ABOUT AUTHORS:
Kanzaria S. H. , Patel D. V., Patel K.V., Gandhi T. R.
Anand Pharmacy College.
Anand, Gujarat
*sainikak@yahoo.in
ABSTRACT:
Objective: To evaluate anti-inflammatory effect of Capparis aphylla Roth. (CA) using experimental animal models. Material and method: Female Wistar rats (200 to 250gm) were randomly allocated to six groups (n=6). Except group-I (normal control), arthritis was induced in animals of all groups by injection of 0.2 ml Complete Freund’s Adjuvant (6mg/ml) on day one. Additionally Group III (Std) and Group IV–VI (CA-1, CA-2 and CA-3) received Indomethacin (100mg/kg), CA (300 mg/kg ; 240 mg/kg and 190 mg/kg) from day 1 to day 21 orally. Paw volumes of both sides (measured by plethysmography) was recorded on day 0, 7, 14, 21. On 7th, 14th, 21stdays the severity of the secondary lesions was evaluated by measuring body weight, arthritic index, Erythrocyte Sedimentation Rate(ESR), Rheumatoid factor(RF), C- Reactive Protein(CRP), Albumin/Globulin(A/G) ratio, X-ray, histopathology of synovial joints. Result: Capparis Aphylla Roth. significantly prevented the freund’s adjuvant induced changes in body weight, serum A/G ratio , arthritic Index, paw edema, ESR, RF, CRP. the results were comparable with the standard drug indomethacin. Conclusion: Antiinflammatory activity of Capparis aphylla Roth. can beattributed to COX-II inhibition leading to decrease in inflammatory mediator.
REFERENCE ID: PHARMATUTOR-ART-1774
INTRODUCTION
Rheumatoid arthritis (RA) is a common chronic inflammatory autoimmune disease of unknown origin. It affects ∼1% of the population and causes the irreversible functional and anatomical joint damage. The have key factors of inflammation and destruction in the rheumatoid joint namely intracellular signaling and proliferation, adhesion, infammation, matrix degradation and angiogenesis that result in a persisting vicious circle. In addition to proinflammatory cytokines, acute phase response (like CRP), abnormal immunoglobulin levels and high rheumatoid factors (RFs) are also considered to be a modulating event during infammatory and immunogenic reaction. . [CHEMICO BIOLOGICAL INTERATION]
Bone loss is a common feature of various inflammatory arthritis Bone erosions results from the activation of an inflammatory response that increases the number and activity of osteoblasts. The complete freund”s adjuvant induced arthritis model represents a systemic inflammatory disease with bone and cartilage changes similar to those observed in RA. The common pathological features of adjuvant arthritis in rats and RA inhuman are joint swelling associated with cellular and pannus invasion of the joint space and bone resorption. Strong bone loss after intense arthritis is induced when adjuvant is injected into the foot pad. The major site of irreversible tissue damage originates from synovium lining the joint capsule with cartilage and bone, which is often termed the “pannus” and this is a characteristic feature of RA. [IJPD, CURATIVE EFFECT]
Capparis aphylla Roth. is commonly known as Caper berry, Karira, Kerada, Karer, Kair , belonging to the family Capparidaceae. Capparis aphylla Roth. has been used traditionally as an anti-inflammatory agent for enlarged cervical glands, sciatica, rheumatoid arthritis, swellings, skin eruptions, ringworm. [C.P. KHAR] Secondly Capparis aphylla Roth. root bark contains spermidine alkaloids which is known for its use in inflammations, asthma and gout.[C.P. KHAR] The different parts of C.aphylla are considered to be analgesic, diaphoretic, alexeteric, laxative, antihelminthes, antibacterial, antifungal, antiviral, and good in cough, asthma, ulcers, boils, vomiting, piles and all inflammations.[ANTIHYPERGLYCEMIC, ANTIOXIDENT]
Consequently, the present study has been undertaken to illustrate the beneficial outcome of the drug capparis aphylla Roth.in adjuvant induced arthritic rat model.
MATERIALS & METHODS.
· Preparation of extract of Capparis aphylla Roth.
3.2 kg root bark of capparis deciduas were percolated three times with ethyl alcohol at room temperature for 2-3 weeks. The solvent from the combined yellowish brown percolate was removed under reduced pressure. 38 g of dark brown viscous residue was taken up in 1N hydrochloric acid and taken out exhudatively with ethyl acetate to remove neutral substances. The aqeous acidic solution was basified with ammonia, yielding yellow precipitates. These yellow precipitates was then extracted with chloroform which on evaporation gave the alkaloid concentrate. The column chromatography of the chloroform extract was carried out in neutral alumina oxide and eluted with a mixture of ethyl acetate:methanol(4:1) and thus isolated the yellow coloured . The yellow compound so obtained contained traces of impurities.
The dragendroffs reagent, methanol, alumina oxide, ethyl acetate, Na azide, Na citrate, succnic acid, bromocresol, brij-95, formaline, formic acid, freund’s adjuvant, carrageenan were obtained from Astron chemical, ahmedabad,Gujarat, India. The total protein kit, RF latex kit, CRP kit were obtained from Coral clinical system, Gujarat,India.
* Animals
Female Wistar albino rats weighing 200–250 g were used. The animals were fed ad libitum and housed in a room with a controlled ambient temperature (22 ±2 °C), humidity (50% ± 10%), and a 12-h light/dark cycle. Animals were acclimated to the housing conditions and handled for 3–4 days before experiments. All experimental procedures were conducted according to the OECD Guidelines for the Care and Use of Laboratory Animals . The experimental protocols were approved by the Ethical Committee on Animal Care.
* Preparation of drug solution:
Accurately weighed quantity of dried extract was suspended in distilled water to prepare the appropriate stock solution of the drug i.e. 190mg/kg, 240mg/kg, 300mg/kg. Indomethacin 100mg/kg was also suspended in distilled water. The doses were administered by selecting theappropriate concentration of the stock solution by orally.
* Animal Grouping
Rats were divided into 6 equal groups (6 rats for each). Group 1 Normal Control group: rats received pure drinking water and regular food ; Group 2 Model Control group: Freund’s adjuvant; Group 3 Standard group : CFA+ Indomethacin 100mg/kg; Group 4 test group: CFA+MECA 190mg/kg; Group 5 test group: CFA+ MECA 240mg/kg; Group 6 test dose: CFA+ MECA 300mg/kg. Animals were treated with drugs for 21 days.
* Induction of Complete Freund’s Adjuvant Arthritis
- Experimental procedure [Schorlemmer HU,et.al, 1999, 474.]
Female Wistar rats (180 to 250 g ) was be randomly allocated to seven groups containing six animals each. Animals in Group I(normal control) received distilled water for the entire 21 day study period. Arthritis was be induced in animals of Group III- VII on day one by injection of 0.2 ml complete Freund’s adjuvant (It consisted of 6 mg Mycobacterium butyricum being suspended in heavy paraffin oil to give a concentration of 6 mg/ml) in sub plantar region of the left hind paw. Group III(Std),Group IV(test drug 190 mg/kg), Group V(test drug 240 mg/kg), Group VI (test drug 300mg/kg ). Paw volumes of both sides and body weight was be recorded on the day of injection, whereby paw volume was be measured by plethysmography. On day 7,14,21 the volume of the injected paw was be measured indicating the primary lesion and the influence of therapeutic agents on this phase. The severity of the induced adjuvant disease was be followed by measurement of the non injected paw (secondary lesions) with a plethysmometer. At the end of study ,on 21 days following parameters was be evaluated.
1. Body weight[Lin, H-S Hu C-Y,2007, 150, 862–872]
Body weight of each animal was be measured on the day of CFA administration, and later, on 7th, 14th and 21st days. The mean percentage reduction in body weights with respect to that on day of CFA administration was be calculated for each drug treated group and compared with that of disease control group.
2. Arthritic Index [Arthur Yin Fana, 121,2009 366–371.
On day 21, the severity of the secondary lesions was be evaluated visually and graded all to following scheme.:
|
ORGANS |
INDICATIONS |
SCORING |
|
EARS |
Absence of nodules& redness |
0 |
|
|
Presence of nodules& redness |
1 |
|
NOSE |
No swelling of connective tissue |
0 |
|
|
Intensive swelling of connective tissue |
1 |
|
TAIL |
Absence of nodules |
0 |
|
|
Presence of nodules |
1 |
|
FORE PAWS |
Absence of inflammation |
0 |
|
|
Inflammation of at least one jointq |
1 |
|
HIND PAWS |
Absence of inflammation |
0 |
|
|
Slight inflammation |
1 |
|
|
Moderate inflammation |
2 |
|
|
Marked inflammation |
3 |
arthritic score
An arthritic index for each animal was be calculated as the sum of these scores. The average scores for each group of drug treated animals was be compared with that of disease control animals
3. Paw edema[Magari Katsue, 2003 139, 927–934.]
Paw inflammation was be quantified based on paw swelling . The volume of the left hind paw was be measured before and after arthritis induction by a mercury displacement method using a plethysmometer for rats. Paw swelling was be expressed as % inhibition of using following formula:
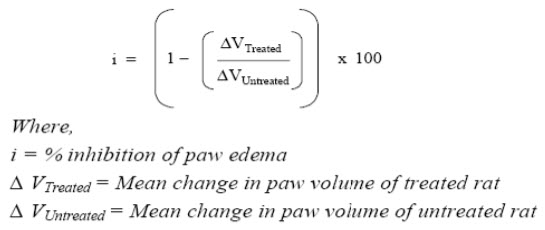
Evaluation:
a).For primary lesions:The percent inhibition of paw volume of the injected left paw over vehical control was be measured at day 7.
b).For secondary lesions:The percent inhibition of paw volume of the non-injected right paw over control was be measured at day 21.
4. Erythrocyte Sedimentation Rate ( ESR) [Mythilypriyaa Rajendran,1997,61, 1861 – 1878]
Higher erythrocyte sedimentation rate is indicator of inflammatory disorder.
Method: WESTERGREN’S METHOD
Requirements:
1)Westergren’s pipette and Westergren’s stand (2) Oxalate bulb, (3) Sodium Citrate solution
Westergren’s pipette: It has a bore of 3mm and a length of 300 mm. It is marked with graduations from 0 to 200. It is open from both the ends.
Westergren’s Stand: It can accommodate six pipettes. There is base containing six grooves each containing a rubber cushion to prevent leakage of blood from Westergren’s pipette. The stand allows the pipettes to remain in exactly vertical position.
Procedure:
1)A sample of blood (about 3 ml) was be obtained by puncturing retro-orbital plexus and was be mixed with 3.8% sodium citrate solution in proportion of four parts of blood to one part of citrate soon. The mixing of blood was be done by rotating the sample gently between the palms of hands.
2) The blood was be sucked slowly up to the mark zero in the Westergren’s tube.
3) The tube was be set upright in the Westergren’s stand, taking care that no blood escapes. The tube was be fixed with the help of screw cap.
4)At the end of one hour and two hours, the upper level of red blood cell column was be read. It was be indicator of mm of clear plasma or ESR.
5. Serum Rheumatoid Factor (RF) [Jung Hyun-Ju,2005, 359–367]
Principle Of The Method:
The RF-Turbilatex is a quantitative turbidimetric test for the measurement of RF in human serum or plasma. Latex particles coated with human gammaglobulin are agglutinated when mixed with samples containing RF. The agglutination causes an absorbance change, dependent upon the RF contents of sample that can be quantified by comparison from a calibrator of known RF concentration.
Preparations:
|
Diluent (R1) |
Tris buffer 20 mmol/L, pH 8.2 Sodium azide 0.95g/L |
|
Latex (R2) |
Latex particles coated with human gamma globulin, pH 7.4 Sodium azide 0.95g/L |
|
RF-CAL |
Calibrator. Human serum. The RF concentration is stated on the vial label. |
|
Optional |
Ref.: 1102114 Control serum ASO/CRP/RF level L Ref.: 1102115 Control serum ASO/CRP/RF Level H. |
Reagents for RF test
For RF Calibrator:
RF Calibrator was be reconstituted with 2.0 ml of distilled water. It was be mixed gently and brought to room temperature for 10 minutes before use.
For Calibration curve (range from 20 to 160 IU/ml):
The following RF calibrator dilutions was be prepared in the normal saline. The concentration of the RF calibrator was be multiplied by the corresponding factor stated in table below to obtain the RF concentration of each dilution.
|
Calibrator dilution |
1 |
2 |
3 |
4 |
5 |
6 |
|
Calibrator RF (ml) |
--- 100 |
10 90 |
25 75 |
50 50 |
75 25 |
100 --- |
|
Factor |
0 |
0.1 |
0.25 |
0.5 |
0.75 |
1.0 |
For one point calibration (linear range up to 100 IU/ml):
Preparation for RF calibrator dilution: 30ml RF calibrator + 70 ml normal saline The RF calibrator concentration was be multiplied by 0.33 to obtain the RF concentration of the diluted calibrator.
Procedure:
1. The reagents and the photometer (cuvette holder) was be brought to 37°C.
2. The following assay conditions was be described:
Wavelength: 650 nm (600-650 nm)
Temperature: 37°C
Cuvette light path: 1 cm
3. The instrument was be adjusted to zero with distilled water.
4. The following reagents was be pipette into a cuvette and mixed.
5. Absorbance was be read after 2 minutes (A2) of the sample addition.
|
|
Ablank |
A2 Calibrator/ Sample |
|
NaCl 9.0 g/l (ml) |
7.0 |
- |
|
Calibrator or sample (ml) |
- |
7.0 |
|
R1: Diluent (ml) |
0.9 |
0.9 |
|
R2: Latex (ml) |
0.1 |
0.1 |
Calculations:
For calibration curve:
Calculated the absorbance difference (A2- Ablank reagent) of each point of the calibration curve and plotted the values obtained against the RF concentration of each calibrator dilution. Rheumatoid factor concentration in the sample was be calculated by interpolation of its (A2-Ablank reagent) in the calibration curve.
For one point calibration:
[(A2-Ablank reagent) sample] / [(A2-Ablank reagent) calibrator] X Diluted calibrator concentration = IU/mL RF
The reading of RF factor of all the groups obtained was be compared with the control animals and was be expressed as IU/mL RF.
Reference Values :
Normal values up to 20 IU/mL. Each laboratory should establish its own reference range.
6. Serum C - reactive protein (CRP) [Mythilypriyaa Rajendran,1997,61, 1861 – 1878.]
Principle:
C-reactive protein is measured by turbidimetry method of analysis. Turbidimetry is the measurement of the reduction in light transmission caused by particle formation. Light transmitted in forward direction is detected. The amount of light absorbed by suspension of particles depends on specimen concentration and on the particle size. Solution requiring quantitation by turbidimetry is measured using visible spectrophotometers.
Reagents:
Reagent 1: Diluent
Tris Buffer 20 mmol/L
Sodium Azide 0.95g/L pH 8.2
Reagent 2: Latex reagent
Suspension of Latex particles coated with anti human CRP
Sodium Azide 0.95g/L
Reagent 3: CRP Calibrator
Lyophillized Human Serum
CRP concentration on label
Calibration:
The assay was be calibrated to the reference material CRM 470/RPPHS.
Reagent Preparation & Stability:
Working Reagent: Contents of the Latex Reagent vial was be gently mixed and working reagent was be prepared as,
1 ml Latex Reagent + 9 ml Diluent
This working reagent was be stable for 30 days at 2-8° C.
CRP Calibrator: Contents of vial was be reconstituted with 1.0 ml of distilled water and kept for 10 minutes before use.
This CRP calibrator was be stable for 30 days at 2-8° C or 3 months at -30° C.
Samples:
Fresh serum is preferred, though samples stored for 8 days at 2-8° C or 3 months at -20° C may also be used.
Equipments required:
Photometer,
Liquid dispensing system
General laboratory equipments.
Procedure:
Assay protocol
Wavelength : 540 nm (530-550nm)
Blank : Distilled water
Temperature : 37° C
Cuvette Path Length : 1 cm
|
Dispense |
Calibrator |
Samples |
|
Working Reagent |
500 µL |
500 µL |
|
Calibrator Sample |
3 µL |
3 µL |
Reagents for CRP test
Blank reading was be adjusted with distilled water. Working reagent and Calibrator/Sample was be pipetted out as per the pipetting system. Absorbance was be measured immediately (A1) and exactly after 2 minutes (A2) of sample addition.
Auto analyzer was be suitably programmed in fixed time mode with a nominal delay of 10 sec and a read interval of 120 sec.
Calculation:
(A2-A1) sample X Calibrator Concentration
(A2-A1) Calibrator
Reference Values
Normal values up to 6 mg/L.
Each laboratory should establish its own reference range.
7. Serum A/G ratio [Mythilypriyaa Rajendran,1997,61, 1861 – 1878.]
Estimation of Protein Contents:
Principle:
Proteins, in an alkaline medium, bind with the cupric ions present in the biuret reagent to form a blue-violet coloured complex; the intensity of the color formed is directly proportional to the amount of protein present in the sample.
Reagents:
Reagent A: Biuret reagent.
Reagent B: Protein standard.
Pipette into clean dry test tubes labeled as blank, standard and test.
|
Addition Sequence |
Blank |
Standard |
Test |
|
Biuret Reagent |
1.0 |
1.0 |
1.0 |
|
Distilled water |
0.02 |
- |
- |
|
Protein standard |
--- |
0.02 |
- |
|
Sample |
--- |
--- |
0.02 |
Mix well and incubate at 37oC for 10 min. or at room temperature for 30 min. Measure the absorbance of the standard, test sample against the blank within 60 min at 550nm.
Calculation:
Total proteins in gm/ dl = (Abs T/ Abs S) X 8
Normal Range: 6-8gm/dl
Determination of Serum Albumin: (Godkar. 2005)
Name of the method: Bromocresol green method.
Principle: Albumin present in serum binds specifically with bromocresol green at pH 4.1 to form green colored complex, intensity of which can be measured colorimetrically by using 640 nm.
Normal Range: 3.3-4.8 g/dL.
Preparation of reagents:
1) Albumin reagent (ready to use): It is prepared by mixing following chemicals in 900 ml distilled water.
a) Succinic acid: 8.85 gm
b) Bromocresol green: 108 mg
c) Sodium azide: 100 mg
d) Brij-35: 4.0 ml
pH of this solution is adjusted by using 1 N sodium hydroxide to 4.1. Final volume is made to one litre by using distilled water. It is stable at room temperature for one year.
2) Albumin standard (4gm/dl): Bovine albumin 4.0 gm in 100 ml of normal saline containing 0.1 gm/dl sodium azide. It is stable at 2-8°C for one year.
Procedure:
Mono step method
- Take three test tubes and labeled them as test, standard and blank.
- Add 5 ml of albumin reagent in each of the test tube.
- Then add 0.05 ml of serum, albumin standard and distilled water respectively.
- Mix thoroughly and keep at room temperature (25°C+ 5° C) for exactly 10 minutes.
- Measure the intensities of the test and standard by setting at 100% T, by using 640 nm (red filter).
Calculation:
Serum albumin g/dl = O.D. test/ O.D. Std. x 4
Determination of Globulins:
Normal range = 1.8-3.6 g/dl
A/G ratio = serum albumin (g/dl) / serum globulins (g/dl)
Normal range = 1.2:1 to 2:1
8. Histopathological analysis [chemico biologicalinteraction, [ijpd, curative effect on rat]
The proximal interphalangeal joint of the hind paw of the rats were removed and separated from the surrounding tissues and weighed. The joints fixed in 10% formalin were decalcified, sectioned and finally stained with haematoxylin and eosin to examine the histopathological changes during the experimental period in all the groups under light microscope.
Cartilage and bone destruction by pannus formation was scored ranging from 0, no change; 1, mild change (pannus invasion within cartilage); 2, moderate change (pannus invasion into cartilage/subchondral bone); 3, severe change (pannus invasion into the subchondral bone); and vascularity (0, almost no blood vessels; 1, a few blood vessels; 2, some blood vessels; 3, many blood vessels). Histopathological changes in the knee joints were scored in the femur region on 5 semiserial sections of the joint, spaced 70 μm apart. Scoring was performed on decoded slides by two observers, as described earlie
9. Radiological analysis[chemico biologicalinteraction, [ijpd, curative effect on ra]
Before sacrificing the animals, X-rays were taken at the joints of the hind paw of the animals for evaluating the bone damage. Radiographs were taken using X-ray apparatus and industrial X-ray ?lm. The X-ray apparatuswas operated at 220Vwith a 40V peak, 0.2 s exposure time, and a 60 cm tube-to-?lmdistance for anterior–posterior projection.
The following radiograph criteria were considered: These scores (destroyed or intact joint) were used as a quantal test for bone necrosis. Radiographs were carefully examined and abnormalities were graded as follows:
(i) Periosteaic reaction, 0 - 3 (none, slight, moderate, marked);
(ii) Erosions, 0 - 3 (none, few, many small, many large);
(iii) Joint space narrowing, 0 - 3 (none, minimal, moderate, marked);
(iv) Joint space destruction, 0 - 3 (none, minimal, extensive, ankylosis).
Bone destruction was scored on the patella as described previously.
RESULTS
A. Body Weight Change
The body weight of the control rats progressively increased while the gain in body weight of arthritic rat was retarded significantly. Drug treated group showed progressive increase in body weight.
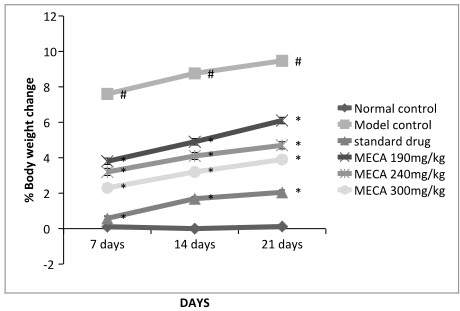
Figure: Effect of methanolic extract of Capparis aphylla Roth. on % body weight change.
Values are expressed as mean ± SEM. N=6.Values are statistically evaluated using ANOVA analysis followed by Dunnett’s test. Significant values were compared with #P<0.05 normal control Vs model control & *P<0.05 model control Vs all MECA groups
B. Arthritic Index
In this study, the incidence of inflammation was 100% on administration of freund’s adjuvant (i.e all the rats responded with an arthritic scored >1), if the rats were not treated with anti inflammatory agent. There was a difference in the severity of inflammation being less in treated group and this could be attributed to the action of standard or MECA.
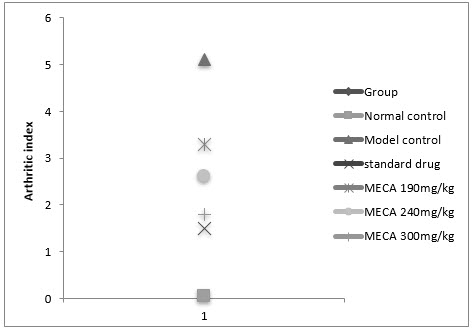
Figure :Effect of methanolic extract of Capparis aphylla Roth. on arthritic index.
Values are expressed as mean ± SEM. N=6.Values are statistically evaluated using ANOVA analysis followed by Dunnett’s test. Significant values were compared with #P<0.05 normal control Vs model control & *P<0.05 model control Vs all MECA groups
C. Paw Volume
An intraplantar injection of CFA into the left hind paw produced a more marked inflammatory response in the model control animals than the normal control(Fig 4.4.3).Seven days after CFA injection, the edema volume were significantly higher in model control animal than normal control animals
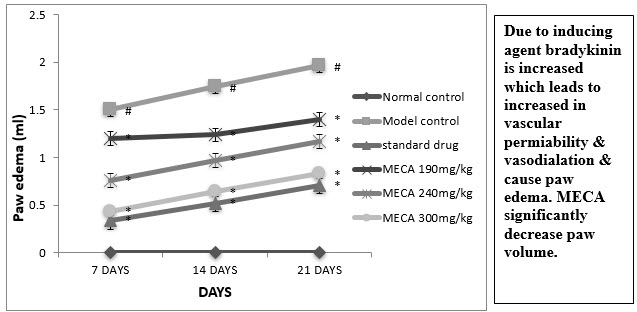
Figure : Effect of methanolic extract of Capparis aphylla Roth. on paw edema.
Values are expressed as mean ± SEM. N=6.Values are statistically evaluated using ANOVA analysis followed by Dunnett’s test. Significant values were compared with #P<0.05 normal control Vs model control & *P<0.05 model control Vs all MECA groups
D. Erytrocyte Sedimentation Rate (ESR)
The indomethacin, MECA 190 mg/kg, MECA 240 mg/kg and MECA 300 mg/kg treated groups ESR was significantly lower as compared to model control group (Fig4.4.4).
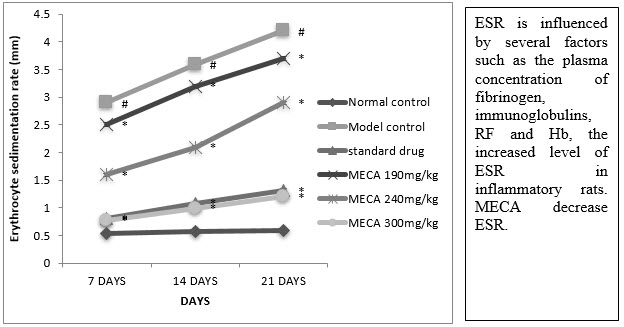
Figure :Effect of methanolic extract of Capparis aphylla Roth. on ESR.
Values are expressed as mean ± SEM. N=6.Values are statistically evaluated using ANOVA analysis followed by Dunnett’s test. Significant values were compared with #P<0.05 normal control Vs model control & *P<0.05 model control Vs all MECA groups
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
E. Serum Rheumatoid Factor (RF)
A significant reduction in RF was observed in indomethacin, MECA 190 mg/kg, MECA 240 mg/kg) and MECA 300 mg/kg treated group as compared to model control group (Fig 4.4.5).
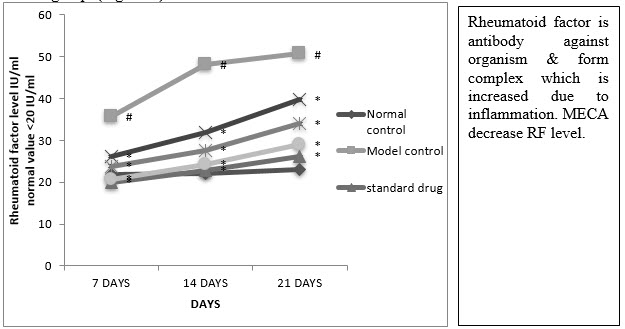
Figure :Effect of methanolic extract of Capparis aphylla Roth. on serum rheumatoid factor.
Values are expressed as mean ± SEM. N=6.Values are statistically evaluated using ANOVA analysis followed by Dunnett’s test. Significant values were compared with #P<0.05 normal control Vs model control & *P<0.05 model control Vs all MECA groups
F. Serum C-Reactive Protein (CRP)
A significant reduction in CRP was observed in indomethacin, MECA 190 mg/kg, MECA 240 mg/kg and MECA 300 mg/kg treated group as compared to model control group. (Fig4.4.6)
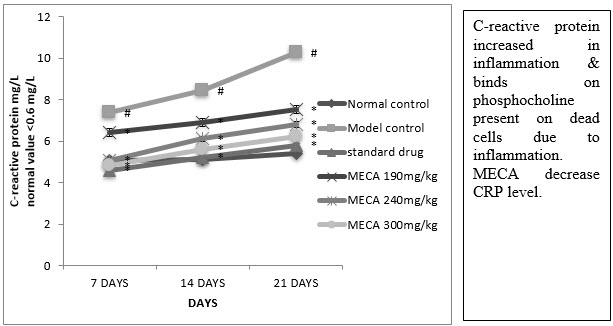
Figure : Effect of methanolic extract of Capparis aphylla Roth.on serum CRP.
Values are expressed as mean ± SEM. N=6.Values are statistically evaluated using ANOVA analysis followed by Dunnett’s test. Significant values were compared with #P<0.05 normal control Vs model control & *P<0.05 model control Vs all MECA groups
G. Serum albumin globulin ratio (A/G ratio) :
There was a significant decrease in total protein albumin level, A/G ratio but significant increase in the level of the globulin by indomethacin in CFA inflammatory rats. the above change were brought back to near normal levels by MECA 190 mg/kg, MECA 240 mg/kg, MECA 300 mg/kg. Fig (4.4.7).
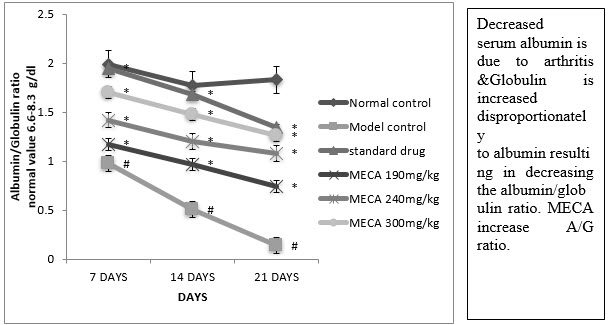
Figure :Effect of methanolic extract of Capparis aphylla Roth. on serum A/G ratio.
Values are expressed as mean ± SEM. N=6.Values are statistically evaluated using ANOVA analysis followed by Dunnett’s test. Significant values were compared with #P<0.05 normal control Vs model control & *P<0.05 model control Vs all MECA groups
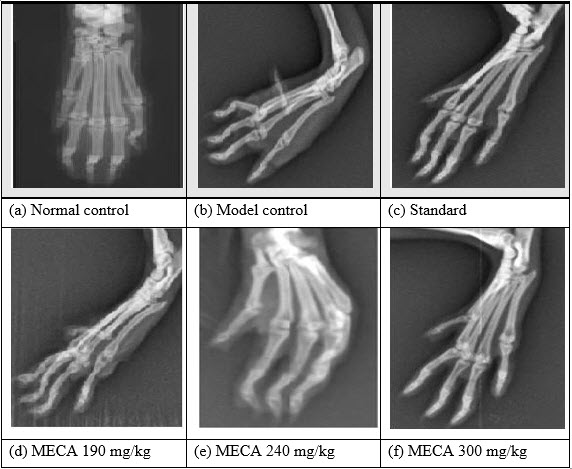
H. Effect of MECA on radiological changes of control and experimental animals [IJPD, CURATIVE EFFECT ON RA]
Bone destruction, which is a common feature of adjuvant arthritis, was examined by radiological analysis. Freund’s Complete Adjuvant treated rats had developed definite joint space narrowing of the intertarsal joints, diffuse soft tissue swelling that included the digits, diffuse demineralization of bone, marked periosteal thickening, and cystic enlargement of bone and extensive erosions produced narrowing or pseudowidening of all joint spaces and bending of phalangeal joints. In contrast, in rats treated with MECA attenuate abnormalities consisted of asymmetric soft tissue swelling and small erosions, periosteal thickening, and minimal joint space narrowing, predominantly localized to the proximal areas of the paws. The drug control rats showed the normal architecture of phalangeal joints as that of control rats.
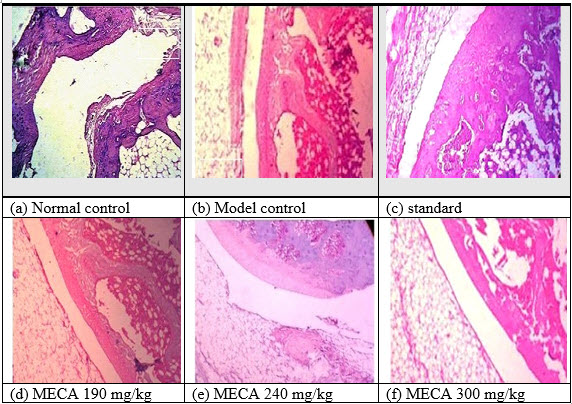
I. Effect of MECA on histopathological changes of control and experimental animals[IJPD, CURATIVE EFFECT ON RA]
As shown in Fig 5 a [NC]:Histology of synovial joint of normal control rat with intact morphology of synovium and synovial lining Fig 5 b [MC]: FA induced disease control rat showed plenty of lymphocytic infiltration [↓]in synovial lining with severe inflammation and marked angiogenesis [?] studied with proliferation of synovial cells [↓↓]Fig 5 c [SD]]: indomethacin 100mg/kg treated rats showed significant protection with mild lymphocytic infiltration [↓] with no evidence of thickening of synovial lining and angiogenesis Fig 5 d MECA 190mg/kg: MECA 190mg/kg treated rats showed milder angiogenesis [?], lymphocytic infiltration [↓] and synovial lining thickening[↓↓]. Fig 5 e MECA 240 mg/kg: MECA 240 mg/kg treated rats showed milder angiogenesis [?], synovial lining thickening [↓↓] with no evidence of lymphocytic infiltration. Fig 5 f MECA 300mg/kg: MECA 300mg/kg treated rats sho
DISCUSSION
Rheumatoid Arthritis is an autoimmune disorder, the immunologically mediated complete Freund’s adjuvant induced arthritic model of chronic inflammation is considered as the best available experimental model of RA. [IJPD, CURATIVE EFFECT ON RA]
Complete Freund’s adjuvant-induced arthritis is a model of chronic polyarthritis with features that resemble RA. Therapeutic efficiency of herbal drug like Glycerhhiza glabra & moschus moschipus were mainly investigated in the rat adjuvant arthritis model. Evaluation of the inflammatory stratus in RA is reflected inflammation in the hind paw. Progression of disease in MECA treated group shows reduction in edema in dose dependent manner as compare to tissues control animals. Symmetric involvement of small hand joints (especially proximal interphalangeal and metacarpophalangeal), foot joints (metarsophalangeal), wrists, elbows, and ankles is typical, but initial manifestations may occur in any joint. Inflammation and / or nodules are observed on ears, nose, and tail, fore paws and hind paws. Arthritic index is the average of the score given to severity of the lesions in these places. This gives full picture of the disease. MECA treated animal showed significant lesser arthritic index as compared with disease control animals. Prominent immunological abnormalities that may be important in pathogenesis of RA include immune complexes are found in joint fluid cells and in vasculitis. Plasma cells produce antibodies e.g., rheumatoid factor (RF) that contribute to these complexes. Serum rheumatoid factor (RF) is the immunological expression of an individual's immune system reaction to the presence of an immunoglobulin molecule that is recognized as "non-self." This response to the "non-self" immunoglobulin results in the presence of immune complexes. These, in turn, bind complement and may eventually lead to synovium, cartilage, and bone destruction. Higher the levels of serum rheumatoid factor, higher are the development of inflammation. Serum rheumatoid factor (RF) measures the amount of antibody IgM titer present in the serum. MECA treated animal showed significantly lesser serum RF when compared to disease control animals. [IJPD, CURATIVE EFFECT ON RA]
Bone destruction, which is a common feature of adjuvant arthritis, was examined by radiological analysis. Adjuvant treated rats had developed definite joint space narrowing of the intertarsal joints, diffuse soft tissue swelling that included the digits, diffuse demineralization of bone, marked periosteal thickening, and cystic enlargement of bone and extensive erosions produced narrowing or pseudowidening of all joint spaces. Despite a similar clinical course of arthritis, disease control rats suffered from more pronounced bone destruction than MECA treated group. [IJPD, CURATIVE EFFECT ON RA]
RA is characterized by synovial tissue leukocyte ingress and angiogenesis. The disease is thought to occur as an immunological response to as yet unidentified antigen. Even in early RA, some of the earliest histological observations are blood vessels. A mononuclear infiltrate characterizes the synovial tissue along with a luxuriant vasculature. Angiogenesis is integral to formation of the inflammatory pannus and without angiogenesis, leukocyte ingress could not occur. Changes in the density of blood vessels in the synovium and alterations in endothelial proliferative responses in RA have been shown in a range of studies. The number of synovial blood vessels has been found to correlate with hyperplasia of synovial cells, infiltration of mononuclear cells, and indices of joint tenderness. Histopathology study of synovial joint showed that treatment with MECA Group decreased vascularity, lymphocytic infiltration with less rheumatoid inflammation and angiogenesis, with no thickening of synovial membrane and absence of lymphoid follicles. As compared to disease control and orally treated animals. [IJPD, CURATIVE EFFECT ON RA]
The value of the acute phase reactant CRP as an indicator of RA disease activity and progression is well recognize. The increased level of CRP in arthritic rats supports the earlier reports demonstrating an abnormal elevation of plasma CRP in AIA rats and in RA patients that might be primarily due to the increased activation of proinflammatory cytokines such as IL-1 and TNF- in combination with IL-6 and moreover, the promoter genes for CRP are highly sensitive and greatly potentiate their induction. The increased level of CRP in turn activates the vascular epithelium thereby accelerating the acute phase response in the blood. Besides that, the increased CRP level might enhance the monocyte activation in arthritic rats, since binding sites for CRP have been identified in monocytes and CRP also increases the mRNA expression of IL-1 and TNF-by human macrophages, thereby augmenting the inflammatory response. The interpretation in turn supports the present findings showing increased level of these cytokines in arthritic conditions. [CHEMICO BIOLOGICALINTERACTION]
As a result, it might be proposed that the presence of high concentration of circulating APP reflect the degree of cytokine stimulation, the intensity of inflammation, as this high concentration of APP was associated with a more severe progressive course of the disease, characterized by intense bone resorption . [CHEMICO BIOLOGICALINTERACTION]
The X-ray appearance of RA joints commonly referred to as diminished joint space is the hallmark of arthritis. In AIA rats, erosion representing bony destruction was evident on bone unprotected by cartilage, since they are exposed directly to cytokines such as TNF-and IL-1,which stimulate the chondrocytes to produce proteolytic enzymes such as collagenases, glycohydrolases and neutral proteases degrading the cartilage. As a result, the pannus invades the joint and subchondral bones and eventually the joint is destroyed and undergoes fibrous fusion or ankylosis. These changes were reverted back to near normal upon KA treatment than the sole SA treatment. This is further supported by histopathological and electron microscopic changes in control and experimental rats. [CHEMICO BIOLOGICALINTERACTION]
Our data suggested that MECA possesses significant antiarthritic activity. The possible mode of anti- arthritic activity of Methanolic extract of MECA appears to be, Possessing anti–inflammatory activity showed in arthritic parameters like Paw edema, Arthritic index, CRP, body weight, serum A/G ratio, Rheumatoid factor, improving bone erosion. [IJPD, CURATIVE EFFECT ON RA]
CONCLUSION
In the light of the above results it might me concluded that the drug MECA exhibited a potent antiarthritic effect by reducing the pathological lesions via down regulating the levels of proinflammatory cytokines thereby reducing the levels of acute phase proteins and also via its enhanced immunomodulatory property. The improved effect of MECA might be attributed to the combined interactions of the spermidine alkaloids like caparicine.
REFERENCES
1. Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE,,"Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation", February 2007, 112-115.
2. Abbas A.B., Lichtman A.H.,"Ch.2 Innate Immunity,” Basic Immunology. Functions and disorders of the immune system, (2009), 978-1-416-4688-2.
3. Lees M, Taylor DJ and Woolley DE, “Mast cell proteinases activate precursor forms of collagenase and stromelysin, but not of gelatinases A and B”, Eur J Biochem, (1994), 223, 171 – 177.
4. Lee DM, Weinblatt ME, “Rheumatoid Arthritis“,Lancet, 2001, 358, 903–911.
5. Kobayashi Y, Shirahase H, Zhao H-F, Kakizoe E and Okunishi H, “Effects of orally available prodrug of cromoglycic acid on collagen-induced arthritis mice”, Folia Pharmacol Jpn,, 1999,114, 154-158.
6. ACR, “American College of Rheumatology subcommittee on rheumatoid arthritis guidelines”; Guidelines for the management of rheumatoid arthritis, 2002 Update, 46, 328–346.
7. Bansback NJ, Regier DA, Ara R, Brennan A, Shojania K, Esdaile JM et al., “An overview of economic evaluations for drugs used in rheumatoid arthritis: focus on tumour necrosis factor-alpha antagonists”, Drugs ,2005, 65,473–496.
8. Muller-Ladner U, Gay RE and Gay S, “Structure and function of synoviocytes”, In Arthritis and Allied Conditions, 13th edn,, 1997, pp 243 – 254. ,
9. Molly RG, Mannick JA, Rodrick ML.,”Cytokines, sepsis and immunomodulation.’, Br J Surg, 1993, 80,289-97.
10. Klinkhoff A, “Biological agents for rheumatoid arthritis”, Drugs, 2004, 64, 1267–1283.
11. Weinblatt ME, “Treatment of rheumatoid arthritis. In Arthritis and Allied Conditions”, 13th edition, Edited by Koopman WJ, 1997, pp 1131 – 1141.
12. Mottonen T, Hannonen P, Leirisalo-Repo M, Nissila M, Kautiainen H, Korpela M., “Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis”, a randomized trial, Lancet 1999, 353, 1568–73.
13. Venalis A, Dadoniene J., “Treatment of severe rheumatoid arthritis combining methotrexate, azathioprine and sulphasalazine with antimalar drugs”, Acta medica Lituanica, 1996, 2, 57–62.
14. Pincus T, Breedveld FC and Emery P, “Does partial control of inflammation prevent long-term joint damage? Clinical rationale for combination therapy with multiple disease-modifying antirheumatic drugs”, Clin Exp Rheumatol, 17, Suppl 18, 1999, S2 – S7.
15. Tugwell P, Welch V, Suarez-Almazor M, Shea B, Wells G., “Efficacy and toxicity of old and new disease modifying antirheumatic drugs”, Ann Rheum Dis, 2000, 32–5.
16. Kammer GM., “The adenylate cyclase-cAMP-protein kinase A pathway and regulation of the immune response” Immunol Today 1988, 9, 222-9.
17. Sever A, Rapson NT, Hunter C A, Liew FY., “Regulation of tumor necrosis factor production by adrenaline and ,3-adrenergic agonists”, J Immunol 1992, 148:3441-5.
18. Dobbins DE, Soika CY, Premen AI et al., “Blockade of histamine and bradykinin-induced increases in lymph flow, protein concentration, and protein transport by terbutaline in vivo”, Microcirc Res, 1982, 2, 127-50.
19. Noel C, Copin M, Hazzan M et al., “Immunomodulatory effect of pentoxiphylline during human allograft rejection”, Transplanation, 2000, 69(6), 1102-7.
20. Neuner P, Klosner G, Schauer E et al., ”Pentoxiphyline in vivo down regulates the release of IL-1β, IL-6, IL-8 and tumornecrosis factor α by human peripheral blood mononuclear cells”, Immunology, 1994, 83,262.
21. Kunkel SL, Wiggins RC, Chensue SW, Larrick J., “Regulation of macrophage tumor necrosis factor production by prostaglandin E2”, Biochem Biophys Res Commun, 1986, 137,404-10.
22. N. Ottah and R. Little, et al., “Biochemical characterization of recombinant fusions of lipopolysaccharide binding protein and bactericidal/permeability-increasing protein”, Implications in biological activity, J Biol Chem,, 1997, pp. 2149–2155.
23. Devi renuka P, kumari krishna S and kokilavani C., “Effect of vitex negundo leaf extract on the free radicals scavengers in complete freund’s adjuvant induced arthritic rats”, Indian journal of clinical biochemistry, 2007 ,143-147
24. Jaijesh P, srinivasan Kk, bhagath kumar P, sreejith G, ciraj Am., “Anti-arthritic property of the plant rubia cordifolia lin.”, Pharmacologyonline,, 2008,1, 107-113
25. Jung Hyun-Ju, Jung Hwan Nama, Jongwon Choib, Kyung-Tae Leec, Hee-Juhn Parka,, “Antiinflammatory effects of chiisanoside and chiisanogenin obtained from the leaves of Acanthopanax chiisanensis in the carrageenan- and Freund’s complete adjuvant-induced rats”, Journal of Ethnopharmacology, 97, 2005 359–367.
26. Kirtikar K.R., Basu B.D., An I.C.S., “Indian Medicinal plants”, Basu, India, 1935, 2nd Ed. p. 700.
27. Kawahito Y, Cannon G, Gulko P et al., “Localization of quantitative trait loci reagulating adjuvant ionduced arthritis in rats: evidence for genetic factors common to multiple auto immune disease”, J Immunol,1998, 161,4411-9.
28. Dinarello C, Moldaver L., “Proinflamatory and antiinflamatory cytokines in rheumatoid arthritis”, Amgen Inc, 2000, 137-138..
29. Corral L, Kaplan G., “Immunomodulation by thalidomide and thalidomide analogues”, Ann Rheum Dis, 1999, 10,1107-13.
30. Biomedical science, “The Basics of acute inflammation”, 2001, 29.
31. Cotran; Kumar, Collins,Robbins, “Pathologic Basis of Disease”, Philadelphia W.B Saunders Company, 1998, 265.
32. Parakrama Chandrasoma, Clive R. T.,”Arthritis”, 2005, 347.
33. Porth, Carol, Hagerstown, MD, “Essentials of pahtophysiology: concepts of altered health states”, Lippincott Williams & Wilkins, 2007, pp. 270.
34. Tortora GJ, Derrickson B., “Principles of anatomy and physiology”, Joint: anatomy, 11th ed., Wiley, 2007,p.258-70.
35. Module JM., “Introduction to Joints”, Retrieved ,2008-01-29.
36. Robbins and cotran, “pathology basis of disease”, vinay kumar, abul.k.abbas, Nelso fausto In: bones, joints and soft tissue tumors. Edition 7th. Elsevier, 2007, P.no:1303-1315
37. Harfindal ET, Helms RA, Gaurley DR, Quan DJ, “Rheumatoid arthritis. Text book of therapeutics drug and disease management”, lippin cott Williams and wilkins., 2006, p.no:1705-1736.
38. Dipiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM,, “Pharmacotherapy and Pathophysiologic approach,In: rheumatoid arthritis”,Edition:5th.appleton and lange., 1999, Page no:1623.
39. Harrison’, “principles of internal medicine”, Eugene Braunwald, Stephen L.Hauser,Anthony S.Fauci,Dan LLongo, J. Larry.joneson In: rheumatoid arthritis. Volume, 2, 15th edition., Mc-Graw-Hill., 2003. p.no:1928-1936.
40. Bautista BB, Boyce E, Koronkowaski M,et al.,”Effects of second line drugs on progression or regression of rhematouid nodules”,J Clin Rheum,, 1995, 1(4),212-218.
41. Carmona L, González-Alvaro I, Balsa A, Angel Belmonte M, Tena X, Sanmartí R, "Rheumatoid arthritis in Spain: occurrence of extra-articular manifestations and estimates of disease severity". Ann. Rheum, Sep, 2003, Dis. 62 (9), 897–900.
42. Mor A, Abramson SB, Pillinger MH., “The fibroblast-like synovial cell in rheumatoid arthritis: a key player in inflammation and joint destruction”, Clin Immunol,. 2005,115:118-28.
43. Silvermann G, Weismans S, “Rutuximab therapy and autoimmune disorders: prospects for antiβ cell therapy”, Arthritis Rheum, 2003, 48: 1484-1492.
44. Dörner T, "Crossroads of B cell activation in autoimmunity: Rationale of targeting B cells," J Rheumatol, 2006, 33:3-11.
45. Baecklund E.et al., "Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis", Arthritis Rheum , 2006,54,692-701.
46. Burmester GR, Stuhlmuller B, Keyzer G, Kinne RW., ”Mononuclear phagocytes and rheumatoid synovitis: mastermind of workhorse in arthritis?”, Arthritis Rheum, 1997, 40, 5-18.
47. Dinarello C, Moldaver L., “Proinflamatory and antiinflamatory cytokines in rheumatoid arthritis”, Amgen Inc, 2000,175-181.
48. Ellingsen T, Buus A, Stemgaard-Pedersen K., “Plasma monocyte chemoattractant protein-1 is a marker for joint inflammation in rheumatoid arthritis”, J Rheumatol, 2001, 28, 41-46.
49. Dinarello C, Moldaver L., “Proinflamatory and antiinflamatory cytokines in rheumatoid arthritis”, Amgen Inc, 2000, 56.
50. Van Snick J, “Interleukin-6 an overview“,Annu Rev Immunol, 1990, 8,253-278.
51. Romano MM, Sironi C, Toniatti N, Polentarutti N, Fruscella P, Ghezzi R, Faggioni W, Luini V, van Hinsbergh S, Sozzani S, et al., “Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment”, Immunity, 1997, 6,315-325.
52. Mihara M, Moriya Y, Kishimoto T, Ohsugi Y, “Interleukin-6 (IL-6) induces the proliferation of synovial fibroblastic cells in the presence of soluble IL-6 receptor”, Br J Rheumatol, 1995, 34, 321-325.
53. Tamura T, Udagawa N, Takahashi N, Miyaura C, Tanaka S, Yamada Y, Koishihara Y, Ohsugi Y, Kumaki K, Taga T, “Soluble interleukin-6 receptor triggers osteoclast formation by interleukin6”, Proc Natl Acad Sci USA, 1993, 90:11924-11928.
54. Chai Z, Gatti S, Toniatti C, Poli V, Bartfai T, “IL-6 gene expression in the CNS is necessary for fever response to LPS or IL-1β: a study on IL-6-deficient mice”, J Exp Med, 1996, 183, 311-316.
55. Streetz KL, Wustefeld T, Klein C, Manns MP, Trautwein C, “Mediators of inflammation and acute phase response in the liver”, Cell Mol Biol, 2001, 47,661-673.
56. Endo H, Akahoshi T, Takagishi K, Kashiwazaki S, Matsushima K., “Elevation interleukin-8 (IL-8) levels in joint fluids of patients with rheumatoid arthritis and the induction by IL-8 of leukocyte infiltration and synovitis in rabbit joints”, Lymphokine Cytokine Res, 1991, 10, 245.
57. Akahoshi T, Wada C, Endo H, Hirota K, Hosaka S, Takagishi K., “Expression of monocyte chemotactic and activating factor in rheumatoid arthritis”, Arthritis Rheum, 1993, 36,762.
58. Hayashida K, Nanki T, Girschick H, Yavuz S, Ochi T, Lypski P., “Synovial stromal cells from rheumatoid arthritis patients attract monocytes by producing MCP-1 and IL-8”, Arthrtitis Res, 2001, 3(2), 118-126.
59. Koch AE., “Angiogenesis: implications for rheumatoid arthritis”, Arthritis Rheum, 1998, 41, 951.
60. Ushio, S., et al., “Cloning of the cDNA for human IFNg-inducin factor, expression in Escherichia coli, and studies on the biologic activities of the protein”,J. Immunol,. 1996 , 156,4274–4279.
61. Gu, Y., et al., “Activation of interferon-gamma inducing factor mediated by interleukin-1b converting enzyme”, Science, 1997, 275,206–209.
62. Okamura, H., Kashiwamura, S., Tsutsui, H., Yoshimoto, T., and Nakanishi, K., “Regulation of interferon-g production by IL-12 and IL-18”, Curr. Opin. Immunol, 1998, 10,259–264.
63. Takeda, K., et al.,”Defective NK cell activity and Th1 response in IL- 18-deficient mice”, Immunity, 1998,. 8,383–390.
64. McInnes IB, al-Mughales J, Field M, Leung BP, Huang FP, Dixon R, Sturrock RD, Wilkinson PC, Liew FY, “The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis”, Nat Med, 1996, 2,175-182.
65. Thurkow EW, van der Heijden IM, Breedveld FC, Smeets TJ, Daha MR, Kluin PM, Meinders AE, Tak PP, “Increased expression of IL- 15 in the synovium of patients with rheumatoid arthritis compared with patients with Yersinia-induced arthritis and osteoarthritis”, J Pathol, 1997, 181,444-450.
66. McInnes IB, Leung BP, Sturrock RD, Field M, Liew FY, “Interleukin- 15 mediates T cell-dependent regulation of tumor necrosis factor-a production in rheumatoid arthritis”, Nat Med, 1997, 3,189-195.
67. Miranda-Carus ME, Balsa A, Benito-Miguel M, Perez de Ayala C, Martin-Mola E, “IL 15 and the initiation of cell contact-dependent synovial fibroblast-T lymphocyte cross-talk in rheumatoid arthritis effect of methotrexate”, J Immunol,2004, 173,1463-1476.
68. Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY, “TLR2 is expressed on activated T cells as a costimulatory receptor”, Proc Natl Acad Sci USA, 2004, 101,3029-3034.
69. Arnason JA and Malone DG, “Role of mast cells in arthritis”, Chem Immunol, 1995, 62, 204 – 238
70. Marone G, “Mast cells in rheumatic disorders: mastermind or workhorse?,” Clin Exp Rheumatol, 1998,16, 245 – 249.
71. Yoffe JR, Taylor DJ and Woolley DE, “Mast-cell products and heparin stimulate the production of mononuclear-cell factor by cultured human monocyte/ macrophages”, Biochem J,1985, 230, 83 – 88.
72. Tetlow LC and Wooley DE, “Mast cells, cytokines, and metalloproteinases at the rheumatoid lesion: dual immunolocalisation studies”, Ann Rheum Dis, 1995, 896 – 903.
73. Schwartz LB, “Mast cells: function and contents”, Curr Opin Immunol, 1994 6, 91 – 97.
74. Yuta Kobayashi and Hideki Okunishi Department of Pharmacology, Shimane Medical University,”Rheumatism”, Izumo 693-8501, Japan Jpn. J. Pharmacol, 2002, 90, 7 – 11.
75. Delves P, Roitt I (eds)., “Encyclopedia of Immunology”, 2nd Ed. San Diego, 1998, 56. .
76. Zucker S, Lysik RM, Zarrabi MH, Greenwald RA, Gruber B, Tickle SP, Baker Ts, Docherty AJ., “Elevated plasma stromelysin level in arthritis”, J Rheumatol, 1994,21, 2329-33.
77. Sarau HM, Ames RS, Chambers J, Ellis C, Elshourbagy N, Foley JJ, Schmidt DB, Muccitelli RM, Jenkins O, Murdock Pr, Herrity NC, Halsey W, Sathe G, Mair AI, Nuthulaganti P, Dytko GM, Buckley PT, Wilson S, Bergsma DJ, Hay DW, “Identification, molecular cloning, expression and characterization of cysteinyl leukotriene receptor”, Mol Pharmacol ,1999,56,657-63.
78. Tripathi KD, “Essential of medical pharmacology.In:anti rheumatic drugs”, Edition 6th, , jaypee brother, 2008,.p.no 202-212.
79. Hansson GK, Edfeldt K, "Toll to be paid at the gateway to the vessel wall", Arterioscler. Thromb. Vasc. Biol.,2005,25 (6), 1085–7.
80. Khare C.P., “Indian medicinal plants”, renewed in 2007, 117-118.
81. Sushila Rathee Permender RatheeDharmender Rathee Deepti Rathe Vikash Kumar, “Phytochemical and pharmacological Potential of Kair (Capparis Decidua)”, 2010, 14-16.
82. Usman g.k., “Phytochemical study on capparis deciduas” ,1980, 6-68.
83. Manavalan r., “Akbarsha m.a. effects of ethanolic extrat of capparis aphylla roth. on testicular steroidogenesis in rats “,pharmacol, 2007 , pg no 1.
84. Pdavid M., MargarnetH., “Nitric oxide a key mediator in the early and late phase of carrageenan induced rat paw inflammation”,pharmacol, 1996, 829-838.
85. Schorlemmer HU, Kurrle R, Schleyerbach R, Bartlett RR., “Disease-modifying activity of malononinitrilamides, derivates of leflunomide’s active metabolite, on models of rheumatoid arthritis”,rheumatol, 1999, 474.
86. Lin, H-S Hu C-Y, Chan H-Y , Liew Y-Y, Huang H-P, Lepescheux, E Bastianelli, R Baron1, G Rawadi And P Cle´Ment-Lacroix, “Anti-Rheumatic Activities Of Histone Deacetylase (Hdac) Inhibitors In Vivo In Collagen-Induced Arthritis In Rodents”, British Journal Of Pharmacology, 2007, 150, 862–872.
87. Zhanga Rui-Xin, Arthur Yin Fana, An-Nan Zhoua, Kamal D. Moudgilb, Zhong-Ze Mac, David Yue-Wei Leec, Harry H.S. Fongd, Brian M. Bermana, Lixing Laoa,, “Extract of the Chinese herbal formula Huo Luo Xiao Ling Dan inhibited adjuvant arthritis in rats”, Journal of Ethnopharmacology 121,2009 366–371.
88. Magari Katsue, Miyata Susumu, Ohkubo Yoshitaka, Seitaro Mutoh & Toshio Goto, “Calcineurin inhibitors exert rapid reduction of inflammatory pain in rat adjuvant-induced arthritis”, British Journal of Pharmacology, 2003 139, 927–934.
89. Mythilypriyaa Rajendran, Shanthib Palanivelu, Sachdanandama Panchanadam, “Ameliorating effect of Kalpaamruthaa, a Siddha preparation in adjuvant induced”, Life Sci, 1997,61, 1861 – 1878.
90. Jung Hyun-Ju, Jung Hwan Nama, Jongwon Choib, Kyung-Tae Leec, Hee-Juhn Parka, “Antiinflammatory effects of chiisanoside and chiisanogenin obtained from the leaves of Acanthopanax chiisanensis in the carrageenan- and Freund’s complete adjuvant-induced rats”, Journal of Ethnopharmacology 97,2005, 359–367.
91. Waltraud Binder & Judith S. Walker, “Effect of the peripherally adjuvant arthritis”, 1998, 647-654.
92. Fleischmann, R., Stern, R., Iqbal, I.,. „Anakinra: an inhibitor of IL-1 for the treatment of rheumatoid arthritis. Expert Opinion on Biological Therapy”, 2004,1333–1344.
93. Chandrashekara, S., Anilkumar, T., Jamuna, S., “Complementary and alternative drug therapy in arthritis”, Journal of the Association of Physicians of India 50, 2002, 225–227.
94. Katschke KJ, Rottman JB, Ruth JH, et al., “Differential expression of chemokine receptors on peripheral blood, synovial fluid and synovial tissue mnocytes/macrophages in rheumatoid arthritis”, Arthritis Rheum,, 2001, 44, 1022-1032.
95. Dolhain, R.J.E.M., Heiden, A.N., Haar, N.T., Breedveld, F.C., and Miltenburg, A.M.M., “Shift towards T lymphocytes with a T helper 1 Cytokine secretion profile in the joints of patients with rheumato rthritis.”, Arthritis Rheum., 1996, 39,1961–1969.
96. Miltenburg, A.M.M., van Laar, J.M, De Kuiper, R., Daha, M.R., and Breedveld, F.C., “T cells cloned from human rheumatoid synovial membrane functionally represent the Th1 subset”, Scand. J. Immuno,1992, 35,603–610.
97. Taurog, J.D., Argentieri, D.C., McReynolds, R.A., “Adjuvant arthritis”, Methods in Enzymology,1988, 162, 339–355.
98. Winder CV, Lembke LA, Stephens MD., ”Comparative bioassay of drugs in adjuvant induced arthritis in rats: flufenamic acid, mefenamic and phenylbutazone”, Arth Rheum, 1969,12,472-81.
99. Somasundaram S, Sadique J, Subramaniam A., “In vitro absorption of 14C leucine during inflammation and the effect of anti-inflammatory drugs in the jejunum of rats”, Biochem Med, 1983,29, 259-64.
100. Hazeena Begum V, Sadique J., “Long term effect of herbal drug Withania Somniferaon adjuvant induced arthritis in rats”, Ind J Exp Biol, 1988, 26, 877-82.
101. Marcelletti J.F., Nakamura R.M., “Assessment of serological markers associated with rheumatoid arthritis. Diagnostic autoantibodies and conventional disease activity markers”, Clin. Appl. Immunol.Rev. 4, 2003, 109–123.
102. Skogh T, Gustafsson D, Kjellberg M, Husberg M, “Twenty eight joint count disease activity score in recent onset rheumatoid arthritis using C reactive protein instead of erythrocyte sedimentation rate,”,Ann. Rheum. Dis. 62, 2003, 681–682.
103. Kim, J.C., Kim, J.K.,. „Summary of Clinical Test”, Kommunsa Publishing Co., Seoul, pp. 242, 298, 303, 1112, 1149.
104. Shmerling RA, Delbanco TL., “The rheumatoid factor: an analysis of clinical utility”, Am J Med, 1991, 91, 528–34.
105. Scott D.L, “Prognostic factors in early rheumatoid arthritis”, Rheumatology 39, 2000, 24–29.
106. McConkey B, Crockson RA, Crockson AP, Nilkinson AR., “The effect of some anti inflammatory drugs on the acute-phase proteins in rheumatoid arthritis”, Q J Med, 1973,32,785-791.
107. Stecher V.J, Connolly K.M, “The relationship between interleukin-1 activity in adjuvant rats and changes in plasma fibronectin, C-reactive protein and albumin levels”, Fed. Proc. 46, 1987, 790.
108. Wong P.K., Campbell I.K., Egan P.J., Ernst M., Wicks I.P., “The role of the interleukin-6 family of cytokines in inflammatory arthritis and bone turnover”, Arthritis Rheum., 48, 2003, 1177–1189.
109. Mortensen R.F., “Macrophages and acute-phase proteins”, Immunol, Ser. 60, 1994, 143–158.
110. Tebo J.M. Mortensen R.F, “Characterization and isolation of a C-reactive protein receptor from the human monocytic cell line”, J. Immunol. 144 ,1990, 231–238.
111. Galve-de Rochemonteix B, Wiktorowicz K, Kushner I, Dayer J.M, “C-reactive protein increases production of IL-1 alpha, IL-1 beta, and TNF-alpha, and expression of mRNA by human alveolar macrophages”, J. Leukoc. Biol. 53, 1993, 439–445.
112. Cawthorne M.A., Palmer E.D., Green J., “Adjuvant-induced arthritis and drug-metabolizing enzymes”, Biochem. Pharmacol. 25, 1976, 2683–2688.
113. Lorber A., Leeb J., Carroll P, Baumgartner W., Hill V., “Protein sulphydryl depression during adjuvant arthritis”, Ann. Rheum. Dis. 34, 1975, 346–349.
114. Lewis E.J., Bishop J., Aspinall S.J., “A simple inflammation model that distinguishes between the actions of anti-inflammatory and antirheumatic drugs”, Inflamm. Res. 47, 1998, 26–35.
115. Lowe J.S., “Serum protein changes in rats with arthritis induced by mycobacterial adjuvant”, Biochem. Pharmacol. 13, 1964, 633–641.
116. Kohn A, Barchet E., “Correlation between albumin permeability and adenosine cyclic monophosphate levels in incubated rat mesentry tissue”, C. R., Seances Soc. Biol. Fil. 170, 1976, 270–330.
117. Fahim A.T., Abd-EI Fattah A.A., Agha A.M., Gad M.Z, “Effect of pumpkin seed oil on the level of free radical scavengers induced during adjuvant-arthritis in rats”, Pharmacol. Res. 31, 1995, 73–79.
118. Sakai A, Hirano T, Okazaki R, Okimoto N, Tanaka K., Nakamura T, “Large-dose ascorbic acid administration suppresses the development of arthritis in adjuvant-infected rats”, Arch. Orthop. Trauma Surg. 119, 1999, 121–126.
119. Baecklund E et al., "Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis," Arthritis Rheum , 2006,54,692-701.
120. Bertram G. Katzung,, “Basic and clinical pharmacology In: antirheumatic drugs.” Edition: 7th, Appleton and lange, 1998.P.no:578-592.
121. Bridges AJ, Malone DG, Jicinsky J, Chen M, Ory P, Engber W and Graziano FM, “ Human synovial mast cell involvement in rheumatoid arthritis and osteoarthritis. Relationship to disease type, clinical activity, and antirheumatic therapy”, Arthritis Rheum 34, 1991,1116 – 1124.
122. Bromley M and Woolley DE, “Histopathology of the rheumatoid lesion. Identification of cell types at sites of cartilage erosion”, Arthritis Rheum 27, 1984, 857 – 863.
123. Cannella AC, O’Dell JR., “Is there still a role for traditional disease-modifying antirheumatic drugs (DMARDs) in rheumatoid arthritis?”, Curr Opin Rheumatol, 2003, 15(3), 185–92.
124. Catrina AI, Trollmo C, af Klint E, Engstrom M, Lampa J, Hermansson Y, Klareskog L, Ulfgren AK, “Evidence that anti-tumor necrosis factor therapy with both etanercept and infliximab induces apoptosis in macrophages, but not lymphocytes, in rheumatoid arthritis joints: extended report”, Arthritis Rheum,, 2005, 52,61-7.
125. Chatham WW, Edberg JC, Kimberley RP., “The role of neutrophils in rheumatoid arthritis: new frontiers in pathogenesis and treatment”, New York, Oxford University Press, Inc, 2000, 101- 112.
126. Cho ML, Yoon CH, Hwang SY, Park MK, Min SY, Lee SH, Park SH, Kim HY, “Effector function of type II collagen-stimulated T cells from rheumatoid arthritis patients: cross-talk between T cells and synovial fibroblasts”, Arthritis Rheum, 2004, 50,776-784.
127. Durai, M., Kim, H.R., Moudgil, K.D., “The regulatory C-terminal determinants within mycobacterial heat shock protein 65 are cryptic and cross-reactive with the dominant self homologs: implications for the pathogenesis of autoimmune arthritis”, Journal of Immunology 173, 2004,181–188.
128. Ellingsen T, Buus A, Stemgaard-Pedersen K.,“Plasma monocyte chemoattractant protein-1 is a marker for joint inflammation in rheumatoid arthritis”, J Rheumatol, 2001, 28, 41-46.
129. Feldmann M, “Pathogenesis of arthritis: recent research progress”, Nat Immunol, 2001, 2,771-773.
130. Feldmann, F., Brennan, F.M., and Maini, R.N.. “Role of cytokines in rheumatoid arthritis”, Ann. Rev. Immunol,. 1996, 14,397–440.
131. Ferrari-Lacraz S, Zanelli E, Neuberg M, Donskoy E, Kim YS, Zheng XX, Hancock WW, Maslinski W, Li XC, Strom TB, Moll T, “Targeting IL-15 receptor-bearing cells with an antagonist mutant IL- 15/Fc protein prevents disease development and progression in murine collagen-induced arthritis”, J Immunol, 2004,173,5818-5826.
132. Firestein GS., “Evolving concepts of rheumatoid arthritis”, Nature 423, 2003, 356–361.
133. Fox DA., “The role of T cells in the immunopathogenesis of rheumatoid arthritis: new perspectives”, Arthritis Rheum, 1997, 40, 598-609.
134. Gately, M.K., et al., “The interleukin-12/interleukin-12 receptor system”, Ann. Rev. Immunol.. 1998, 16,495–521.
135. Gay S, Gay RE, Koopman WJ, “Molecular and cellular mechanisms of joint destruction in rheumatoid arthritis: two cellular mechanisms explain joint destruction?”, Ann Rheum Dis, 1993,S39-S47.
136. Gordon JR, Burd PR and Galli SJ, “Mast cells as a source of multifunctional cytokines”, Immunol Today 11, 1990,458 – 464.
137. Hart PH, Whitty GA, Piccoli DS, Hamilton JA., “Control by IFNgamma and PGE2 and TNFa and IL-i production by human monocytes”, Immunol, 1989, 66,376-83.
138. Jaspers, J.P.M.M., “Van oers, R.J.M. et al Leekers, B.Nine rheumatoid Factor Assays compared”, .J.Clin.Chem.Clin.Biochem., 1988,26,863.
139. Jdattela M., “Biology of disease; biologic activites and mechanisms of action of tumor necrosis factor-a/cachectin”, Lab Invest, 1991, 64:724-42.
140. Kakizoe E, Li S-H, Kobayashi Y, Nishikori N, Dekio S and Okunishi H, “Increases in mast cells and chymase in fibroproliferative paws of collagen-induced arthritic mice”, Inflamm Res, 1999, 48: 318 – 324
141. Katrieb A, Tak PP, Bertuch JV, et al., ”Expression of chemokines and matrix metalloproteinases in early rheumatoid arthritis”, Rheumatology (Oxford), 2001, 40: 988-994.
142. Katz, J.D., Benoist, C., and Mathis, D., “T helper cell subsets in insulin dependent diabetes”, Science, 1995, 268:1185–1188.
143. Kohno, K., and Kurimoto, M., “Interleukin 18, a cytokine which resembles IL-1 structurally and IL-12 functionally but exerts its effect independently of both”, Clin. Immunol. Immunopathol, 1998, 86:11–15.
144. Kraan MC, Haringman JJ, Ahern MJ, Breedveld FC, Smith MD, Tak PP, “Quantification of the cell infiltrate in synovial tissue by digital image analysis”, Rheumatology (Oxford), 2000, 39:43-49.
145. Levi-Schaffer F and Kupietzky A, “Mast cells enhance migration and proliferation of fibroblasts into an in vitro wound”, Exp Cell Res ,1990, 42 – 49.
146. Loh RKS, Jabara HH and Geha RS, “Disodium cromoglycate inhibits S_ to S_ deletional switch recombination and IgE synthesis in human B cells”, J Exp Med, 1994, 180, 663 – 671
147. Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, Smolen J, Emery P, Harriman G, Feldmann M, Lipsky P, “Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial”, ATTRACT Study Group Lancet, 1999, 354.
148. Malone DG, Irani A-M, Schwartz LB, Barrett KE and Metcalfe DD, “Mast cell numbers and histamine levers in synovial fluids from patients with diverse arthritides”, Arthritis Rheum, 1986, 956 –963.
149. Malone DG, Wilder RL, Saavedra-Delgado AM and Metcalfe DD, “Mast cell numbers in rheumatoid synovial tissues-Correlations with quantitative measures of lymphocytic infiltration and modulation by antiinflammatory therapy”, Arthritis Rheum, 1987,130 – 137
150. Mauri, C., Williams, R.O., Walmsley, M., and Feldmann, M., “Relationship between Th1/Th2 cytokine patterns and the arthritogenic response in collagen induced arthritis”, Eur. J. Immunol,. 1996, 26:1511–1518
151. Mc Dermott M., “TNF and TNFβ biology in health and disease”, Cell Biol.,2001,47 (4):619-35
152. Morita, Y., et al., “Expression of interleukin-12 in synovial tissue from patients with rheumatoid arthritis”, Arthritis Rheum, 1998, 41:306–314.
153. Mosmann, T.R., and Sad, S., “The expanding universe of T cell subsets: Th1, Th2 and more”, Immunol. Today, 1996, 17:13–17.
154. Pfeffer K, Matsuyama T, Kunding TM et al., ”Mice deficient for the 55 kD tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection”, Cell (USA), 1993, 73:457-67.
155. Qu Z, Liebler JM, Powers MR, Galey T, Ahmadi P, Huang X-N, Ansel JC, Butterfield JH, Planck SR and Rosenbaum JT, “Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous hemangioma”, Am J Pathol ,1995, 564 – 573
156. Racke, M.K., et al.,”Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease”, J. Exp. Med,. 1961–1966, 180
157. Simon LS, “DMARDs in the treatment of rheumatoid arthritis current agents and future developments”, Int J Clin Pract, 2000, 54: 243–9.
158. Smolen JS, Steiner G, “Therapeutic strategies for rheumatoid arthritis”, Nat Rev Drug Discov, 2003, 2,473-488.
159. Ulfgren AK, Andersson U, Engstrom M, Klareskog L, Maini RN, Taylor PC, “Systemic anti-tumor necrosis factor alpha therapy in rheumatoid arthritis down-regulates synovial tumor necrosis factor alpha synthesis”, Arthritis Rheum, 2000, 43:2391-2396.
160. Ulfgren AK, Grondal L, Lindblad S, Khademi M, Johnell O, Klareskog L, Andersson U, “Interindividual and intra-articular variation of proinflammatory cytokines in patients with rheumatoid arthritis”, Potential implications for treatment, Ann Rheum Dis, 2000, 59:439-447.









