 About Authors:
About Authors:
Dharmendra Kumar*, Bhanu Priya, Sumedha Bansal,
Department of Pharmaceutical Technology, Meerut Institute of Engineering and Technology,
Meerut, Uttar Pradesh, India, 250005
* rvnimiet@gmail.com
Abstract
A new Nasal drug delivery system of Domperidone has been developed as decent drug delivery by using natural mucoadhesive agent extract from Tamarindus indica L. The mucoadhesive strength, pH, viscosity and gelling property of this natural mucoadhesive agent was found to be higher in comparison to synthetic polymers, hydroxy propyl methyl cellulose (HPMC) and carbopol which are conventionally used for similar purpose. In vitro drug release characteristic through franz-diffusion cell using excised bovine nasal membrane was also found to be better in comparison to the above synthetic polymers. Now patient friendly, needle free dosage form may replace the Domperidone injections/ tablets in future.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1731
Introduction:
Generally nose is the important part of body for inhalation process. But when it is used as the route of drug delivery attained the great attraction for various drugs. Because nose provides faster and higher level of drug absorption with possibility of self-administration.[1]
The aim of this study was to develop a nasal delivery system of domperidone with a natural mucoadhesive agent extracted from tamarind seeds.
An adult nasal cavity has about a 20 ml capacity with a large surface area (about 180 cm2) for the drug absorption afforded by the microvillus present along the pseudo-stratified columnar epithelia cells of the nasal mucosa. [2]
A new nasal drug delivery system of domperidone has been developed with a natural mucoadhesive agent from Tamarindus indica L. The mucoadhesive strength, viscosity and gelling property of this natural mucoadhesive agent was found to be higher in comparison to synthetic polymers.
Domperidone is a potent antiemetic, antimigraine drug effective for preventing different kinds of emesis. Its conventional dosage form such as tablet and suspension give poor bioavailability (18%) due to extensive first pass effect, whereas on rapid i.v. injectionit has been shown to cause cardiac arrhythmias [3]. In these conditions, the intranasal delivery seems to be an attractive alternative.
The large surface area of the nasal mucosa affords a rapid onset of therapeutic effect, potential for direct-to-central nervous system delivery, no first-pass metabolism, and non-invasiveness; all of which may maximize patient convenience, comfort, and compliance [4]. However, the limitations of a nasal delivery include: potential local tissue irritation; rapid mucociliary clearance of the therapeutic agent from the site of deposition resulting in a short span of time available for absorption; low permeability of the nasal membrane for the larger macromolecules; presence of proteolytic enzymes that may cause degradation in the nasal cavity; limited formulation manipulation for changing drug delivery profiles; and possible presence of pathological conditions such as colds or allergies which may alter nasal bioavailability [5]. Strategies to overcome these limitations include: the use of bioadhesive polymers that increase residence time of the formulation in the nasal cavity thereby improving absorption; the use of nontoxic enhancers to improve the permeability of the nasal membrane [6,7].
Materials and Methods
Chemicals:
Domperidone, acetone and other required materials provide by department of pharmaceutics MIET Meerut.
Plant material:

The seeds of Tamarind were purchased from local market, Ghanghauli, Super Noida, India. The seeds were identified and authenticated by MIET Meerut
Extraction and Isolation of Mucoadhesive Material.:
Tamarind seeds (250 g) were soaked in double distilled water at room temperature and then boiled with sufficient amount of double distilled water under stirring condition in a water bath until slurry was prepared. Then the slurry was cooled and kept in refrigerator overnight to settle out un-dissolved materials. The upper clear solution was decanted off and centrifuged at 1000 rpm for 30 minutes. The supernatant was separated and concentrated at 50-55º C on a water bath to a third of its original volume. Solution was cooled down to room temperature and was poured into thrice volume of acetone by continuous stirring. The precipitate was washed repeatedly with acetone and dried.
Determination of pH and Viscosity:
The pH and viscosity of 1% w/v solution of the tamarind seed extract were measured in Toshcon pH Meter and Toki sangyo Viscometer TV-10, respectively and values were compared with 1% w/v solutions of the synthetic polymers HPMC and carbopol 934.
Characterization of mucoadhesive property of tama-rind extract:
The mucoadhesive property of tamarind seed extract was determined by Shear stress method (8) and Park and Robinson Method (9) and results were compared with synthetic polymers. In this study 0.5% w/v solutions of the polymers were used for shear stress method and 1% w/v solutions of the polymers for Park & Robinson method.
Preparation of Domperidone Solution:
The domperidone solution was prepared by dissolving the drug in a little amount of water and diluting the solution with the nasal solution (11) (0.65% NaCl, 0.04% KH2PO4, 0.09% K2 HPO4 and 0.02% benzalkonium chloride).
Formation of Drug-Polymer Gel:
Domperidone was dissolved in small quantity of solvent. Nasal solution was added to the drug in a constant stirring condition. The required amount of tamarind extract or synthetic polymer (HPMC or carbopol 934) was added to the drug solution and stirred on a magnetic stirrer until the gel is formed.
Preparation of excised epithelial tissue from nasal mucosa:
Bovine nasal mucosa was obtained from the local slaughterhouse. After removing the skin, the nose was stored on ice in buffer solution during transport to the laboratory (10). The septum wall was fully exposed by a longitudinal incision through the lateral wall of the nose. The septum mucosa was carefully removed from the underlying bone by cutting along the whole septum and pulling the mucosa off the septum with homeostatic forceps. The cavity mucosa was also carefully removed from the conchae and lower cavity using homeostatic forceps after exposing the cavity each side of the septum. The mucosal tissues were then immediately immersed in Ringer’s Solution.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Result and discussion
Determination of pH and Viscosity:
The pH and viscosity of 1% w/v solution of the tamarind seed extract were measured in Toshcon pH Meter and Toki sangyo Viscometer TV-10, respectively and values were compared with 1% w/v solutions of the synthetic polymers HPMC and carbopol 934.
pH range was found between 3.0-7.2. The pH of tamarind extract varied from 6.0 to 7.2, whereas pH of HPMC and carbopol 934 were around 6.5 and 3.0 respectively. As the pH of nasal mucosa varied between 5.5-6.5, all the materials tested were found to be suitable for preparing nasal gels.
It was found that the viscosities of 1% w/v; tamarind extract, HPMC and Carbopol 934 ranged between 35 cp, 3 cp and 12 cp respectively. From the above experimental observations it was found that natural mucoadhesive tamarind extract exhibited a higher viscosity than synthetic polymers (HPMC and carbopol 934) at the same concentration.
Tamarind viscosity > Carbapol viscosity > HPMC viscosity
Characterization of mucoadhesive property of tama-rind extract:
The mucoadhesive property of tamarind seed extract was determined by Shear stress method (8) and Park and Robinson Method (9) and results were compared with synthetic polymers. In this study 0.5% w/v solutions of the polymers were used for shear stress method and 1% w/v solutions of the polymers for Park & Robinson method.
By Shear stress method
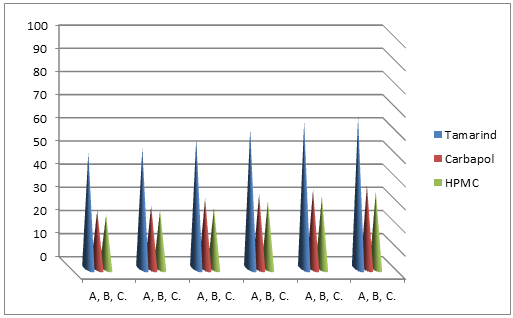
Fig. 2. Comparative result of bioadhesive property of the natural mucoadhesive extracts of Tamarind with synthetic polymers HPMC and carbopol 934 by shear stress method. All the polymers used in 0.5% w/v solution. Experimental temperature was maintained at 37°C±1. Values are expressed as the mean of 6 observations. Weight required (gm) vertical axis, time (minutes) horizontal.
By Park & Robinson method :
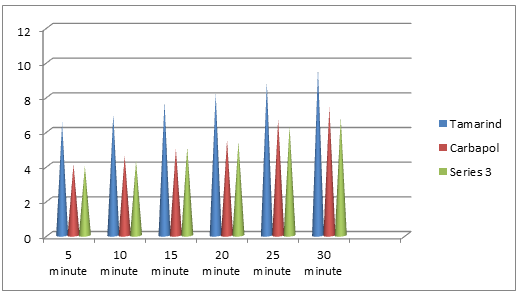
Fig-3. Comparative result of mucoadhesive property of the natural mucoadhesive extracts of Tamarind with synthetic polymers HPMC and carbopol 934 by Park & Robinson method. All the polymers used in 1% w/v solution. Experimental temperature was maintained at 37°C±1.
Release studies of drug from gel:
The franz diffusion cell (12) was used for the drug release study. The diffusion chamber with an exposed tissue surface area of 2.54cm2 is filled with 100 ml of phosphate buffer solution of pH 6.0. The excised nasal mucosal membrane was secured over the mouth of the upper tube keeping mucus side exposed to gel. For equilibration the mucosae were Pre-incubated with preheated buffer for ~30 min. Domperidone containing gel (1mg/ml) placed on the membrane were dispersed in 100 ml of phosphate buffer solution of pH 6.0 and stirred at a constant rate by a PTFE-coated magnetic bar at 600 min-1. Cells were kept under a constant oxycarbon flow (95% O2, 5% CO2). Throughout the studies the buffer solution in the chamber was maintained at 37°C connecting the franz diffusion cell with water bath. At pre-determined period of time 1ml of sample was taken and simultaneously replaced with same volume of pre-warmed (37°C) fresh buffer solution. The collected samples were diluted suitably and drug concentration estimated using Spectrophotometer.
In-vitro release study:
It was observed that 100% drug was released in case of natural mucoadhesive agent (tamarind) containing domperidone within 11-12 hours. (Fig. 4).

Conclusion:
Conclusion was reached that mucoadhesive agent extract from tamarind seed more suitable at low concentration than other mucoadhesive agent such as Carbapol, HMC. Natural mucoadhesive agent is edible, it is easily biodegradable and non-allergic like many synthetic agents used for similar purpose.Domperidone is given in the form of injection or tablet to many patients in hospitals and nursing homes. But these are not suitable due various factors such as first pass metabolism; clearance etc. and nasal gel formation is the best formulation for anti-emetic domperidone. This work will definitely add a new dimension to newer drug delivery systems that is nasal gel drug delivery system. So in future for domperidone tablet or injection will not require nasal gel gives sufficient absorption with very low toxic effect.
ACKNOWLEDGEMENT
I heartedly acknowledge D2 pharmaceutical’s for providing platform for conducting this research work. Dipin Kaushik, Aditi Singhal And Guru Mandela (researcher) Ghanghauli for spend the time in my practical work to check out the mistakes.
References
1. Kumar Dharmendra, Bansal Sumedha . Nasal Drug Delivery System: A Complete Review bioscience (Pharma tutor) 2013 Pharmatutor-ART-1593.
2. Kumar Dharmendra, Bansal mayank, Nasal Gel Formulation: A Review TGJPR 2012. Pg.303-310.
3. Tripathi K.D., Essentials of Medical Pharmacology. Newdelhi, 2008, pg. 496.
4. Shinde V., Mali K., Remeth J., Havaldar V., Mahajan N., Journal of Pharmacy Research. 2008 ,1, pg. 88-96.
5. Chien Y., Su K., Crit Rev Ther Drug Carr Syst: 1987, 4, pg. 67–194.
6. Bertram U., Bodmeier R., European Journal of Pharmaceutical Sciences.2006,27, pg. 62-71.
7. Rajpoot A.K et al Mucoadhesive, Thermosensitive Prologned Release Insitu Nasal Gel of Domperidone IJDFR volume 2011, p.g 201-224.
8. Rao YM, Vani G and Bala Ramesha Chary R. Design and evaluation of mucoadhesive drug delivery systems. Ind. Drugs. 1998; 35: pg. 558-565.
9. Park K & Robinson R, Bioadhesive platforms for oral-controlled drug delivery: method to study bioadhesion, Int. J. Pharm, 1984;19: pg.107-27.
10. Schmidt MC, Simmen D, Hilbe M, Boderke P, Ditzinger GN, Sandow JR, Lang S, Rubas W and Merkle HP. Validation of Excised Bovine Nasal Mucosa as In Vitro Model to Study Drug Transport and Metabolic Pathways in Nasal Epithelium. J. Pharm. Sci. 2000;89: pg. 396-407.
11. Loyd V, Allen Jr. Compounding Nasal Preparations. Secundum Artem. 1998;7:1-6.
12. Frantz SW. Instrumentation and methodology for in vitro skin diffusion cells in methodology for skin absorption. In: Methods for Skin Absorption (Kemppainen BW,Reifenrath WG, Eds), CRC Press, Florida, 1990: pg. 35-59.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









