 About Authors:
About Authors:
DIPAK KUMAR DASH
M.PHARM, PHARMACEUTICS
HIMALAYAN PHARMACY INSTITUTE, SIKKIM
SUSPENSION
Pharmaceutical suspension may be defined as coarse dispersions in which insoluble solids are suspended in a liquid medium. Insoluble solids may have a size range from 10 to 1000 µm.
STABILITY OF SUSPENSION
It is important to understand that suspensions are kinetically stable, but thermodynamically unstable, system.
Physical stability is defined as the condition in which the particles remain uniformly distributed throughout the dispersion without any signs of sedimentation. It is difficult to achieve this condition. Hence the definition can be restated as –if the particles settle they should be easily resuspendable by moderate amount of shaking.
[adsense:336x280:8701650588]
Reference ID: PHARMATUTOR-ART-1093
EFFECT OF PARTICLE SIZE ON STABILITY
When left undisturbed for a long period of time the suspension particles will aggregate, sediment, eventually cake. When a suspension is very well dispersed (i.e., deflocculated), the particles will settle as small individual particles. This settling will be very slow and will result in a low-volume, high-density sediment that may be difficult or impossible to redisperse. When the particles are held together in a loose open structure, the system is said to be in the state of flocculation. Particle size of the active agent plays a key role in the physical stability and bioavailability of the drug product.
The rate of sedimentation, agglomeration, is affected by particle size. The most efficient method of producing small particle size is dry milling. However, wet milling may be desirable for potentially explosive ingredients.
DEFLOCCULATED SUSPENSION
In this system solids are present as individual particles. These system have a shorter shelf life ,but have greater bioavailability when compared to flocculated systems.
FLOCULATED SUSPENSION
In this system particles aggregate themselves by chemical bridging. These folks are light fluffy conglomerates which are held together by weak vanderwall force of attraction.
Flocculating Agents
flocculating agents decreases zeta potential of the suspended charged particle and thus cause aggregation (flock formation) of the particles.
Examples of flocculating agents are:
• Neutral electrolytes such as KCl, NaCl. , Surfactants, Polymeric flocculating agents.
• Sulfate, citrates, phosphates salts
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
EFFECT ON SEDIMENTATION
If the particle size is reduced to half of its original sizes the rate of sedimentation decreases by a factor of four.
Theory of Brownian movement: Brownian movement of particles prevents sedimentation. if the size of the particles is about 2 to 5 micro meter.
When dispersed particles are in contact with an aqueous solution of an electrolyte, the particles may selectively adsorb one charge species. If the adsorbed species is an anion, the particles will be overall negatively charged. The ions that give the particle its charge, anions in this case, are called potential-determining ions or co-ions. Remaining ionic species in the solution are the rest of the anions and the total number of cations added. This means, there will be excess cations than anions in the dispersion medium. These cations having a charge opposite to that of the potential-determining ions are known as counter-ions or gegenions. They are attracted to the negatively charged surface by electric forces. Gegenions also repel the approach of any further anions to particle surface, once the initial adsorption is complete. These electric forces and thermal motion keeps an equal distribution of all the ions in solution. It results in an equilibrium condition where some of the excess cations approach the surface and the rest of the cations will be distributed in decreasing the amounts as one moves away from the charged surface.
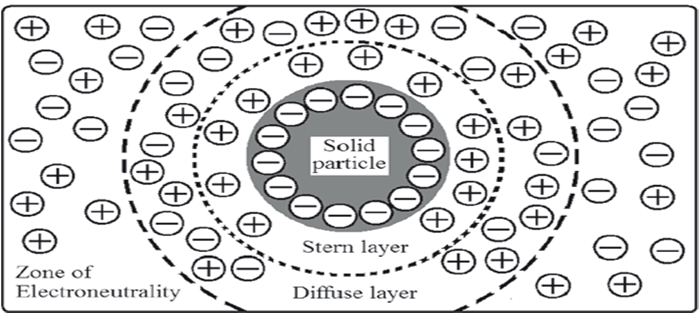
ELECTRICAL DOUBLE LAYER THE SOLID LIQUID MEDIUM INTERFACE
Dispersion of particle involves the intimate contact of the solids with water as vehicle. This is a critical and difficult step because most solids are hydrophobic in nature. In addition when finely devided powders are selected for reason of better physical stability ,they adsorbs a lot of air which is difficult to expel.as a result solids float on the surface of the vehicles, through there density are high.
Contact angle (O) comment
90 float
0 complete wetting
180 insignificant wetting
NEARST EQUATION & ZETA POTENTIAL
The electric double layer is formed in order to neutralize the charged particles in a suspension. The difference in electric potential between the actual or true surface of the particle and the electro neutral region is referred to as the surface or electro thermodynamic or Nernst potential (E). Hence, Nernst potential is controlled by the electrical potential at the surface of the particle due to the potential determining ions. The potential difference between the shear plane and the electro neutral region is known as the electro kinetic or zeta (z) potential. While Nernst potential has little influence in the formulation of stable suspension, zeta potential has significant effect on it. Zeta potential governs the degree of repulsion between adjacent, similarly charged solid dispersed particles. If the zeta potential is reduced below a certain value, which depends on the specific system under investigation, the attractive forces between particles due to vanderWaals’ force, overcome the forces of repulsion and the particles come together to form floccules. This phenomenon is known as flocculation.
References
1) Lachman L.,Liberman H.A. and Kanig J.L., “ TheTheory and Practice of Industrial Pharmacy” , 3rd edition ,Published by Varghese Publishing House, P.- 462-464
2) Martin A., “Physical Pharmacy”, 4th edition, published by B.I. waverly Pvt Ltd New Delhi P.- 42-43
3) Remington ,“The science and practice of pharmacy”, 21st edition, volume-1, published by-lippincott Williams & wilkins, P.-288-290
4) phares.biz/pdf/ solid dispersions_V1.pdf
5)Factsheet_solubility_Development_Poorly_Soluble_Compounds_Nov2006.pdf
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org









