 ABOUT AUTHORS:
ABOUT AUTHORS:
P.PRATHYUSHA*
Department of Pharmaceutics,
Raghavendra Institute of Pharmaceutical and Educational Research,
Anantapur, A.P, India.
* prathyusha2012@gmail.com
ABSTRACT
In the present investigation, an attempt was made to formulate the Oral Controlled Release Matrix Tablets of Glibenclamide in order to improve efficacy, reduce the frequency of administration, reduce dose related side effects and better patient compliance. Four formulations were prepared by wet granulation method using Ethyl cellulose and as a polymer in the ratio of 1:1, 1:2 and 1:4 and Conventional Tablets by using Starch mucilage as a granulating agent. The formulated granules showed satisfactory flow properties. All the tablets formulation showed acceptable pharmaco technical properties and complied with pharmacopoeial standards. The tablets were evaluated for weight variation, hardness, friability, drug content and invitro dissolution studies. In- vitro release studies were carried out at pH1.2 for first 2 hrs followed by phosphate buffer at pH7.4 over a period of 12hrs using USP dissolution apparatus. F3 shows good initial release of 25.2% in 1 hr and may extend release of 96.8% in 12 hrs. Different release models like zero order, first order, higuchi, Hixson-Crowell and krosmeyer-peppas were applied to invitro drug release data in order to evaluate the drug release mechanisms and kinetics. Formulated matrix tablets were compared with conventional and marketed (diaonil 5mg) formulations. Matrix tablets with optimum concentration of Ethyl cellulose were successfully developed and evaluated.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1681
INTRODUCTION
Diabetes mellitus is a chronic metabolic disorder characterized by high glucose concentration in blood, caused by Insulin deficiency, often combined with Insulin resistance. Glibenclamide is an oral hypoglycemic agent, which is a drug for the treatment of patients with Non-Insulin Dependent Diabetes Mellitus (NIDDM) 2. Glibenclamide inhibits ATP sensitive potassium channels in pancreatic beta cells. This inhibition causes depolarization in cell membrane, then calcium channels to open which are voltage dependent which causes an increase in intracellular calcium in the beta cell, which stimulates insulin release. Glibenclamide is a weak acid of pKa 6.3 and practically insoluble in water and acidic solutions.90% are bound to plasma proteins. A dose of 2.5-5mg is taken once daily. Therefore to reduce frequency of administration and improve patient compliance, a controlled release matrix dosage form of Glibenclamide is desirable. Hydrophilic polymer matrix systems are widely used in oral controlled drug delivery systems because of their flexibility to obtain a desirable drug release profile, cost effectiveness, and broad regulatory acceptance. Ethyl cellulose a choice for formulation of hydrophilic matrix system, providing robust mechanism, choice of viscosity grades, non-ionic nature, consistent reproducible release profiles, cost effectiveness and utilization of existing conventional equipment and methods.
The aim of present work is to formulate and evaluate uncoated Ethyl cellulose based Glibenclamide matrix tablets in different ratios by using Starch mucilage as granulating agent and Magnesium stearate as lubricant and talc as glidant. Another objective of this work is toevaluate drug release data using various kinetic models and to determine the mechanism of drug release.
MATERIALS AND METHODS
Glibenclamide was obtained as a gift sample from Pharma fabrikon, Madurai, Tamilnadu, India. Ethyl cellulose was procured from SD-Fine chem., Mumbai, India. Lactose and Starch from Nice chemicals, talc and Magnesium stearate etc. were of analytical reagent grade.
PREPARATION OF MATRIX TABLETS
Controlled release matrix formulations of Glibenclamide were prepared with Ethyl cellulose polymer by using different drug-polymer ration of 1:1(F1), 1:2(F2) and 1:4(F3). Conventional tablets(F4) of Glibenclamide were also formulated with out polymer. Here Lactose and Talc were used as diluent and Magnesium stearate was used as lubricant. Matrix tablets were prepared by wet granulation method. Required quantity of Drug, Ethyl cellulose and Lactose weighed, mixed well and passed through sieve no-60, powder mixture was wetted with sufficient quantity of Starch mucilage. The wet mass was passed through sieve no-16 and obtained granules were dried at 600c for 30 min. the dried belts granules were passed through sieve no-22. It was weighed and lubricated using Magnesium stearate and Purified Talc. Conventional tablets were also prepared as same as matrix tablets with out using ethyl cellulose. The granules were compressed by using a single stroke tableting punch machine manually(cad Mach). Prior to compression the weight and hardness adjustments were done. The compressed tablets were evaluated for their weight variation, hardness, friability, drug content and in-vitro drug release.
|
S.NO |
INGREDIENTS |
MATRIX TABLETS (F1) |
MATRIX TABLETS (F2) |
MATRIX TABLETS (F3) |
CONVENTIONAL TABLETS (F4) |
| 1. | Glibenclamide | 5mg | 5mg | 5mg | 5mg |
| 2. | Ethyl cellulose | 5mg | 10mg | 20mg | - |
| 3. | Lactose | 235mg | 230mg | 220mg | 240mg |
| 4. | Starch mucilage | q.s | q.s | q.s | q.s |
| 5. | Magnesium stearate | 1% | 1% | 1% | 1% |
| 6. | Purified talc | 1% | 1% | 1% | 1% |
| Total | 250mg | 250mg | 250mg | 250mg |
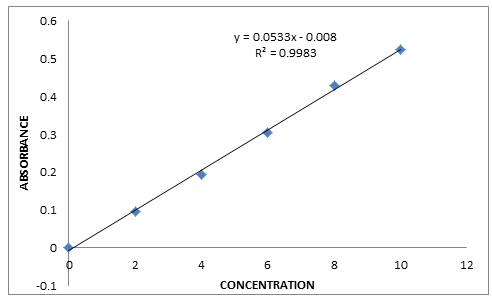
Table-1: Formulation of Glibenclamide Matrix tablets.
EVALUATION OF TABLETS
[adsense:468x15:2204050025]
1.WEIGHT VARIATION:- The Weight variation test were performed by taking 20 tablets individually and collectively weighed in Oahu’s digital balance, the average weight was calculated and the deviation of individual weight from the mean weight was calculated.
2. HARDNESS:- The Tablets hardness has been defined as the force required to break tablet in a diametric compression test. Monsanto hardness tester was employed to determine the tablet hardness between two plungers. The lower plunger was forced against a spring by turning the thread bolt until the tablet fractures. The force of the fracture was recorded. Hardness is determined by force required to fracture – zero force.
3. FRIABILITY:-10 tablets were weighed and rotated for 4 min at one hundred revolutions per minute using Roche friabilator(veego). The tablets were then reweighed and percentage friability was calculated using following formula
%Friability = (Loss in weight/Initial weight) × 100
4. DRUG CONTENT:- The Drug content of the tablets was measured Spectrophotometric ally. For this purpose 5 tablets were weighed and crushed in a mortar and pestle. The % drug content or assay of the tablet was determined by official method.
5. INVITRO DISSOLUTION STUDIES:- The dissolution studies were carried out by using USP type 2 (paddle type) Dissolution apparatus(Electro lab) for both the conventional type and matrix type formulations. The tablets were placed in 900ml of medium which was maintained at 370c. Drug release studies were carried out at pH1.2 for first 2 hrs. followed by phosphate buffer at pH7.4 over a period of 12hrs using USP dissolution apparatus.This was stirred at 1000rpm and samples of 1ml were withdrawn at 1,2,3,4,5,6,8,9,10 and 12 hours. For the conventional tablets the samples were withdrawn at 15,30,45,60,90,120 min. The drug release were determined by measuring the absorbance of tablets at 340nm in Uv-Spectrophotometer. Similarly the dissolution behaviour for the amount of drug release in a particular time and percentage was calculated by using the following formula,
Percentage release = (conc. × dilution factor × bath volume/ drug content) ×100
PROCEDURE FOR THE DEVELOPMENT OF CALIBRATION CURVE OF GLIBENCLAMIDE:- A stock solution of Glibenclamide (1mg/1ml) by dissolving 100mg standard drug in 100ml of distilled water. From this stock solution, 0.2,0.4,0.6,0.8,1.0 mg/ml concentrations were prepared by diluting with appropriate quantities of distilled water. Its λmax was analysed using Uv-Spectrophotometer(Perkins) at 340nm. The prepared solutions were measured at 340nm. The standard curve between Concentration (vs.) Absorbance was plotted.
Figure-1 Calibration curve of Glibenclamide.
DETERMINATION OF RELEASE KINETICS
The dissolution data obtained was fitted to Zero order, first order, Higuchi, Hixson Crowell and Korsmeyer Peppas equations to understand the rate and mechanism of Glibenclamide release from the prepared formulations and commercial product. The release kinetics parameters for formulations studied in a PH 7.4phosphate buffer. The correlation coefficients were calculated and used to find the fitness of the data.
Zero Order Kinetic
It describes the system in which the drug release rate is independent of its concentration.
Mt = Mo + Ko t
First Order Kinetic
It describes the drug release from the systems in which the release rate is concentration dependent.
log Mt = log Mo + kt/ 2.303
Higuchi Model
It describes the fraction of drug release from a matrix is proportional to square root of time.
Mt = Mo+ kHt1/2
Korsmeyer-Peppas model
The power law describes the drug release from the polymeric system in which release deviates from Fickian diffusion, as expressed in following equation.
Mt = Mo+ kktn
Where Mt is the amount of drug dissolved in time t, M0 the initial amount of drug, K0 the zero-order release constant, K the first order release constant. KH the Higuchi rate constant, KK the release constant and n is the release exponent, which characterizes the mechanism of drug release. The magnitude of the exponent n indicates the release mechanism as Fickian diffusion, as case II transport, or as anomalous transport.
For cylindrical matrix tablets, if the exponent n = 0.45, then the drug release mechanism is Fickian diffusion, and if 0.45 < n < 0.89, then it is non-Fickian or anomalous diffusion. An exponent value of 0.89 is indicative of Case-II Transport or typical zero-order release.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RESULTS AND DISCUSSION:-
Physical Characterization of the Designed Tablets
The physical appearance, tablet hardness, friability, weight variation, and drug content uniformity of all tablet formulations were found to be satisfactory and reproducible as observed from the data in below Table. Tablet hardness was found to be good (between 4.5 to 5 kg/cm2) depending on the compression force applied, and friability was less than 1% (wt./wt.). The manufactured tablets showed low weight variation and a high degree of drug content uniformity, indicating that wet granulation is an acceptable method for preparing good-quality matrix tablets of Glibenclamide.
|
S.NO |
FORMULATION |
WEIGHT VARIATION |
HARDNESS |
FRIABILITY |
DRUG CONTENT |
|
1. 2. 3. 4. |
F1 F2 F3 F4 |
252±7.5% 251±7.5% 255±7.5% 250±7.5% |
4.5±0.5 4.5±0.5 4.7±0.5 05±0.5 |
1% 0.9% 0.9% 1% |
99.4% 98.6% 96.8% - |
Table-2: Evaluation of Glibenclamide Matrix tablets.
In Vitro Release Studies
The results of dissolution studies indicated that F1(1:1), F2(1:2), and F3(1:4) released 38.9%, 35.4%, and 25.2% of Glibenclamide at the end of 1 h; and 99.4%, 98.6%, and 96.8% of drug at the end of 12 h respectively (Figure 2). From these can be concluded that F3 shows best Controlled release activity than other formulations. Further the release data was fitted to various mathematical models to evaluate kinetics and mechanism of drug release. The regression coefficient obtained by first order kinetics(R2:0.9798,0.9535,0.9807) were compared to zero order kinetics(R2:0.9926,0.9924,0.9906). It is concluded that drug release from all formulations follow zero order release kinetics and all formulations show good regression coefficient in Korsemeyers Pappas model (R2:0.9919,0.9914, 0.9955). So all formulations show diffusion type of drug release mechanisms.
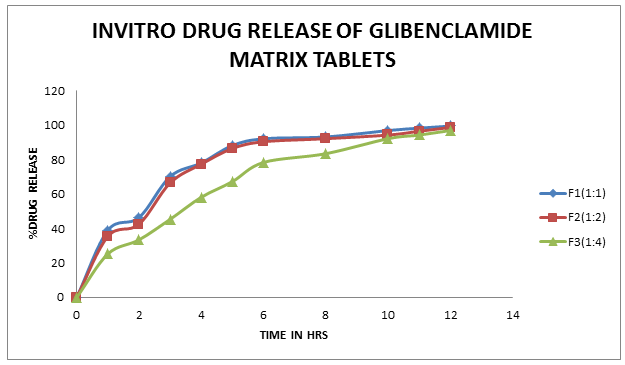
Figure-2 In vitro release of Glibenclamide Matrix tablets.
Conventional tablets show good regression coefficient in zero order drug release kinetics and best fit model is Korsemeyers Pappas model which follows diffusion type of release kinetics.

Figure-3 In-vitro release of Glibenclamide Conventional and Marketed tablets.
|
MODEL |
PARAMETER |
F1 |
F2 |
F3 |
F4 |
| ZERO ORDER | R2 | 0.9926 | 0.9924 | 0.9906 | 0.9235 |
| FIRST ORDER | R2 | 0.9798 | 0.9535 | 0.9807 | 0.9160 |
| HIGUCHI | R2 | 0.9736 | 0.9721 | 0.9540 | 0.9235 |
| HIXSON CROWELL | R2 | 0.9105 | 0.8872 | 0.9855 | 0.9740 |
| KORSMEYERS PEPPAS | R2 | 0.9919 | 0.9914 | 0.9955 | 0.9863 |
Table-3: Drug release Kinetics of Prepared Formulations.
REFERENCES:-
1.Vidyadhara, S and Prasad, J. 2004. Formulation and evaluation of Propranolol hydrochloride oral controlled release matrix tablets. Indian J. Pharm. Sci. 66: 188-192.
2.Majeti, N., Ravi Kumar and Neeraj Kumar. 2001. Polymeric controlled drug-delivery systems perspective issues and opportunities. Drug. Dev. Ind. Pharm. 27: 1-30.
3. Chien Y W, “Novel Drug Delivery Systems,” ed. by Chien Y. W., Marcel Dekker, Inc., New York, U.S.A., 1992, pp. 139—196.
4.Controlled Drug Delivery Concepts and Advances; S.P. Vyas, Roop K. Khar; First Edition; Vallabh Prakashan; 2002; P.155?192.
5.Bodea-A and Leucuta, Optimization of Propranolol Hcl Sustained Release pellets using Box-Behnken design and desirability function., Drug-Dev.-Ind. Pharma., 1998,24(2),145-155.
6.Gilbert Banker., Drug products, Modern pharmaceuticals, 3rd Edition.,1996.,Gilbert, Christopher, Rhodes-T(Eds),pg-22-30.
7. James Swarbick and James, C.Boylan, Encyclopaedia of Pharmaceutical Technology.,1993., Marcel Dekker. Inc.,vol-8.,pp-204-209.
8.Manekar.N.C. and Joshi.S.B., Types of Prolonged action dosage forms., Pharma-Times.,1991,23(4),25-27.
9.Hamdy Abd-el-Kader, Osama Youssef Abdullah and He sham Salem, “Formulation of Controlled Release Baclofen Matrix Tablets: Influence of some Hydrophobic excipients on release rate and in-vitro evaluation” AAPS Pharm SciTech, 2008, DOI:10.1208/s12249-008-9094-0.
10. J.Jeya Ananthi, Umesh Chourasia, A.Lakshmi, M.B Vishwananthan, “Formulation and Evaluation of Controlled Release Matrix Tablets of pentoxifylline” Journal of Pharmacy Research , 2009,Vol-2. Issue1.
11.Sourabh Jain, SK Yadav and UK Patil, “Preparation and Evaluation of Sustained Release Matrix Tablet of Furosemide using Natural polymers”, Research Pharm and Tech.1(4),2008,Page 374-376.
12. Raghavendra Rao N.G, Gandhi Sagar, Patel Tarun, “Formulation and Evaluation of Sustained Release Matrix Tablets of Tramadol Hcl.”, International journal pharmacy and Pharmaceutical Sciences,2009, 1(1), pg60-70.
13. Ganesh Kumar, V.Juyal, P.P Badoni, “Formulation and Evaluation of Matrix Tablets of Acarbose”, Drug invention Today,2010,2(5),264-267.
14.Potu Apparao , Jyothi,VeeraReddy Prabhakar Reddy, Jukunti Raju and Burra Shashidher, “Formulation and Evaluation of gum based Matrix Tablets of Lamivudine”, Pelagia Research library,2011,3(3):176-192.
15.Syed Namath Ulla, An up Kumar Roy, Martard Kulkarni, Vinod Kumar SM, “Formulation and Evaluation of Sustained Release Matrix Tablets of Lornoxicam”, International Journal of Drug development and Research, 2011, vol3,issue1.
16. wikipedia.org/wiki.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









