About Authors:
A.D.Taranalli, Dilip Patoliya*, Sonal Chanchlani, Vishal Patel, Keyur Makadiya
Department of Pharmacology and Pharmacokinetics,
K.L.E. University’s College of Pharmacy, Belgaum.
*Dilip_patoliya@yahoo.com
ABSTRACT:
Ulcerative colitis is a chronically recurrent inflammatory bowel disease of unknown origin in which oxidative stress has been implicated. The main aim of the present study was to evaluate possible protective effect of alcoholic extract of Aloe Vera against the drug induced model of colitis in rats. Induction of colitis by colonic administration of drugs(Acetic acid, Indomethacin and TNBS) cause severe microscopic inflammation of colon after 24 hrs of administration of one of any above inducing agent, as assessed by the macroscopically. Microscopically colonic tissue showed ulceration, oedema and inflammatory cell infiltration. Biochemical estimation studies revealed decrease in serum levels of lactate Dehydrogenase (LDH) may be due to increase in Colonic concentration of tumor necrosis factor α (TNF- α) and neutrophill infiltration index. Oxidative stress was indicated by reduced glutathione concentration (GSH) as well as increase in levels of lipid peroxidase (LPO) and myeloperoxidase (MPO) activity in colonic tissue. Pretreatment with Aloe Vera extract at a dose of (400mg/kg/day, orally),10 days before induction of colitis increase level of serum LDH and GHS, while colonic concentration of LPO and MPO was increased. It also showed decrease in macroscopic score. The findings of the present study provide evidence that Aloe Vera extract may be beneficial in inflammatory bowel disease.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1450
INTRODUCTION:
Ulcerative colitis and Crohn’s disease are chronic, relapsing, immunologically mediated disorders that are collectively referred to as inflammatory bowel diseases (IBD). Inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis (UC) is an immune-mediated chronic intestinal disorder, characterized by rectal bleeding and diarrhea resulting in disruption of the epithelial barrier, and the formation of epithelial ulceration (Silva et al., 2010).Etiology and pathogenesis of IBD remain obscure, although environmental factors, in combination with genetic factors, are suggested to be involved in its pathogenesis (Fiocchi, 1998; Loftus, 2004). Furthermore, prolonged and chronic UC may progress to colorectal cancer. Although substantial progress has been made in the treatment of the disease, no definitive therapies until now are available for this disorder, and limiting drug-induced toxicity is a continuous challenge. Most of the treatment modalities are used for UC including anti-inflammatory therapy (5-aminosalicylic acid and glucocorticosteroids), immunomodulatory agents (Azathioprine, Mercaptopurines and cyclosporine) (Kozuch and Hanauer, 2008).However, these drugs have many serious side effects and the recrudescence rates of IBD are rather high.
[adsense:468x15:2204050025]
Prolonged or inadequate activation of the intestinal immune system participates in the pathological events of chronic mucosal inflammation (Sartor, 1997). Tumor necrosis factor-α (TNF-α) is a key immunoregulatory cytokine that plays a pivotal role both in experimental and clinical studies of ulcerative colitis and amplifies the inflammatory response by activating a cascade of immune responses (Dionne et al., 1997; Ardizzone and Bianchi, 2005; Kozuch and Hanauer, 2008).it has been generally accepted that over expression of pro-inflammatory mediators such as reactive oxygen mediators, neutrophil infiltration, and cytokines contribute to the inflammatory cascade in pathological process of colitis (Yao et al., 2010). Among these pro-inflammatory mediators, inflammatory cytokines play a crucial role in the development of UC .Pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6) significantly increased in the colonic mucosa in UC. In particular, TNF-α, which is induced, synthesized and secreted from macrophages, Lymphocytes, and polymorphonuclear neutrophils, are regarded as the most prominent “first-line” cytokines (Bumgart D C et al., 1997).Reactive oxygen metabolites (ROMs) produced and released by Immune cells play an important role in the physiopathology of UC. Oxidative stress and its consequent lipid peroxidation could aggravate free radicals chain reactions, disrupt the integrity of intestinal mucosa barrier, and activate inflammatory mediators. It has been shown that colonic lipid peroxidase (LPO) contents increased both in human and experimental animal studies (Garrido et al., 2000). The levels of LPO were often used as an indication of oxidative damage and as a marker for free radicals-induced lipid peroxidation. Several investigations showed that constitutive production of nitric oxide (NO) played the protective role in immune-mediated tissue injury. However, large quantities of NO produced following the up-regulation of inducible nitric oxide synthase (iNOS) in epithelial cells has been closely associated with IBD (Wang et al., 2009). Overproduced NO can react with superoxide and inhibit key enzymes in the mitochondrial election transport chain, leading to the production of potent cytotoxic oxidant, peroxynitrite that can increase iNOS through NF-B and decrease prostacyclin synthase (Yamada and Specian, 1993). Therefore, excessive NO can accelerate process of UC.
Aloe is a dried juice of leaves of Aloe barbidensis Miller belongs to familyLiliaceae.In traditional medicine Aloe Vera is used as abortificient, adjuvant activity, alkalizing activity, analgesic, as a local anesthetic, anti asthmatic, antiburn effect, anti complement activity, antifrtility activity, antifungal,, anti hypercholesteromia, Anti hyperglycemic, anti inflammatory, antilecopenic activity, antipyretic activity, anti tumor activity, antiulcer42, wound healing, in hair fall therapy, histamine release inhibition, and uterine stimulation 35, 36, 41-51. It has been prove that aloe Vera exudates shows antioxidant activity on diabetic rats42. It also shown the protective effect on lidocane induced hepatotoxicity and genotoxicity in rats.
A number of recent studies have renewed interest in Aloe Vera for the treatment of chronic inflammatory conditions. To date, however, the possible modulatory role of Aloe Vera in colon inflammation has not been yet verified; hence we aimed in the current investigation to evaluate the possible modulating effect of Aloe Vera extract on acetic acid-induced ulcerative colitis model in rats.
MATERIALS AND METHODS:
Chemicals: -
Sulfasalazine was obtained from Wallec pharmaceutical Pvt. Ltd., Goa, Indomethacin from Indoco pharmaceutical Pvt. Ltd. Mumbai, as a gift sample. While acetic acid, carbachol, TNBS obtained from Himedia laboratories pvt.ltd. and LDH kit from ERBA Diagnostics, Germany.
Animals: -
Male wistar rats of seven weeks old (150-200 g) were obtained from Venkateshwara Enterprises, Bangalore, Karnataka were used for the study. They were housed under standard laboratory conditions and fed with standard rodent diet and water ad libitum. All the experimental procedures were carried out in accordance with committee for the purpose of control and supervision of experiments on animal (CPCSEA) guidelines. The study was reviewed and approved by the Institutional Animal Ethical Committee (IAEC Reg. No.: 627/02/a/CPCSEA), J. N. Medical College, KLE University, Belgaum, Karnataka.
Preparation of plant Extract:-
Aloe Vera Leaves were collected and dried at room temperature for 9 to 10 days. Then the dried plant powdered and extracted by alcohol using soxhelet apparatus. The plant extract was filtered and evaporated to remove alcohol content and to form a powder.
Induction of colitis:-
In this study we used three models of colitis. Rats were randomly divided in to five groups for each model (n= 6). Experimental colitis was produced in model 1, 2, 3 with acetic acid, Indomethacin and TNBS respectively. In brief, rats were lightly anesthetized with anesthetic ether. Then a flexible polyethylene catheter with an external diameter of 2mm was inserted into the colon via the canula to induced colitis. Rats were held in a head –down position for 5 min after the instillation of inducing agent, in order to distribute the agents in the entire colon.
Assessment parameters:-
LPO estimation:-
LPO level of the colon tissues was determined by the thiobarbituric acid method (Ohkawa et al., 1979). Briefly, the colon tissues were homogenized for 10 min in10 volumes of ice-phosphate buffer. After centrifuged at 5000 rpm/min for 10 min at 4 ?C, LPO levels in the supernatant were measured at 532nm using UV spectrophotometer.
GSH estimation:-
GSH level of the colon tissues was measured by the Ellaman’s reagent method. In this method the colon tissue were homogenized for 10 min in 10 volumes of ice cold phosphate buffer and then allow to centrifuged at 5000 rpm/min for 10 min. 50 µl of Ellaman’s Reagent and 2.5 ml reaction buffer were added in each test tubes. In test samples 250 µl of colon homogenate and in blank sample 250 µl of reaction buffer was added. Mix well and incubate it at room temperature for 15 min. then measure the absorbance at 412nm on UV spectroscopy.
MPO estimation:-
MPO activity, marker neutrophil infiltration was performed. Pieces of inflamed tissue (rat colon) were taken. The tissue was rinsed with ice cold saline, bottled dry, weighed and excised. Minced tissue was homogenized in 10 volume of ice cold potassium phosphate buffer (pH 7.4), using tissue homogenizer. The homogenate was centrifuged at 3500 rpm for 30 min at 40 C. The supertant was discarded. 10 ml of ice cold 50mM potassium buffer (pH 6.0) , containing 0.5% hexadecyl trimethyl ammonium bromide(HETAB) and 10mM EDTA was added to the pellet. It was then subjected to one cycle of freezing and thawing and brief (15 s) of sonication. After sonication solution was centrifuged at 15,000 rpm for 20 min. Myeloperoxidase activity was measured spectrophotometically as follows: 0.1 ml of supertant was combined with 2.9 ml of 50mMphosphate buffer containing 0.167 mg/ml o-dianisidine hydrochloride and 0.0005% H2O2. The change in absorbance was measured spectrophotometically at 460nm.
Serum LDH estimation:-
Serum lactate dehydrogenase was measured by using serum, indicator of tissue damage by using commercially available diagnostic kit (ERBA Diagnostics, Germany).
Colon contractility study:-
Colitis is associated with dysmotility and decrease colonic contractility. The lowest part of distal colon was cut and placed in kerb’s solution at pH 7.4 containing Nacl, Kcl, Cacl2, Mgso4, KH2Po4, NaHCo3, and glucose. The solution was saturated with carbogen gas. The muscle strip was set up in 40 ml organ bath and contraction is recorded using fixed force transducer-Biopac data Acquisition system. A resting tension of 1 g was applied and the tissues were allowed to stabilize for 30 min, during which the bath fluid was changed at least once. After stabilization tissue viability was checked by adding 80mM Kcl. A non cumulative dose response curve was studied with different concentration of carbachol concentration; the contractile response was allowed to reach a peak before washing out and left to relax to the base line. This procedure was continued until maximum response was achieved. The maximum response was expressed as mg/mg tissue weight89,90, 91.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Macroscopic scoring:-
A 6 cm of colon extending proximally from 4 cm above the anal margin was removed, split longitudinally and macroscopic appearances of the colonic mucosa scored on a scale 0-4 as follows:
Score/points Particulars
0 no macroscopic change
1 mucosal erythema alone
2 mild mucosal oedema, slight bleeding or small erosions
3 moderate oedema, bleeding ulcers or erosions
4 Severe ulceration/erosions, oedema and tissue necrosis.
Statistical analysis:-The results were expressed as meanstandard deviation (Mean ± SD). Statistical analysis was performed with graph pad prism statistical software. One way ANOVA analysis followed by Dunnett’s test was used for data analysis. Differences were considered significant when P < 0.001.
Results:-
Acetic acid induced colitis:-
Aloe Vera treatment showed significant changes in all parameters assessed.
Macroscopic score:- After induction of colitis, there was a macroscopic evidence of extensive colonic mucosal injury as assessed by the macroscopic score. As shown in table.1, there was a significant increase of macroscopic score in acetic acid treated group as compare to normal. Pre-treatment with aloe Vera extract showed a decrease in macroscopic score as compare to the acetic acid treated group. Similarly, Sulfasalazine treated group also showed decrease in macroscopic score.
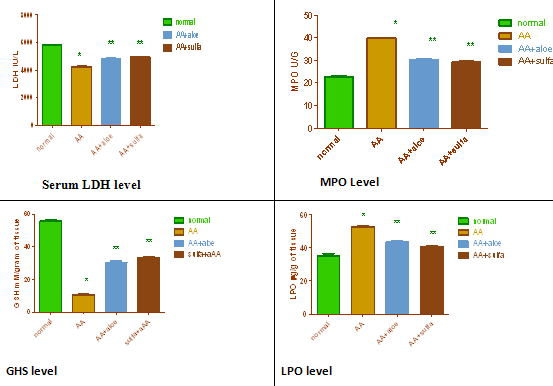
Serum LDH levels:- As shown in fig.1 , serum LDH level was significantly decrease in Acetic acid treated group as compare to normal group. Pre-treatment with Aloe Vera extract, significantly increase the elevated level of serum LDH level when compared with acetic acid treated group. As well as sulfasalazine treated group also increase the serum LDH levels.
Myeloperoxidase (MPO) assay:-MPO activity, a marker neutrophil infiltration was significantly increase with Acetic acid treated group as compared to normal group (fig.2). Aloe Vera treated group as well as sulfaselazine group showed significantly decrease in MPO levels as compared to acetic acid treated group.
Reduced glutathione (GSH) assay:- As shown in fig.3, GHS level was significantly decrease in Acetic acid treated group as compare to normal group. Pre-treatment with Aloe Vera extract, significantly increase the elevated level of GHS level when compared with acetic acid treated group. As well as sulfaselazine treated group also increase the GSH levels.
Lipid peroxidase (LPO) assay:- LPO level was significantly increase in acetic acid treated group as compare to normal group (fig.4). Whereas the aloe Vera treated group shows significantly decrease in LPO level than the acetic acid treated group. Also sulfasalazine treated group shows significantly decrease LPO level as compare to acetic acid treated group.
Table .1.Effect of Aloe Vera on Acetic acid induced model of colitis.
|
Treatment group |
GSH |
LPO |
LDH |
MPO |
Macroscopic score |
|
Normal |
55.86 |
35.06 |
5826 |
22.56 |
0.0 |
|
Acetic acid treated |
10.53 |
52.34 |
4257 |
39.78 |
4.7±0.32* |
|
Aloe Vera+ Acetic acid |
30.25 |
43.45 |
4797 |
30.23 |
3.80±0.30** |
|
Acetic acid +sulfasalazine |
33.32 |
40.72 |
4951.3 |
29.30 |
3.01±0.28** |
Figure.1. Effect of Aloe Vera extract on Acetic induced model of colitis
Values are expressed as mean ± S.E.M for 6 animals in each group.
*p < 0.001 when compare with normal group.
**p<0.001 when compare with Value are Acetic acid treatment group and normal group.
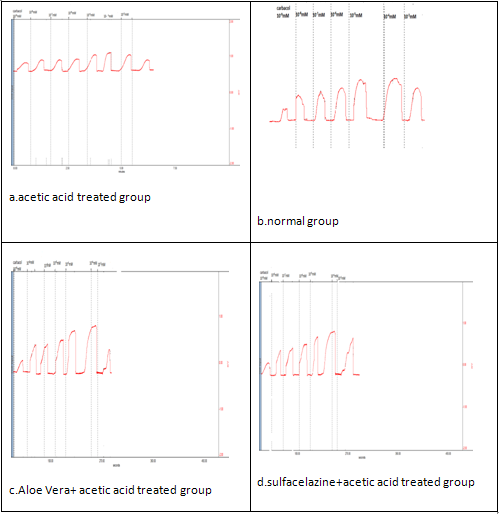
Figure.2.Colon contractility study using carbachol:-
|
Parameters |
Normal |
Acetic acid |
A.A+ Aloe Vera |
A.A +sulfasalazine |
|
Emax |
263.50±18.53 |
95.20±16.48* |
165±12.62** |
177.4±28.20** |
|
pD2 |
-6.582±0.30 |
-6.599±0.33 |
-6.391±0.02 |
-6.577±0.19 |
Indomethacin induced model of colitis:-
Table.2. Effect of Aloe Vera extract on Indomethacin induced model of colitis.
|
Treatment group |
GSH(mM/g) |
LPO(µg/g) |
LDH(IU/l) |
MPO(U/g) |
Macroscopic score |
|
Normal |
55.86±2.47 |
35.06 |
5826 |
22.56 |
0.0 |
|
Indomethacin |
16.74±1.23* |
52.34 |
2637 |
33.95 |
4.50±0.22* |
|
Aloe Vera +Indomethacin |
27.55±0.79** |
39.42 |
5009 |
25.7 |
3.50±0.23** |
|
Sulfasalazine + Indomethacin |
52.470±0.8** |
40.90 |
5132 |
23.6 |
3.01±0.28** |
Figure .3. Effect of Aloe Vera extract on Indomethacin induced model of colitis.
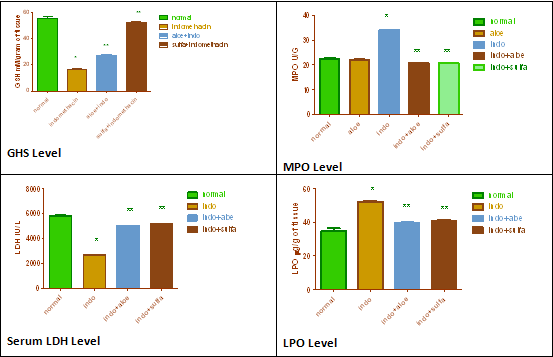
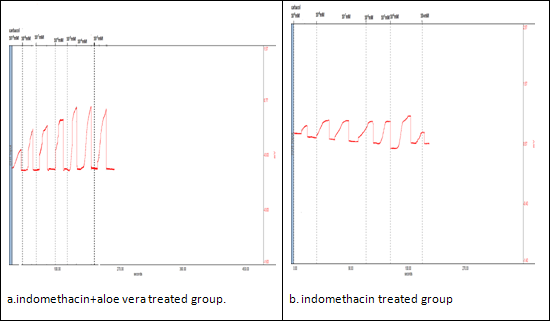
Figure. 4. Colon contractility study by carbachol in Indomethacin induced model of colitis.
|
Parameters |
Normal |
Indomethacin |
Indo+ aloe vera |
Indo+ sulfasalazine |
|
Emax |
263.50±18.53 |
94.23±5.9 |
164.2±12.62** |
179.5±22** |
|
pD2 |
-6.62±0.30 |
-6.545±0.33 |
-6.577±0.02 |
-6.455±0.19 |
Values are expressed as mean ± S.E.M for 6 animals in each group.
*p < 0.001 when compare with normal group.
**p<0.001 when compare with Value are Indomethacin treatment group and normal group.
TNBS induced model of colitis:-
Table.3. Effect of Aloe Vera on TNBS induced model of colitis.
|
Treatment group |
GSH(µg/g) |
LPO(µg/g) |
LDH(IU/L) |
MPO(U/G) |
Macroscopic score |
|
Normal |
55.86 |
35.06 |
5826 |
22.56±0.84 |
0.0 |
|
TNBS treated |
8.85 |
54.78 |
2423.5 |
37.98 |
4.58±0.31* |
|
Aloe Vera +TNBS |
21.63 |
40.93 |
4636.2 |
28.18 |
3.59±0.32** |
|
Sulfaselazine +TNBS |
25.56 |
39.80 |
5118 |
27.53 |
3.08±0.22** |
Figure .5. Effect of Aloe Vera extract on Indomethacin induced model of colitis.
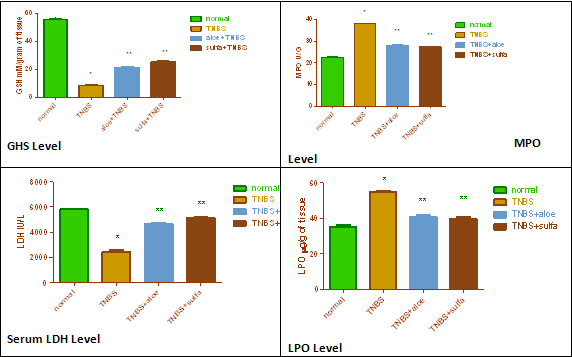
Figure.6. Colon contractility study by carbachol in TNBS induced model of colitis.
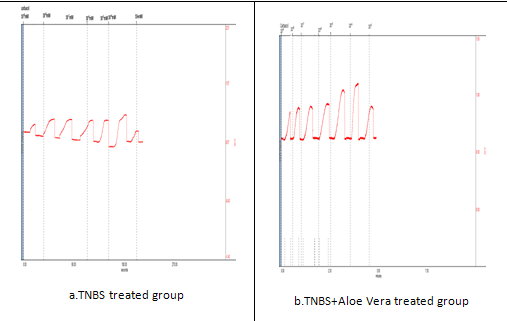
|
parameters |
Normal |
TNBS |
TNBS +Aloe Vera |
TNBS+ sulfaselazine |
|
Emax |
263.50±18.53 |
260.50±17.9* |
278.6±12** |
264±15.5* |
|
pD2 |
-6.582±0.30 |
-6.599±0.28* |
-6.619±0.33** |
-6.535±0.22* |
Values are expressed as mean ± S.E.M for 6 animals in each group.
*p < 0.001 when compare with normal group.
**p<0.001 when compare with Value are TNBS treatment group.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Discussion:-
Acetic acid induced model of colitis in rats(study I):- The present study demonstrates that Aloe Vera prevents tissue damage in rat model of colitis induced by acetic acid, as verified from its effects on macroscopic score, biochemical estimation, and contractility studies. Increase in Macroscopic score, LPO level and MPO level.It decreases Serum LDH level as well as GSH level,along with intestinal dysmotility and disorientation of colonic structure. Pre- treatment with Aloe Vera extract leads to, Decreasing in macroscopic score , LPO and MPO level. It increases serum LDH and GSH level. It also restored reduced colonic contractility.
Acetic acid induced colitis is one of the commonly used experimental models for screening drug active against the entry of protonated form of the acid into epithelium, where it dissociates to IBD. The mechanism by which acetic acid produces inflammation appears to involve liberate protons causing intracellular acidification those most likely accounts for the epithelial injury observed. The inflammatory response initiated by acetic acid includes activation of cyclooxygenase and lipooxygenase pathways and generation of inflammatory mediators like prostaglandins and leukotrienes. Excess production of reactive oxygen metabolites e.g. superoxide, hydroxyl radical, hydrogen peroxide, hypoclorus acid and oxidant derivatives such as N- chloramines are detected in the inflamed mucosa and may be pathogenic in IBD. Also, there is increase in proinflammatory cytokine TNF-α production in colonic mucosa after acetic acid instillation. Cytokines are important to gastrointestinal host defence, but their over production may cause excessive gut inflammation and intestinal motility disorders. Moreover, it induces the production of other cytokines including adhesion molecules, arachidonic acid metabolites and activation of immune and non immune cells17, 30.
In consistent with previous reports , in the present study, intrarectal administration of acetic acid produced massive necrosis , ulcerated mucosa, increased infiltration of inflammatory cells and intestinal dysmotility30,34,36. Various studies report that drugs like pyrrolidinedithiocarbamate, curcumin, celecoxib, nimesulide, gigcobiloba and copaifera langsdorffii are found to be effective in IBD. Due to either their anti TNF-α, anti oxidant and anti-inflammatory property. In view of these reports, in the present study, the beneficial effect of Aloe Vera extract on acetic acid induced colitis may be due to either of anti TNF-α, anti oxidant and anti-inflammatory property.
Indomethacin induced colitis in rats study II):-
In consistent with previous reports , in the present study, administration of Indomethacin lead to development of acute intestinal inflammation, manifested by thickening of the bowel wall, mesenteric hemorrhage, mesenteric adhesion and multiple mucosal ulcers of the small intestine.
Indomethacin, a non-selective COX inhibitor produces enterocolitis in rats which characterised by linear ulceration, thickening and transmural inflammation. The mechanism of Indomethacin induced enterocolitis have not been fully illustrated, but previous reports suggest that , inhibition of protective prostaglandins PGE1, PGE2 and prostacylin (PG12) may be one of the mechanism by which Indomethacin induces injury. In addition, bacteria and bacterial products, biliary secretion and food intake have been demonstrated to be important for the development of intestinal lesions31.
In present study, Aloe Vera showed beneficial effect against Indomethacin induced colitis in rats. Aloe Vera prevents tissue damage in rat model of colitis induced by Indomethacin in a dose dependent manner, as verified from its effects on macroscopic score, biochemical estimation, and contractility studies. It showed a decrease in the macroscopic score. Significant decrease in serum LDH and GHS activity was observed. It also showed significantly increase in LPO and tissue MPO level. This may be due to its antimicrobial properties and gut flora to plays an important role in the pathogenesis of enterocolitis. Hence Aloe Vera was effective in Indomethacin induced model of colitis in rats.
Trinitro benzene sulfonic acid induced colitis in rats TNBS):-
In consistent with previous reports, in the present study, administration of TNBS lead to development of colitis in rats. In present study, Aloe Vera showed beneficial effect against TNBS induced colitis in rats. Aloe Vera prevents tissue damage in rat model of colitis induced by, as verified from its effects on macroscopic score, biochemical estimation, and contractility studies. It showed a decrease in the macroscopic score. Significant decrease in serum LDH and GHS activity was observed. It also showed significantly increase in LPO and tissue MPO level.
The role of reactive metabolites of oxygen and nitrogen in the pathophysiology of IBD has been well reported in humans as experimental models of colitis 6,10,33,34. The inflammatory process in the intestine is probably derived from the chronic presence of numerous, activated, MPO-containing phagocytes in the inflamed intestine. These are responsible for the overproduction of reactive oxygen species that overwhelm the antioxidant.
Defenses, such as glutathione and its related enzymes that normally participle in protecting colonic tissue loss of activity. Free radical generation has been proposed as playing an important early role in the pathogenesis of IBD, and could contribute to the initial neutrophil infiltration in the inflamed colonic mucosa6. The recruitment and activation of these cells result in an increase in free radical production that overwhelms the tissue’s antioxidant protective mechanisms, resulting in a situation of oxidative stress, which definitively perpetuates colonic inflammation 42.
Hence Aloe Vera extract shows protective effect against the TNBS induced colitis in rats may be due to its antioxidant property.
Conclusion:-
In conclusion the present study indicates the beneficial effect of Aloe Vera extract in Acetic acid, Indomethacin and TNBS induced model of colitis and may be comparable to that of sulfaselazine. Further study is required to explore the different possible mechanism of actions and proper clinical investigation should be carried out to confirm the same activity in human disease.
References:-
-
Bamias G, Nyce MR, Rue S, Cominelli F. New concepts in the pathophysiology of inflammatory bowel disease. Ann international med. 2005; 143:895-904.
-
Head KA, Jureka JS. Inflammatory bowel disease part-1: ulcerative colitis- pathophysiology and conventional and alternative treatment option. Altern med review. 2003; 8(3): 247-83.
-
Baumgart DC. What’s new in inflammatory bowel disease in 2008? World J. gastroenterology 2008; 14(3):329-30.
-
Loftus E. clinical epidemiology of inflammatory bowel disease: incidence, prevalence and environmental influences. J. gastroenterology 2004; 126: 1504-17.
-
Kozuch PL, Hanauer SB. Treatment of inflammatory bowel disease: A review of medical therapy. World J. Gastroenterology 2008; 14(3): 354-77.
-
Rajasekaran Subbiah, Kasiappan Ravi, Karuran Sivagnanam. Beneficial effect of Aloe Vera leaf gel extract on lipid profile status in rats with streptozotocin induced diabetes. J.clinical and experimental pharmacology and physiology 2006; 33:232-37.
-
Zandi Keivan, Moloud Abbas Zadeh, Kohzad Sartavi, Rastian Zahra. Antiviral activity of Aloe Vera against herpes simples virus type2: in vitro study. African J. biotechnology 2007; 6(15):1770-73.
-
Etim OE, Farombi EO, Usoh IF, Akpan EJ. The protective effect of Aloe Vera juice on the lindane induced hepatotoxicity and genotoxicity. J. pharma. Sci.2006; 19(4):333-337.
-
Harputluogulu Murat M. M, Dmirel Ulvi, Yucel Neslihan, et al. The effect of gingko biloba extract on acetic acid induced colitis in rats. Turkish journal of gastroenterology 2006; 17(13):177-182.
-
Ardizzone Sundro. Ulcerative colitis. J. Gastroenterology 2003:1-8.
-
Ardizzone Sundro. Drug used in Ulcerative colitis. J. Gastroenterology 2003:1-8.
-
Crohn’s and colitis foundation of America, Inc. 1996-2002. http://www.ccfa.org/physician/colitisb.html.
-
National digestive diseases information clearinghouse, 2 information way Bethesda,MD:2893-3570. https://www.niddk.nih.gov/health/digest/pubs/colitis/colitis.htm.
-
Corrao G, Tragnose A, Caprilli R, et al. risk of inflammatory bowel disease attributable to smoking , oral contraception and breast feeding in Italy: a nationwide case- control study. Cooperative Investigation of the Italian group for study of the colon and rectum (GISC). Int. J Epidemiology 1998; 27:397-404.
-
Handrickson B A, Gokhale R, Cho J H. Clinical aspects and pathophysiology of IBD. Clinical microbiology reviews, 2002; 15(1).
-
B Joseph, Kirsner. Inflammatory bowel disease part II: Clinical and therapeutic aspects. J. gastroenterology 1991; 105: 671-732.
-
Thomas T Macdonald, Simon H Murch. Etiology and pathogenesis of cronic inflammatory bowel disease. Baillierr’s Clinical gastroenterology 1994; 8(1):1-34.
-
Goodman & Gilman’s the pharmacological basis therapeutics. 11th edi. McGraw- hill medical publishing division 2006:1009-1018.
-
Russell J Greene, Harris D Norman. Pathology and therapeutics for pharmacist: A basis for clinical pharmacy practice. 3rd edi. Published by pharmaceutical press 2008: 114-124.
-
Silbernagl Stefan, Lang Florian. Color Atlas of Pathophysiology. Thieme Flexibook: 134-170.
-
Craig C, Stitzel M. Morden pharmacology with clinaical application. 480-482.
-
Robin, Charles E, Douglas C, Jon C, James M. Pathophysiology basis of disease. 7th edi. 1200-1260.
-
Daniel C, Carding R Simon. Inflammatory bowel disease: cause and immunobiology. J.Gastroenterology-1, 2007; 125:1627-41.
-
Dense Silvio, Miquel Sans, Claudio Fiocchi. Inflammatory bowel disease: the role of environmental factors. Autoimmunity reviews.2004; 3: 394-400.
-
Stegk J P, Ebert B, Martin J. Expression profile of human 11β- hydroxyl steroid dehydrogenases type1 and type 2 in inflammatory bowel diseases. J. molecular and cellular endocrinology 2009; 301: 104-08.
-
Yu Wei, Lin Zhen wu, John gerrit, Wang Yunhua et al. Gene regulated by NKx2-3 in siRNA- mediated knockdown B-cells: implication of endothelin-1 in inflammatory bowel disease. J. Molecular genetics and Metabolism.2010; 100:88-95.
-
Wallace J L. Nitric oxide releasing mesalamine: potential utility for treatment of inflammatory bowel disease. J. digestive and liver disease 2003; 35:535-40.
-
ehealthmd.com/library/ulcerativecolitis/UC_tests.html.
-
National digestive disease information clearinghouse.[cited 2008 Nov 02]: Available at URL: niddk.nih.gov.
-
emedicine.medscape.com/179037-overview.
-
Gionchetti P, Amandini C, Rizzello F et al. probiotics –role in inflammatory bowel disease. J. digestive and liver disease 2002; 34:958-82.
-
Wirtz Stefan, Markus F. mouse model of inflammatory bowel disease. Advanced drug delivery review 2007; 59:1073-83.
- Blumberg Richard. Lawrence Saubermann, Stober Warren. Animal model of mucosal inflammation and their relation to human inflam









