyl derivatives were also prepared. Some of these derivatives were evaluated for antimicrobial activity in vitro. It was found that all the selected compounds exhibit antimicrobial activity and some of these compounds had a broad spectrum of activity. (Figure No.16)

Where, Ar – 3-Br-C6H4, 4-Br-C6H4, 4-NO2-C6H4
Figure No.16: 2 -[4-N-2-acylhydrazinocarbonyl) aniline]-3-methyl quinoxalines
P) Antihistaminic activity:39
Sridevi C. et al synthesized phenyl pyrazolo benzimidazole quinoxaline. All the synthesized compounds were screened for their antihistaminic activity.Some were shown good % protection of antihistamic activity. ( Figure No.17)
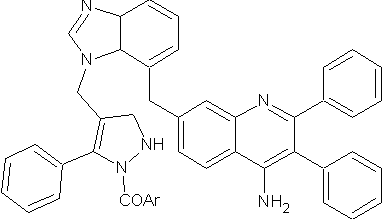
Figure No.17: Phenyl pyrazolo benzimidazole quinoxaline.
Q) Antiproliferativeactivity:40
Chung H et al were synthesizedA series of 6-arylamino-2,3-bis(pyridin-2-yl)-7-chloro-quinoxaline-5,8-diones and evaluated for theirinhibitory activity on rat aortic smooth muscle cell proliferation. They were observed that The quinoxaline-5,8-diones exhibited a potent antiproliferativeactivity. (Figure No.18)
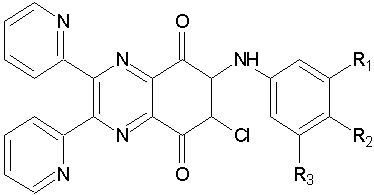
Figure No.18: 6-arylamino-2, 3-bis(pyridin-2-yl)-7-substituted -quinoxaline-5,8-diones
Conclusion:
The literature reveals that quinoxaline has diverse biological potential, and the easy synthetic routes for synthesis have been attention of the chemists, pharmacologists and researchers. The anticancer and anticonvulsant activities are the most encouraging activities for the pharmacists. Also the research on antitubercular activity has given positive results. By the present scenario and due to their wide range of applications, these compounds have received a great deal of attention in connection with their synthesis and it can be concluded that quinoxaline have a great potential. In conclusion a wide variety of biological activity of quinoxaline has been described. Quinoxaline moiety containing 1,4-di-N oxide shows broad spectrum of activity against wide number of bacterial species and also exhibited antituberculosis activity.
Acknowledgement:
Authors are thankful to The Principal, Appasaheb Birnale College of Pharmacy, Sangli. We would like to give our sincere thanks to Prof. Shirote P. J. for his valuable suggestions and encouragement from time to time.
References:
1. wikipedia.org/wiki/quinoxalines
2. NikamSS., CordonJ.J., OrtwineDF., Design and synthesis of novel quinoxaline-2,3-dione AMPA/GlyN receptor antagonis , Journal of Medicinal Chemistry, 1999, 42: 2266-71.
3. Sridevi CH., Balaji K., Naidu A.,Antimicrobial Evaluation and Synthesis of Some Phenylpyrazolo benzothiazoloquinoxaline Derivatives, E-Journal of Chemistry,2010,7(1) : 234-238.
4. Urquiola C.Vieites M.,Aguirre G.,Improving anti-trypanosomal activity of 3-aminoquinoxaline- 2-carbonitrile N1, N4-dioxide derivatives by complexation with vanadium,Bioorganic & Medicinal Chemistry, 2006, 14: 5503–5509.
5. Harmenberg J., Wahren B.,Antiherpes virus Activity and Mechanism of Action of Indolo-(2,3-b)Quinoxaline and Analogs, Antimicrobial Agents and Chemotherapy, 1988,32: 1720-1724.
6. Zarranz B., Jaso M., Lima LM.,Antiplasmodial activity of 3-trifluoromethyl-2-carbonylquinoxaline di-N-oxide derivatives, Rev. Bras. Cienc. Farm., 2006, 42 : 55-67
7. Xia H., Wang F., YU K.,Novel cyclophilin D inhibitors derived from quinoxaline exhibit highly inhibitory activity against rat mitochondrial swelling and Ca2+ uptake/ release, Acta Pharmacologica Sinica, 2005,26 (10): 1201–1211.
8. Chung HJ., Jung OJ.,Synthesis and biological evaluation of quinoxaline-5, 8-diones that inhibit vascular smooth muscle cell proliferation,Bioorganic & Medicinal Chemistry Letters, 2005, 15: 3380–3384.
9. Bailly C., Echepare S., Gago F., Recognition elements that determine affinity and sequence-specific binding to DNA of 2QN, a biosynthetic bis-quinoline analogue of echinomycinJornal of Anti-Cancer Drug Des., 1999,15: 291.
10. Sato S., Shirator O., Katagiri K.,Mode of action of quinoxaline antibiotics: Interaction of quinomycin A with deoxyribonucleic acid. Antibiot J., 1967, 20: 270.
11. Srinivas C., Sesha C. Kumar S.,Efficient, convenient and reusable polyaniline-sulfate salt catalyst for the synthesis of quinoxaline derivatives,Journal of Molecular Catalysis, 2007: 227–230.
12. Brown DJ., Taylor EC., The Chemistry of Heterocyclic Compounds Quinoxalines supplement II, John Wiley and Sons, New Jersey, 2004.
13. Jeon MK., Hyun DS., Gong YD.,Solid-phase synthesis of quinoxaline derivatives using 6-amino-2,3-dichloroquinoxaline loaded on AMEBA resin,Tetrahedron Letters, 2005, 46: 4979–4983.
14. Antoniottia S., and Duach E.,Direct and catalytic synthesis of quinoxaline derivatives from epoxides and ene-1,2-diamines,Tetrahedron Letters, 2002, 43: 3971–3973.
15. More SV., Sastry MN., Yao CF., Cerium (IV) ammonium nitrate (CAN) as a catalyst in tap water: A simple, proficient and green approach for the synthesis of quinoxalines, Green Chemistry, 2005, 91-95.
16. More S.M., Sastry M.N., and Yao C.F.,Molecular iodine: a powerful catalyst for the easy and efficient synthesis of quinoxalines, Tetrahedron Letters, 46, 2005, 6345–6348.
17. Sithambaram S.,Ding Y.,Li W.,Shen X.,Manganese octahedral molecular sieves catalyzed tandem process for synthesis of quinoxalines,Green Chemistry, 2008, 10: 1029–1032.
18. Dong F., Kai G., Zhenghao F., Xinli Z.,A practical and efficient synthesis of quinoxaline derivative catalyzed by task-specific ionic liquid,Catalysis Communications, 2008, 9: 317–320.
19. Zhang X., Wang Z, X., Sun Y.J.,Synthesis of quinoxaline derivatives catalyzed by PEG-400, Chinese Chemical LettersChinese Chemical Letters, 21, 2010: 395–398.
20. HeraviM.M., Bakhtiari K., Tehrani H.M., Facile synthesis of quinoxaline derivatives using O-iodoxybenzoic acid (IBX) at room temperature,ARKIVOC, 2006,(xvi): 16-22.
21. Shinde DB, Kotharkar SA.,Lead Oxide (PbO) Mediated Synthesis of Quinoxaline,Journal of the Iranian Chemical Society, 2006, 3(3): 267-271.
22. Ajaikumar S. Pandurangan, A.Efficient synthesis of quinoxaline derivatives over ZrO2/MxOy (M = Al, Ga, In and La) mixed metal oxides supported on MCM-41 mesoporous molecular sieves, Applied Catalysis A: General, 2009, 357: 184–192.
23. Yan L., Liu F., Dai G., An efficient synthesis of quinoxaline derivatives from 4-chloro-4-deoxy-a-D-galactose and their cytotoxic activities, Bioorganic & Medicinal Chemistry Letters, 2007, 17,: 609–612
24. Vicente E., Villar R., Burguete A., Solano B., Aldana I., Cho A., Robert M., Efficacy of Quinoxaline-2-Carboxylate 1,4-Di-N-Oxide Derivatives in Experimental Tuberculosis Experimental Tuberculosis, antimicrobial agents and chemotherapy, 2008, 3321–3326.
25. Carta A., Paglietti G., Nikookar ME., Sanna P., Sechi L., Novel substituted quinoxaline 1,4-dioxides with in vitro antimycobacterial and anticandida activity, Eur. J. Med. Chem., 37, 2002, 355–366.
26. Jaso A., Zarranz B., Aldana I., Monge A., Synthesis of new 2-acetyl and 2-benzoyl quinoxaline 1,4-di-N-oxide derivatives as anti-Mycobacterium tuberculosis agents, European Journal of Medicinal Chemistry, 38, 2003, 791-/800.
27. Vicente E., Lima LM., Bongard E., Charnaud S., Villar R., Perez-Silanes S., Aldana I., Vivas L., Monge A., Synthesis and structureeactivity relationship of 3-phenylquinoxaline1,4-di-N-oxide derivatives as antimalarial agents, European Journal of Medicinal Chemistry, 20, 2007, 1-8.
28. Szekelyhidi Z., Pato J., Waczek F., Banhegyi P., Hegymegi-Barakonyi B., Hafenbradl D., Obert S., Synthesis of selective SRPK-1 inhibitors: Novel tricyclic quinoxaline derivatives,Bioorganic & Medicinal Chemistry Letters, 15, 2005, 3241–3246.
29. Liu C., Wang B., Li W., Yun LH., Liu Y., Su RB, Li J., Design, synthesis, and biological evaluation of novel 4-alkylamino-1-hydroxymethylimidazo[1,2-a]quinoxalines as adenosine A1 receptor antagonists,Bioorganic & Medicinal Chemistry, 12, 2004, 4701–4707.
30. Iwashita A., Hattori K., Yamamoto H., Ishida J., Kido Y., Miyake H., Kinoshita T., Warizaya M., Ohkubo M., Matsuoka N., Mutoh S., Discovery of quinazolinone and quinoxaline derivatives as potent and selective poly(ADP-ribose) polymerase-1/2 inhibitors, FEBS Letters, 579, 2005, 1389–1393.
31. Peter kleim J., Rosner M., Winkler I., Paessens A., Kirsch R., Arnold E., Ries G., Selective pressure of a quinoxaline nonnucleoside inhibitor of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT) on HIV-1 replication results in the emergence of nucleoside RT-inhibitor-specific HIV-1 mutants, Proc. Natl. Acad. Sci. USA, 93, 1996, 34-38,
32. Wagle S., Adhikari AV., Kumari NS., Synthesis of some new 4-styryltetrazolo[1,5-a] quinoxaline and1 substituted-4-styryl[1,2,4]triazolo[4,3-a]quinoxaline derivatives as potent anticonvulsants, European Journal of Medicinal Chemistry, 44, 2009, 1135-1143.
33. Hashem A., Gouda M., Badria F., Synthesis of some new pyrimido[20,10:2,3]thiazolo[4,5-b]quinoxaline derivatives as anti-inflammatory and analgesic agents, European Journal of Medicinal Chemistry 45 ,2010, 1976–1981.
34. Myers MR., He W., Hanney B., Setzer N., Maguire MP., Zulli A., Needle S.,Potent Quinoxaline-Based Inhibitors of PDGF ReceptorTyrosine Kinase Activity.Part 1: SAR Exploration andEffective Bioisosteric Replacement of a Phenyl Substituent,Bioorganic & Medicinal Chemistry Letters, 13, 2003, 3091- 3095.
35. Moarbess G., Masquefa CD., Bonnard V., Paniagua SG., Vidal JR., Bonnet P., In vitro and in vivo anti-tumoral activities of imidazo[1,2-a]quinoxaline, imidazo[1,5-a]quinoxaline, and pyrazolo[1,5-a]quinoxaline derivatives, Bioorganic & Medicinal Chemistry, 16, 2008, 6601–6610.
36. Weng Q., Wang D., Guo P., Fang L., Hu Y., Yang B., Q39, a novel synthetic Quinoxaline 1,4-Di-N-oxide compound with anti-cancer activity in hypoxia, European Journal of Pharmacology, 581, 2008, 262–269.
37. Masquefa CD., Moarbess G., Khier S., David N., Paniagua SG., Bressolle F., BonnetP., New imidazo[1,2-a]quinoxaline derivatives: Synthesis and in vitro activity against human melanoma, European Journal of Medicinal Chemistry, 44, 2009, 3406–3411.
38. Refaat HM., Moneer AA., Khalil OM., Synthesis and Antimicrobial Activity of Certain Novel Quinoxalines,Archives Pharm Research, 27, 2004, 1093-1098,.
39. Sridevi CH., Balaji K., Naidu A., Sudhakaran R.,Antimicrobial Evaluation and Synthesis of Some Phenylpyrazolo benzothiazoloquinoxaline derivatives, E-Journal of Chemistry, 2009, 6(3), 866-870.
40. Chung HJ., Jung OJ., Chae DM., Hong SY., Chung KH., Leea SK., Ryu CK.,Synthesis and biological evaluation of quinoxaline-5,8-diones that inhibit vascular smooth muscle cell proliferation,Bioorganic & Medicinal Chemistry Letters, 15, 2005, 3380–3384.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









