 About Author:
About Author:
AJEET*
Department of Pharmaceutics
S. D. College of Pharmacy and Vocational Studies,
Muzaffarnagar, U.P. India Pin: 251001
*ajeet_pharma111@rediffmail.com
INTRODUCTION
Amongst the various routes of drug delivery, oral route is perhaps the most preferred to the patient and the clinician alike. However, peroral administration of drugs has disadvantages such as hepatic first pass metabolism and enzymatic degradation within the GI tract, that prohibit oral administration of certain classes of drugs especially peptides and proteins. Consequently, other absorptive mucosae are considered as potential sites for drug administration. Transmucosal routes of drug delivery (i.e., the mucosal linings of the nasal, rectal, vaginal, ocular, and oral cavity) offer distinct advantages over peroral administration for systemic drug delivery. These advantages include possible bypass of first pass effect, avoidance of presystemic elimination within the GI tract, and, depending on the particular drug, a better enzymatic flora for drug absorption.
The nasal cavity as a site for systemic drug delivery has been investigated by many research groups and the route has already reached commercial status with several drugs including LHRH and calcitonin. However, the potential irritation and the irreversible damage to the ciliary action of the nasal cavity from chronic application of nasal dosage forms, as well as the large intra- and inter-subject variability in mucus secretion in the nasal mucosa, could significantly affect drug absorption from this site. Even though the rectal, vaginal, and ocular mucosae all offer certain advantages, the poor patient acceptability associated with these sites renders them reserved for local applications rather than systemic drug administration.[1-12]
The oral cavity, on the other hand, is highly acceptable by patients, the mucosa is relatively permeable with a rich blood supply, it is robust and shows short recovery times after stress or damage, and the virtual lack of Langerhans cells makes the oral mucosa tolerant to potential allergens. Furthermore, oral transmucosal drug delivery bypasses first pass effect and avoids pre-systemic elimination in the GI tract. Within the oral mucosal cavity, delivery of drugs is classified into three categories: (i) sublingual delivery, which is systemic delivery of drugs through the mucosal membranes lining the floor of the mouth, (ii) buccal delivery, which is drug administration through the mucosal membranes lining the cheeks (buccal mucosa), and (iii) local delivery, which is drug delivery into the oral cavity.[13-16]
REFERENCE ID: PHARMATUTOR-ART-1286
[adsense:336x280:8701650588]
Overview of oral mucosa
Structure
The oral mucosa is composed of an outermost layer of stratified squamous epithelium. Below this lies a basement membrane, a lamina propria followed by the submucosa as the innermost layer. The epithelium is similar to stratified squamous epithelia found in the rest of the body in that it has a mitotically active basal cell layer, advancing through a number of differentiating intermediate layers to the superficial layers, where cells are shed from the surface of the epithelium. The epithelium of the buccal mucosa is about 40-50 cell layers thick, while that of the sublingual epithelium contains somewhat fewer. The epithelial cells increase in size and become flatter as they travel from the basal layers to the superficial layers.[17]
The turnover time for the buccal epithelium has been estimated at 5-6 days, and this is probably representative of the oral mucosa as a whole. The oral mucosal thickness varies depending on the site: the buccal mucosa measures at 500-800 µm, while the mucosal thickness of the hard and soft palates, the floor of the mouth, the ventral tongue, and the gingivae measure at about 100-200 µm. The composition of the epithelium also varies depending on the site in the oral cavity. The mucosae of areas subject to mechanical stress (the gingivae and hard palate) are keratinized similar to the epidermis. The mucosae of the soft palate, the sublingual, and the buccal regions, however, are not keratinized. The keratinized epithelia contain neutral lipids like ceramides and acylceramides which have been associated with the barrier function. These epithelia are relatively impermeable to water. In contrast, non-keratinized epithelia, such as the floor of the mouth and the buccal epithelia, do not contain acylceramides and only have small amounts of ceramide. They also contain small amounts of neutral but polar lipids, mainly cholesterol sulfate and glucosyl ceramides. These epithelia have been found to be considerably more permeable to water than keratinized epithelia.[18-21]
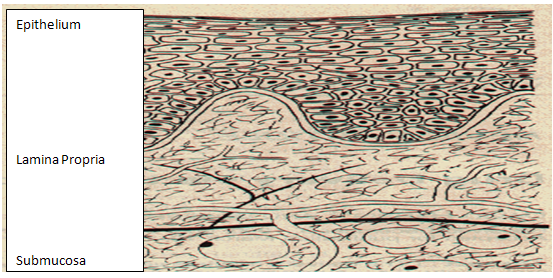
Figure 1. Structure of the oral mucosae[18]
Permeability
The oral mucosae in general is a somewhat leaky epithelia intermediate between that of the epidermis and intestinal mucosa. It is estimated that the permeability of the buccal mucosa is 4-4000 times greater than that of the skin. As indicative by the wide range in this reported value, there are considerable differences in permeability between different regions of the oral cavity because of the diverse structures and functions of the different oral mucosae. In general, the permeabilities of the oral mucosae decrease in the order of sublingual greater than buccal, and buccal greater than palatal. This rank order is based on the relative thickness and degree of keratinization of these tissues, with the sublingual mucosa being relatively thin and non-keratinized, the buccal thicker and non-keratinized, and the palatal intermediate in thickness but keratinized.[18,22]
It is currently believed that the permeability barrier in the oral mucosa is a result of intercellular material derived from the so-called ‘membrane coating granules’ (MCG). When cells go through differentiation, MCGs start forming and at the apical cell surfaces they fuse with the plasma membrane and their contents are discharged into the intercellular spaces at the upper one third of the epithelium. This barrier exists in the outermost 200µm of the superficial layer. Permeation studies have been performed using a number of very large molecular weight tracers, such as horseradish peroxidase and lanthanum nitrate. When applied to the outer surface of the epithelium, these tracers penetrate only through outermost layer or two of cells. When applied to the submucosal surface, they permeate up to, but not into, the outermost cell layers of the epithelium. According to these results, it seems apparent that flattened surface cell layers present the main barrier to permeation, while the more isodiametric cell layers are relatively permeable. In both keratinized and non-keratinized epithelia, the limit of penetration coincided with the level where the MCGs could be seen adjacent to the superficial plasma membranes of the epithelial cells. Since the same result was obtained in both keratinized and non-keratinized epithelia, keratinization by itself is not expected to play a significant role in the barrier function. The components of the MCGs in keratinized and non-keratinized epithelia are different, however. The MCGs of keratinized epithelium are composed of lamellar lipid stacks, whereas the non-keratinized epithelium contains MCGs that are non-lamellar. The MCG lipids of keratinized epithelia include sphingomyelin, glucosylceramides, ceramides, and other nonpolar lipids, however for non-keratinized epithelia, the major MCG lipid components are cholesterol esters, cholesterol, and glycosphingolipids. Aside from the MCGs, the basement membrane may present some resistance to permeation as well, however the outer epithelium is still considered to be the rate limiting step to mucosal penetration. The structure of the basement membrane is not dense enough to exclude even relatively large molecules.[19,23-24]
Buccal mucosa as site of Drug Delivery
There are three different categories of drug delivery within the oral cavity (i.e., sublingual, buccal, and local drug delivery). Selecting one over another is mainly based on anatomical and permeability differences that exist among the various oral mucosal sites. The sublingual mucosa is relatively permeable, giving rapid absorption and acceptable bioavailabilities of many drugs, and is convenient, accessible, and generally well accepted. The sublingual route is by far the most widely studied of these routes. Sublingual dosage forms are of two different designs, those composed of rapidly disintegrating tablets, and those consisting of soft gelatin capsules filled with liquid drug. Such systems create a very high drug concentration in the sublingual region before they are systemically absorbed across the mucosa. The buccal mucosa is considerably less permeable than the sublingual area, and is generally not able to provide the rapid absorption and good bioavailabilities seen with sublingual administration. Local delivery to tissues of the oral cavity has a number of applications, including the treatment of toothaches, periodontal disease, bacterial and fungal infections, aphthous and dental stomatitis, and in facilitating tooth movement with prostaglandins.[18,25-30]
Even though the sublingual mucosa is relatively more permeable than the buccal mucosa, it is not suitable for an oral transmucosal delivery system. The sublingual region lacks an expanse of smooth muscle or immobile mucosa and is constantly washed by a considerable amount of saliva making it difficult for device placement. Because of the high permeability and the rich blood supply, the sublingual route is capable of producing a rapid onset of action making it appropriate for drugs with short delivery period requirements with infrequent dosing regimen. Due to two important differences between the sublingual mucosa and the buccal mucosa, the latter is a more preferred route for systemic transmucosal drug delivery. First difference being in the permeability characteristics of the region where the buccal mucosa is less permeable and is thus not able to give a rapid onset of absorption (i.e. more suitable for a sustained release formulation). Second being that, the buccal mucosa has an expanse of smooth muscle and relatively immobile mucosa which makes it a more desirable region for retentive systems used for oral transmucosal drug delivery. Thus the buccal mucosa is more fitted for sustained delivery applications, delivery of less permeable molecules, and perhaps peptide drugs.[18,23]
Similar to any other mucosal membrane, the buccal mucosa as a site for drug delivery has limitations as well. One of the major disadvantages associated with buccal drug delivery is the low flux which results in low drug bioavailability. Various compounds have been investigated for their use as buccal penetration enhancers in order to increase the flux of drugs through the mucosa. Since the buccal epithelium is similar in structure to other stratified epithelia of the body, enhancers used to improve drug permeation in other absorptive mucosae have been shown to work in improving buccal drug penetration. Drugs investigated for buccal delivery using various permeation/absorption enhancers range in both molecular weight and physicochemical properties. Small molecules such as butyric acid and butanol, ionizable low molecular weight drugs such as acyclovir, propranolol, and salicylic acid, large molecular weight hydrophilic polymers such as dextrans, and a variety of peptides including octreotide, leutinizing hormone releasing hormone (LHRH), insulin, and a-interferon have all been studied.[31-40]
A series of studies on buccal permeation of buserelin and fluorescein isothiocyanate (FITC) labelled dextrans reported the enhancing effects of di- and tri-hydroxy bile salts on buccal penetration. Their results showed that in the presence of the bile salts, the permeability of porcine buccal mucosa to FITC increased by a 100-200 fold compared to FITC alone. The mechanism of penetration enhancement of FITC-labelled dextrans by sodium glycocholate (SGC) was shown to be concentration dependent. Below 10 mM SGC, buccal permeation was increased by increasing the intercellular transport and at 10 mM and higher concentrations by opening up a transcellular route. Gandhi and Robinson investigated the mechanisms of penetration enhancement of transbuccal delivery of salicylic acid. They used sodium deoxycholate and sodium lauryl sulfate as penetration enhancers, both of which were found to increase the permeability of salicylic acid across rabbit buccal mucosa. Their results also supported that the superficial layers and protein domain of the epithelium may be responsible for maintaining the barrier function of the buccal mucosa.[36-37,41-42]
[adsense:468x15:2204050025]
Buccal Drug Delivery Systems
Other than the low flux associated with buccal mucosal delivery, a major limitation of the buccal route of administration is the lack of dosage form retention at the site of absorption. Consequently, bioadhesive polymers have extensively been employed in buccal drug delivery systems. Bioadhesive polymers are defined as polymers that can adhere onto a biological substrate. The term mucoadhesion is applied when the substrate is mucosal tissue. Polymers which can adhere to either hard or soft tissue have been used for many years in surgery and dentistry. Diverse classes of polymers have been investigated for their potential use as mucoadhesives. These include synthetic polymers such as monomeric a cyanoacrylate, polyacrylic acid, hydroxypropyl methylcellulose, and poly methacrylate derivatives as well as naturally occurring polymers such as hyaluronic acid and chitosan. Other synthetic polymers such as polyurethanes, epoxy resins, polystyrene, and natural-product cement have also been extensively investigated.[17,27,51-55]
In general, dosage forms designed for buccal administration should not cause irritation and should be small and flexible enough to be accepted by the patient. These requirements can be met by using hydrogels. Hydrogels are hydrophilic matrices that are capable of swelling when placed in aqueous media. Normally, hydrogels are crosslinked so that they would not dissolve in the medium and would only absorb water. When drugs are loaded into these hydrogels, as water is absorbed into the matrix, chain relaxation occurs and drug molecules are released through the spaces or channels within the hydrogel network. In a more broad meaning of the term, hydrogels would also include water-soluble matrices that are capable of swelling in aqueous media, these include natural gums and cellulose derivatives. These ‘pseudo-hydrogels’ swell infinitely and the component molecules dissolve from the surface of the matrix. Drug release would then occur through the spaces or channels within the network as well as through the dissolution and/or the disintegration of the matrix. The use of hydrogels as adhesive preparations for transmucosal drug delivery has acquired considerable attention in recent years.[56]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
REVIEW OF LITERATURE
Parikj Bhavik Anjankumar (2011) has designed and evaluated mucoadhesive bi-layered buccal devices comprising a drug containing mucoadhesive layer and a drug free backing membrane. Bilaminated films composed of mixture of drug (Valsartan) and chitosan, with hydroxyl- propylmethylcellulose (15 cps) and backing layer (ethyl cellulose). Films were fabricated by solvent casting technique and were evaluated for thickness, drug content uniformity, bio-adhesion strength, percent, swelling index, folding endurance and in vitro drug release. A combination of chitosan and hydroxylpropylmethylcellulose (1:1) using propylene glycol (50% by weight of polymer) as plasticizer gave promising results. The optimized film exhibited an In vitro drug release of approximately 90% in 5 hrs along with satisfactory bio-adhesive strength[57].
Rohit Chaudhary et. al. (2010) has designed and evaluated mucoadhesive bilayered buccal devices comprising a drug containing mucoadhesive layer and a drug free backing membrane. Bilaminatd patches composed of mixture of drug (Methotrexate) and sodium alginate alone or in combination with sodium carboxy methylcellulose ,Polyvinylpyrrolidine and carbopol 934 and backing membrane (Ethyl cellulose).The patches were fabricated by solvent casting technique and were evaluated for In-Vitro and Ex-Vivo drug release. The patches were evaluated for film weight uniformity, thickness, swelling index, surface pH, mucoadhesive strength and mucoadhesive time and folding endurance. A combination of sodium alginate with carbopol-934 and glycerol as plasticizer gives promising results. The optimized patch exhibit an in vitro release of 82% through cellophane membrane and 70.78 % through buccal mucosa with satisfactory mucoadhesive strength and mucoadhesive time.[58]
G.A. Khairnar and F. J. Sayyad (2010) reviewed the highlights of development of mucoadhesive polymers in buccal drug delivery. Buccal delivery of the desired drug using mucoadhesive polymers has been the subject of interest since the early 1980s. Advantages associated with buccal drug delivery have rendered this route of administration useful for a variety of drugs. This article covers the anatomy of oral mucosa, mechanism of drug permeation, characteristics and properties of the desired polymers, new generation of the mucoadhesive polymers[59].
A polymeric film composed of Carbopol, Poloxamer and hydroxypropyl methylcellulose was prepared by Myung Kwan Chun et. al. (2010) to develop a buccal patch and the effects of composition of the film on adhesion time, swelling ratio, and dissolution of the film were studied. The effects of plasticizers or penetration enhancers on the release of triamcinolone acetonide (TAA) were also studied. The hydrogen bonding between Carbopol and Poloxamer played important role in reducing swelling ratio and dissolution rate of polymer film and increasing adhesion time. The swelling ratio of the composite film was significantly reduced and the adhesion time was increased when compared with Carbopol film. As the ratio of Poloxamer to hydroxypropyl methylcellulose increased from 0/66 to 33/33, the release rate of TAA decreased. However, no further significant decrease of release rate was observed beyond the ratio of 33/33. The release rate of TAA in the polymeric film containing polyethylene glycol 400, a plasticizer, showed the highest release rate followed by triethyl citrate, and castor oil. The release rate of TAA from the polymeric film containing permeation enhancers was slower than that from the control without enhancers. Therefore, these observations indicated that a preparation of a buccal patch is feasible with the polymeric film composed of Cabopol, Poloxamer and hydropropyl methylcellulose.[60]
Buccal patches for the delivery of atenolol using sodium alginate with various hydrophilic polymers like carbopol 934 P, sodium carboxymethyl cellulose, and hydroxypropyl methylcellulose in various proportions and combinations were fabricated by Surya Adhikari et. al. (2010) using solvent casting technique. Various physicomechanical parameters like weight variation, thickness, folding endurance, drug content, moisture content, moisture absorption, and various ex vivo mucoadhesion parameters like mucoadhesive strength, force of adhesion, and bond strength were evaluated. An in vitro drug release study was designed, and it was carried out using commercial semipermeable membrane. All these fabricated patches were sustained for 24 h and obeyed first-order release kinetics. Ex vivo drug permeation study was also performed using porcine buccal mucosa, and various drug permeation parameters like flux and lag time were determined.[61]
Bilayer nicotine mucoadhesive patches were prepared and evaluated by Rana Abu Huwaij (2010) to determine the feasibility of the formulation as a nicotine replacement product to aid in smoking cessation. Nicotine patches were prepared using xanthan gum or carbopol 934 as a mucoadhesive polymers and ethyl cellulose as a backing layer. The patches were evaluated for their thickness, weight and content uniformity, swelling behavior, drug–polymers interaction, adhesive properties, and drug release. The physicochemical interactions between nicotine and the polymers were investigated by Fourier transform infrared (FTIR) spectroscopy. Mucoadhesion was assessed using two-arm balance method, and the in vitro release was studied using the Franz cell. FTIR revealed that there was an acid base interaction between nicotine and carbopol as well as nicotine and xanthan. Interestingly, the mucoadhesion and in vitro release studies indicated that this interaction was strong between the drug and carbopol whereas it was weak between the drug and xanthan. Loading nicotine concentration to non-medicated patches showed a significant decrease in the mucoadhesion strength of carbopol patches and no significant effect on the mucoadhesion strength of xanthan patches. In vitro release studies of the xanthan patches showed a reasonable fast initial release profile followed by controlled drug release over a 10-h period.[62]
The aim of Gazzi Shanker et. al. (2010) was concerned with formulation and evaluation of bioadhesive buccal drug delivery of tizanidine hydrochloride tablets, which is extensively metabolized by liver. The tablets were prepared by direct compression using bioadhesive polymers such as hydroxylpropyl methylcellulose K4M, sodium carboxymethyl cellulose alone, and a combination of these two polymers. In order to improve the permeation of drug, different permeation enhancers like beta-cyclodextrin (β-CD), hydroxylpropyl beta-cyclodextrin (HP-β-CD), and sodium deoxycholate (SDC) were added to the formulations. The β-CD and HP-β-CD were taken in 1:1 molar ratio to drug in formulations. Bioadhesion strength, ex vivo residence time, swelling, and in vitro dissolution studies and ex vivo permeation studies were performed. In vitro release of optimized bioadhesive buccal tablet was found to be non-Fickian. SDC was taken in 1%, 2%, and 3% w/w of the total tablet weight. Stability studies in natural saliva indicated that optimized formulation has good stability in human saliva. In vivo mucoadhesive behavior of optimized formulation was performed in five healthy male human volunteers and subjective parameters were evaluated.[63]
Deelip Derle et. al. (2009) has formulated and evaluated mucoadhesive bi-layer buccal tablets of propranolol hydrochloride tablets using the bioadhesive polymers such as sodium alginate and carbopol 971 P along with ethyl cellulose as an impermeable backing layer. The tablets were evaluated for weight variation, thickness, hardness, friability, surface pH, mucoadhesive strength, swelling index, in vitro drug release. Tablets containing sodium alginate and carbopol 971 P in the ratio of 5:1 showed the maximum percentage of in vitro drug release without disintegration in 12 hours. The swelling index was proportional to sodium alginate content and inversely proportional to carbopol 971 P content. The surface pH of all tablets was found to be satisfactory, close to neutral pH; hence, no irritation would observe with these tablets. The mechanism of drug release was found to be zero-order kinetics.[64]
Subhash V. Deshmane et. al. (2009) have the objective of present work was to characterize the effect of chitosan with PVP K-30 on water soluble drug by preparing mucoadhesive buccal patch. Each formulated batch was subjected to various evaluation parameters. The swelling percentage was found to be function of solubility of drug and PVP K-30. The mucoadhesive strength, vapour transmission and in-vitro released of water soluble drug through water insoluble chitosan base matrix were found satisfactorily. The physical appearance of buccal patch was examined by scanning electron microscopy. The released kinetic model best to fit for the optimized batch was Hixson Crowell, indicating that the drug release from systems in which there is a change in the surface area and the diameter of particles present in dosage form[65].
Chandra Sekhar Kolli et. al. (2008) have the aim of this investigation was to develop and evaluate mucoadhesive buccal patches of prochlorperazine (PCPZ). Permeation of PCPZ was calculated in vitro using porcine buccal membrane. Buccal formulations were developed by solvent-casting technique using hydroxy propylmethyl cellulose (HPMC) as mucoadhesive polymer. The patches were evaluated for in vitro release, moisture absorption and mechanical properties. The optimized formulation, based on in vitro release and moisture absorption studies, was subjected for bioadhesion studies using porcine buccal membrane. In vitro flux of PCPZ was calculated to be 2.14 ± 0.01 µg. h –1 .cm –2 and buccal absorption was also demonstrated in vivo in human volunteers. In vitro drug release and moisture absorbed was governed by HPMC content. Increasing concentration of HPMC delayed the drug release. All formulations followed Zero order release kinetics whereas the release pattern was non-Fickian. The mechanical properties, tensile strength (10.28 ± 2.27 kg mm –2 for formulation P3) and elongation at break reveal that the formulations were found to be strong but not brittle. The peak detachment force and work of adhesion for formulation P3 were 0.68 ± 0.15 N and 0.14 ± 0.08 mJ, respectively. The results indicate that suitable bioadhesive buccal patches of PCPZ with desired permeability and suitable mechanical properties could be prepared.[66]
Supriya Sidhaye et. al. (2008) have the purpose of this study was to develop and optimize formulations of mucoadhesive bilayered buccal patches of sumatriptan succinate using chitosan as the base matrix. The patches were prepared by the solvent casting method. Gelatin and polyvinyl pyrrolidone (PVP) K30 were incorporated into the patches, to improve the film properties of the patches. The patches were found to be smooth in appearance, uniform in thickness, weight, and drug content; showed good mucoadhesive strength; and good folding endurance. A 32 full factorial design was employed to study the effect of independent variables viz. levels of chitosan and PVP K30, which significantly influenced characteristics like swelling index, in-vitro mucoadhesive strength, in vitro drug release, and in-vitro residence time. Different penetration enhancers were tried to improve the permeation of sumatriptan succinate through buccal mucosa. Formulation containing 3% dimethyl sulfoxide showed good permeation of sumatriptan succinate through mucosa. Histopathological studies revealed no buccal mucosal damage. It can be concluded that buccal route can be one of the alternatives available for administration of sumatriptan succinate.[67]
A buccal patch for systemic administration of carvedilol in the oral cavity has been developed by Y. Vamshi Vishnu et. al. (2007) using two different mucoadhesive polymers. The formulations were tested for in vitro drug permeation studies, buccal absorption test, in vitro release studies, moisture absorption studies and in vitro bioadhesion studies. The physicochemical interactions between carvedilol and polymers were investigated by Fourier transform infrared (FTIR) Spectroscopy. According to FTIR the drug did not show any evidence of an interaction with the polymers used and was present in an unchanged state. XRD studies reveal that the drug is in crystalline state in the polymer matrix. The results indicate that suitable bioadhesive buccal patches with desired permeability could be prepared. Bioavailability studies in healthy pigs reveal that carvedilol has got good buccal absorption. The bioavailability of carvedilol from buccal patches has increased 2.29 folds when compared to that of oral solution. The formulation AC5 (HPMC E 15) shows 84.85 + 0.089% release and 38.69 + 6.61% permeated through porcine buccal membrane in 4 hr. The basic pharmacokinetic parameters like the C max , T max and AUC total were calculated and showed statistically significant difference (P<0.05) when given by buccal route compared to that of oral solution[68].
Mucoadhesive buccal patches containing propranolol hydrochloride were prepared by Vishnu M. Patel (2007) using the solvent casting method. Chitosan was used as bioadhesive polymer and different ratios of chitosan to PVP K-30 were used. The patches were evaluated for their physical characteristics like mass variation, drug content uniformity, folding endurance, ex vivo mucoadhesion strength, ex vivo mucoadhesion time, surface pH, in vitro drug release, and in vitro buccal permeation study. Patches exhibited controlled release for a period of 7 h. The mechanism of drug release was found to be non-Fickian diffusion and followed the first-order kinetics. Incorporation of PVP K-30 generally enhanced the release rate. Swelling index was proportional to the concentration of PVP K-30. Optimized patches (F4) showed satisfactory bioadhesive strength of 9.6 ± 2.0 g, and ex vivo mucoadhesion time of 272 minutes. The surface pH of all patches was between 5.7 and 6.3 and hence patches should not cause irritation in the buccal cavity. Patches containing 10 mg of drug had higher bioadhesive strength with sustained drug release as compared to patches containing 20 mg of drug. Good correlation was observed between the in vitro drug release and in vitro drug permeation with a correlation coefficient of 0.9364. Stability study of optimized patches was done in human saliva and it was found that both drug and buccal patches were stable.[69]
Vishnu Patel et. al. (2007) have developed formulations and systematically evaluated in vitro performances of buccoadhesive patches of propranolol hydrochloride using the hydrophobic polymer Eudragit L-100 as the base matrix. The hydrophilic polymers Carbopol 934 and polyvinyl pyrrolidone (PVP) K30 were incorporated into the Eudragit patches, to provide the patches with bioadhesive properties and to modify the rate of drug release. The patches, which were prepared by the solvent casting method, were smooth and elegant in appearance; were uniform in thickness, weight, and drug content; showed no visible cracks; and showed good folding endurance. A 32 full factorial design was employed to study the effect of independent variables like hydrophilic polymers Carbopol 934 and PVP K30, which significantly influenced characteristics like swelling index, ex vivo mucoadhesive strength, in vitro drug release, and ex vivo residence time. A stability study of optimized Eudragit patches was done in natural human saliva; it was found that both drug and buccal patches were stable in human saliva. It can be concluded that the present buccal formulation can be an ideal system to improve the bioavailability of the drug by avoiding hepatic first-pass metabolism.[70]
A novel delivery system has been developed by Marta Corbonits et. al (2004) for testosterone replacement.This formulation, COL-1621 (Striant), a testosterone-containingbuccal mucoadhesive system, has been shown in preliminary studiesto replace testosterone at physiological levels when used twicedaily. Therefore, the current study compared the steady-statepharmacokinetics and tolerability of the buccal system witha testosterone-containing skin patch (Andropatch or Androderm)in an international multicenter study of a group of hypogonadalmen.
Sixty-six patients were randomized into two groups; one appliedthe buccal system twice daily, whereas the other applied thetransdermal patch daily, in each case for 7 d. Serum total testosteroneand dihydrotestosterone concentrations were measured at d 1,3 or 4, and 6, and serially over the last 24 h of the study. Pharmacokineticparameters for each formulation were calculated, and the twogroups were compared. The tolerability of both formulationswas also evaluated.
Thirty-three patients were treated with the buccal preparation,and 34 were treated with the transdermal patch. The averageserum testosterone concentration over 24 h showed a mean of18.74 nmol/liter (SD =; 5.90) in the buccal system group and12.15 nmol/liter (SD =; 5.55) in the transdermal patch group (P< 0.01). Of the patients treated with the buccal system,97% had average steady-state testosterone concentrations withinthe physiological range (10.41–36.44 nmol/liter), whereasonly 56% of the transdermal patch patients achieved physiologicaltotal testosterone concentrations (P < 0.001 between groups).Testosterone concentrations were within the physiological rangein the buccal system group for a significantly greater portionof the 24-h treatment period than in the transdermal patch group(mean, 84.9% vs. 54.9%; P < 0.001). Testosterone/dihydrotestosteroneratios were physiological and similar in both groups. Few patientsexperienced major adverse effects from either treatment. Nosignificant local tolerability problems were noted with thebuccal system, other than a single patient withdrawal. We concludethat this buccal system is superior to the transdermal patch inachieving testosterone concentrations within the normal range.It may, therefore, be a valuable addition to the range of choicesfor testosterone replacement therapy.[71]
Mucoadhesive patches for delivery of salbutamol sulphate were prepared by L. Panigrahi et. al. (2004) using polyvinyl alcohol, hydroxypropylmethyl cellulose and chitosan. Mechanical property, swelling and bioadhesive characteristics were detemined for both plain and medicated patches. Mechanical properties were determined in presence of carbopol and polyvinylpyrrolidone. The results showed an increase in swelling after addition of salbutamol sulphate to the plain formulation. This was attributed that the salbutamol sulphate modifies the way water is bound to or taken by the polymer. A decrease in residual time was observed for polyvinyl alcohol and citosan containing formula. High drug release was obtained from polyvinyl alcohol compared to the hydroxypropylmethylcellulose. Physical characteristics of the studied patches showed promising with good bioadhesion[72].
Deirdre faye Vaughan (2003) have the purpose of this study was to establish pharmacokinetic parameters and the bioavailability of albuterol and butorphanol when administered intravenously and buccally. Three dogs weighing 20 kg were studied. Each received albuterol and butorphanol by buccal and intravenous administration. Blood samples were collected and analyzed by ELISA. Values for pharmacokinetic parameters were determined using non-compartmental modeling.
For albuterol, extrapolated C max and C o after buccal and IV administration were 10.28 ± 2.77 and 57.74 ± 9.04 ng/ml, respectively. Volume of distribution was 2.13 ± 1.30 L/kg and clearance was 4.73 ± 3.91 ml/min/kg. A significant difference existed between the disappearance rate constant of buccal and intravenous albuterol administration. The half-lives of buccal and IV albuterol were 160.96 ± 24.19 and 364.20 ± 115.20 min, respectively. The bioavailability of buccally administered albuterol was 35%. Maximal concentration (C max ) and C o after buccal and IV butorphanol administration were 6.66 ± 1.65 and 8.24 ± 5.55 ng/ml, respectively. Volume of distribution was 27.58 ± 10.14 L/kg and Cl was 137.87 ± 19.55 ml/min/kg. The half-life of buccally administered butorphanol was 259.15 ± 33.12 min and 172.12 ± 94.95 min for intravenous butorphanol. The bioavailability of buccally administered butorphanol was 606%. The buccal patch used in this study achieved systemic concentrations for both albuterol and butorphanol. Further studies are needed to determine if therapeutic drug concentrations can be achieved with the buccal patch and if the patch can result in clinical efficacy.[73]
Mucoadhesive patches for delivery of cetylpyridinium chloride (CPC) were prepared by Noha adel nafee (2003) using polyvinyl alcohol (PVA), hydroxyethyl cellulose (HEC) and chitosan. Swelling and bioadhesive characteristics were determined for both plain and medicated patches. The results showed a remarkable increase in radial swelling (S D) after addition of the water-soluble drug (CPC) to the plain formulae. A decrease in the residence time was observed for PVA and chitosan containing formulae. Higher drug release was obtained from PVA patches compared to HEC ones, while both are non-ionic polymers. A considerable drop in release was observed for chitosan formulae after the addition of water-soluble additives, polyvinyl pyrrolidone (PVP) and gelatin. Ageing was done on PVA formulae; the results showed there was no influence on the chemical stability of CPC, as reflected from the drug content data. Physical characteristics of the studied patches showed an increase in the residence time with storage accompanied with a decrease in drug release. This may be due to changes in the crystal habit of the drug as well as to slight agglomeration of the polymer particles.[74]
Within the oral mucosal cavity, the buccal region offers an attractive route of administration for systemic drug delivery. The mucosa has a rich blood supply and it is relatively permeable. Amir H. Shojaei (1998) have the objective of this article to review buccal drug delivery by discussing the structure and environment of the oral mucosa and the experimental methods used in assessing buccal drug permeation/absorption. Buccal dosage forms will also be reviewed with an emphasis on bioadhesive polymeric based delivery systems.
Amongst the various routes of drug delivery, oral route is perhaps the most preferred to the patient and the clinician alike. However, peroral administration of drugs has disadvantages such as hepatic first pass metabolism and enzymatic degradation within the GI tract, that prohibit oral administration of certain classes of drugs especially peptides and proteins. Consequently, other absorptive mucosae are considered as potential sites for drug administration. Transmucosal routes of drug delivery (i.e., the mucosal linings of the nasal, rectal, vaginal, ocular, and oral cavity) offer distinct advantages over peroral administration for systemic drug delivery. These advantages include possible bypass of first pass effect, avoidance of presystemic elimination within the GI tract, and, depending on the particular drug, a better enzymatic flora for drug absorption.
The nasal cavity as a site for systemic drug delivery has been investigated by many research groups and the route has already reached commercial status with several drugs including LHRH and calcitonin. However, the potential irritation and the irreversible damage to the ciliary action of the nasal cavity from chronic application of nasal dosage forms, as well as the large intra- and inter-subject variability in mucus secretion in the nasal mucosa, could significantly affect drug absorption from this site. Even though the rectal, vaginal, and ocular mucosae all offer certain advantages, the poor patient acceptability associated with these sites renders them reserved for local applications rather than systemic drug administration. The oral cavity, on the other hand, is highly acceptable by patients, the mucosa is relatively permeable with a rich blood supply, it is robust and shows short recovery times after stress or damage, and the virtual lack of Langerhans cells makes the oral mucosa tolerant to potential allergens. Furthermore, oral transmucosal drug delivery bypasses first pass effect and avoids pre-systemic elimination in the GI tract. These factors make the oral mucosal cavity a very attractive and feasible site for systemic drug delivery.
Within the oral mucosal cavity, delivery of drugs is classified into three categories: (i) sublingual delivery, which is systemic delivery of drugs through the mucosal membranes lining the floor of the mouth, (ii) buccal delivery, which is drug administration through the mucosal membranes lining the cheeks (buccal mucosa), and (iii) local delivery, which is drug delivery into the oral cavity.[75]
C. Li et. al. (1998) have assessed the bioadhesive properties of several different mucoadhesive buccal patches. The patches consisted of custom coformulations of silicone polymers and Carbopol 974P. The contact angle of water was measured for each of the test formulations, using an ophthalmic shadow scope. The corresponding work of adhesion between the water and the patches (W1), and between the patches and freshly-excised rabbit buccal mucosa (W2) was then calculated, using a modification of Dupre's equation. The bioadhesive strength between the patches and excised rabbit buccal mucosa was also assessed. The results of the contact-angle measurements indicated that the contact angle decreased with an increase in the amount of Carbopol in the formulation. Additionally, the calculated values of both W1 and W2 increased with an increase in the amount of Carbopol in the buccal-patch formulations. A correlation (r not equal to 0.9808) was found between the measured contact angle and the calculated values for W2. The direct measurement of the force required to separate a buccal patch from excised rabbit buccal mucosa with the INSTRON demonstrated that the adhesive strength increased with an increase in the amount of Carbopol. This preliminary study has shown that the measurement of contact angles alone may provide a useful technique for estimating the work of adhesion, and may serve as a convenient and rapid screening procedure to identify potential mucoadhesive buccal-patch formulations[76].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table 1. List of compounds used as oral mucosal permeation enhancers
Permeation Enhancer
· 23-lauryl ether
· Aprotinin
· Azone
· Benzalkonium chloride
· Cetylpyridinium chloride
· Cetyltrimethylammonium bromide
· Cyclodextrin
· Dextran sulfate
· Lauric acid
· Lauric acid/Propylene glycol
· Lysophosphatidylcholine
· Menthol
· Methoxysalicylate
· Methyloleate
· Oleic acid
· Phosphatidylcholine
· Polyoxyethylene
· Polysorbate 80
· Sodium EDTA
· Various alkyl glycosides
· Sodium glycodeoxycholate
· Sodium lauryl sulfate
· Sodium salicylate
· Sodium taurocholate
· Sodium taurodeoxycholate
· Sulfoxides
· Sodium glycocholate
Table 2.Related research on mucoadhesive polymers and delivery systems
|
Bioadhesive Polymers Studied |
Investigation Objectives |
|
HPC and CP |
Preferred mucoadhesive strength on CP, HPC, and HPC-CP combination |
|
CP, HPC, PVP, CMC |
Studied inter polymer complexation and its effects on bioadhesive strength |
|
CP, MC and HPMC |
Formulation and evaluation of buccoadhesive controlled release delivery systems |
|
HPC, HEC, PVP, and PVA |
Tested mucosal adhesion on patches with two-ply laminates with an impermeable backing layer and hydrocolloid polymer layer |
|
HPC and CP |
Used HPC-CP powder mixture as peripheral base for strong adhesion and HPC-CP freeze dried mixture as core base |
|
CP, PIP, and PIB |
Used a two roll milling method to prepare a new bioadhesive patch formulation |
|
Xanthum gum and Locust bean gum |
Hydrogel formation by combination of natural gums |
|
Chitosan, HPC, CMC, Pectin, Xantham gum, and Polycarbophil |
Evaluate mucoadhesive properties by routinely measuring the detachment force form pig intestinal mucosa |
|
Hyaluronic acid benzyl esters, Polycarbophil, and HPMC |
Evaluate mucoadhesive properties |
|
Hydroxyethylcellulose |
Design and synthesis of a bilayer patch (polytef-disk) for thyroid gland diagnosis |
|
Polycarbophil |
Design of a unidirectional buccal patch for oral mucosal delivery of peptide drugs |
|
Poly(acrylic acid) and Poly(methacrylic acid) |
Synthesized and evaluated crosslinked polymers differing in charge densities and hydrophobicity |
|
Number of Polymers including HPC, HPMC, CP, CMC. |
Measurement of bioadhesive potential and to derive meaningful information on the structural requirement for bioadhesion |
|
Poly(acrylic acid-co-acrylamide) |
Adhesion strength to the gastric mucus layer as a function of crosslinking agent, degree of swelling, and carboxyl group density |
|
Poly(acrylic acid) |
Effects of PAA molecular weight and crosslinking concentration on swelling and drug release characteristics |
|
Poly(acrylic acid-co-methyl methacrylate) |
Effects of polymer structural features on mucoadhesion |
|
Poly(acrylic acid-co- butylacrylate) |
Relationships between structure and adhesion for mucoadhesive polymers |
|
HEMA copolymerized with Polymeg® (polytetramethylene glycol) |
Bioadhesive buccal hydrogel for controlled release delivery of buprenorphine |
|
Cydot® by 3M (bioadhesive polymeric blend of CP and PIB) |
Patch system for buccal mucoadhesive drug delivery |
|
Formulation consisting of PVP, CP, and cetylpyridinium chloride (as stabilizer) |
Device for oramucosal delivery of LHRH - device containing a fast release and a slow release layer |
|
CMC, Carbopol 974P, Carbopol EX-55, Pectin (low viscosity), Chitosan chloride, |
Mucoadhesive gels for intraoral delivery |
|
CMC, CP, Polyethylene oxide, Polymethylvinylether/Maleic anhydride (PME/MA), and Tragacanth |
Buccal mucoadhesive device for controlled release anticandidal device - CMC tablets yielded the highest adhesive force |
|
HPMC and Polycarbophil (PC) |
Buccal mucoadhesive tablets with optimum blend ratio of 80:20 PC to HPMC yielding the highest force of adhesion |
|
PVP, Poly(acrylic acid) |
Transmucosal controlled delivery of isosorbide dinitrate |
Abbreviations: CP = Carbopol 934P, HPC = Hydroxy propyl cellulose, PVP = Poly(vinyl pyrrolidone), CMC = Sodium carboxymethyl cellulose, HPMC = Hydroxy propyl methyl cellulose, HEC = Hydroxy ethyl cellulose, PVA = Poly(vinyl alcohol), PIB = Poly(isobutylene), PIP = Poly(isoprene), MC = Methyle cellulose
OBJECTIVE OF THE WORK
1. Optimization of concentration of mucoadhesive polymer.
2. Optimization of solvent.
3. Evaluation of selected prepared mucoadhesive buccal patches.
PLAN OF THE WORK
1. Optimization of formulation (Trial and error basis)
2. Evaluation of optimized formulation
I. Patch weight
II. Patch thickness
III. Folding endurance
IV. Surface pH
V. Swelling index
VI. Mucoadhesive time
EXPERIMENTAL WORK
Materials and methods
Methyle cellulose, Polyvinylpyrolidone (PVP) K30, Polyethylene glycol (PEG) 4000 were purchased from Central Drug House (P) Ltd. New Delhi, CDH Laboratory. Methanol and Acetic acid were purchased from Samir Tech-chem Pvt. Ltd., Vadodara.
The mucoadhesive buccal patches were prepared by Solvent casting method. The weighed and measured quantity of Methyle Cellulose/H.P.M.C., P.V.P. and/or P.E.G. and glycerin (in required patches) were taken in solvent i.e. Methanol/Acetic acid in beaker and the mixture was stirred for 5 to 10 minutes, then the dispersion was kept for overnight. Next day the dispersion was poured into petridish and kept on level surface and left untouched for 24 hour.
After changing the solvent instead of methanol/acetic acid we took water. The weighed and measured quantity of Methyle Cellulose, P.V.P. and Glycerin were taken in solvent i.e. Water in beaker and the mixture was stirred for about 15 minutes. Then dispersion was kept untouched for about 2 hours, then poured it into Petridish and kept it in oven at 40 0C to 45 0C for about 6 to 7 hours.
Table 3: Buccal Patch Formulations
|
Formulations |
Units |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
F9 |
F10 |
F11 |
F12 |
|
Methyle cellulose |
(mg) |
300 |
300 |
400 |
400 |
450 |
500 |
500 |
540 |
700 |
800 |
- |
500 |
|
H.P.M.C. |
(mg) |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
500 |
- |
|
P.V.P. K30 |
(mg) |
100 |
150 |
100 |
100 |
160 |
- |
180 |
220 |
250 |
- |
200 |
180 |
|
P.E.G. 400 |
(mg) |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
200 |
- |
|
Methanol |
(ml) |
40 |
20 |
20 |
20 |
30 |
40 |
20 |
30 |
40 |
40 |
- |
- |
|
Distilled water |
(ml) |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
20 |
|
Acetic acid (5 %) |
(ml) |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
20 |
- |
|
Glycerin |
(ml) |
- |
2 |
2 |
- |
2 |
- |
2 |
2 |
2 |
- |
- |
1 |
P.V.P.- Polyvinyl Pyrollidone, P.E.G.- Polyethylene glycol, H.P.M.C.- Hydroxypropylene methyl cellulose
Table 4 : Procedures and observations
|
|
Procedure |
Observations |
|
F1 to F11 |
The weighed and measured quantity of Methyle Cellulose/H.P.M.C., P.V.P. and/or P.E.G. and glycerine (in required patches) were taken in solvent i.e. Methanol/Acetic acid in beaker and the mixture was stirred for 5 to 10 minutes, then the dispersion was kept for overnight. Next day the dispersion was poured into petridish and kept on level surface and left untouched for 24 hour[58,59]. |
F1-Patch formed with slippery surface, brittle nature and good transparency. F2- Patch formed with smooth surface, cracking and no transparency. F3- Patch formed with rough surface, cracking and no transparency. F4-It was completely stuck to the petridish with rough surface with no transparency. F5- Patch formed with smooth surface, cracking and no transparency. F6- Patch formed with slippery surface, hard & brittle with a little transparency. F7- Patch formed with smooth surface and good physical characteristic like folding endurance. F8- Patch formed with rough surface, cracking, no transparency and with smooth tearing. F9- Patch formed with soft rough surface, cracking and no transparency F10- Very thick patch formed with cracking, no transparency but with good folding endurance. F11- No patch formation has been taken, its physical characteristic was very very viscous gel with complete transparency.
|
|
F12 |
The weighed and measured quantity of Methyle Cellulose, P.V.P. and Glycerin were taken in solvent i.e. Water in beaker and the mixture was stirred for about 15 minutes. Then dispersion was kept untouched for about 2 hours, then poured it into Petridish and kept it in oven at 40 0C to 45 0C for about 6 to 7 hours.
|
The patches were formed with good physical appearance, transparency and very good folding endurance. |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Evaluation of mucoadhesive buccal patches[58]
Film weight
For evaluation of film weight, 8 films of (2×2cm2) from each formulation were taken and weighed individually on a digital balance. The results were analyzed for mean and standard deviation.
Thickness
For evaluation of thickness, 8 films (2×2cm2) of each formulation were taken and the film thicknesses were measured by digital thickness gauze. The results were analyzed for mean and standard deviation.
Folding endurance
8 films from each formulation (2×2cm2) were cut by using sharp blade. Folding endurance was determined by repeatedly folding a small strip of film at the same place till it brakes. The number of times, the films could be folded at the same place without breaking will give the value of folding endurance. The results were analyzed for mean and standard deviation.
Surface pH
The surface pH of the patches were determined in order to investigate the possibility of any side effects, in-vivo. An acidic or alkaline pH may cause irritation to the buccal mucosa. It was our attempt to keep the surface pH as close to neutral as possible. For the determination of surface pH, 1 patch (2×2cm2) from each formulation were taken and with the help of pH paper, surface pH have been observed.
Swelling index (S.I.)
For the determination of swelling index (S.I) the pre-weighed 1 patch (2×2cm2) from each formulation were placed in a beaker (containing 20 ml of water). After particular interval of time patches were removed and wiped with tissue paper and weighed.
S.I. = (W 2 -W 1 / W 1) × 100
Where, S.I. is swelling index, W1 is weight of buccal patch before dipping into beaker and W2 is weight of buccal patch after dipping in beaker & wiped.
Mucoadhesive time
The in-vitro mucoadhesive time was determined using disintegration apparatus. The disintegration medium was 800 ml of phosphate buffer (pH 7.4) maintained at 37±2 0C. The segment of buccal mucosa of sheep was glued to the surface of glass slab, which was then vertically attached to the apparatus. Three mucoadhesive films of each formulation were hydrated on one surface with Phosphate buffer (pH 7.4) and the hydrated surface was brought into contact with the mucosal membrane and allowed the apparatus to move up and down. The time required for complete detachment of the film from surface was recorded. The results were analyzed for mean and standard deviation.
Result and discussion
All the 12 different formulations were observed carefully and according to their physical appearance 2 of them were chosen for further evaluations. These 2 formulations were F7 and F12 which shows prominent results. They have been evaluated for weight, thickness, folding endurance, surface pH, swelling index, mucoadhesive strength and mucoadhesive time.
Patch weight
Both the formulations (F7 and F12) show uniformity in weights. The average weights of F7 were found to be 230 mg. and for F12 found to be 63.75 mg. Results are shown as mean ± standard deviation in Table 12.
Patch thickness
Both the formulations (F7 and F12) show uniformity in thickness. The average thickness of F7 was found to be 0.63 mm and for F12 found to be 0.17 mm. Results are shown as mean ± standard deviation in Table 12.
Folding endurance
The folding endurance was measured manually, by folding the film repeatedly at a point till they were broken. The breaking time was considered as the end point and the results are shown in Table 13. The maximum average folding was found to be the high for formulation F12 while the low for F7.
The folding endurance values were found to be optimum.
Surface pH
Considering the fact that acidic or alkaline pH may cause irritation to the buccal mucosa. The surface pH of the buccal films was determined to optimize both drug permeation and mucoadhesion. Attempts were made to keep the surface pH as close as to salivary pH. The pH values of both the formulations were within the range of salivary pH. No significant difference was observed in surface pH for both formulations.
Swelling index
The swelling indices of both formulations were evaluated as per the general protocol. It was observed that the percentage swelling indices of various formulations were in order of F12>F7.
Mucoadhesive time
Both formulations showed satisfactory mucoadhesive time. Formulation F12 showed high mucoadhesive time while formulation F7 shows low mucoadhesive time. Results are shown as mean ± standard deviation in Table 12.
Table 5:Buccal patch formulations
|
Ingredients |
Units |
F7 |
F12 |
|
Methyl cellulose |
(mg) |
500 |
500 |
|
Methanol |
(ml) |
20 |
- |
|
Distilled water |
(ml) |
- |
20 |
|
P.V.P. K30 (Polyvinylpyrollidon) |
( mg) |
180 |
180 |
|
Glycerin |
(ml) |
2 |
1 |
Table 6: Patches weight of formulations
|
Formulations |
Wt. of 1st (mg) |
Wt. of 2nd (mg) |
Wt. of 3rd (mg) |
Wt. of 4th (mg) |
Wt. of 5th (mg) |
Wt. of 6th (mg) |
Wt. of 7th (mg) |
Wt. of 8th (mg) |
Mean ± S.D. (mg) |
|
F7 |
250 |
220 |
240 |
290 |
230 |
180 |
260 |
170 |
230±40 |
|
F12 |
60 |
90 |
60 |
70 |
50 |
70 |
50 |
60 |
63.75±13.02 |
S.D. :- standard deviation
Table 7: Patches thickness of formulations
|
Formulations |
1st (mm) |
2nd (mm) |
3rd (mm) |
4th (mm) |
5th (mm) |
6th (mm) |
7th (mm) |
8th (mm) |
Mean ± S.D. (mm) |
|
F7 |
0.70 |
0.56 |
0.64 |
0.83 |
0.62 |
0.59 |
0.75 |
0.37 |
0.63±0.138 |
|
F12 |
0.16 |
0.19 |
0.17 |
0.15 |
0.16 |
0.17 |
0.14 |
0.26 |
0.17±0.037 |
S.D. :- standard deviation
Table 8: Folding endurance of formulations
|
Formulations |
1st |
2nd |
3rd |
4th |
5th |
6th |
7th |
8th |
Mean ±S.D. |
|
F7 |
113 |
111 |
119 |
127 |
103 |
114 |
110 |
118 |
114.37±7.130 |
|
F12 |
290 |
294 |
280 |
293 |
297 |
287 |
290 |
288 |
289.87±5.166 |
S.D. :- standard deviation
Table 9: Surface pH of formulations
|
Formulations |
pH range |
|
F7 |
6 - 7 |
|
F12 |
6- 7 |
Table 10: Swelling Index(S.I.) of formulations
|
Time (min) |
F7 (%) |
F12 (%) |
|
5 |
3.98 |
4.45 |
|
10 |
6.22 |
5.55 |
|
15 |
8.68 |
11.13 |
|
20 |
12.89 |
18.10 |
Table 11: Mucoadhesive time of formulations
|
Formulations |
1st (s) |
2nd (s) |
3rd (s) |
4th (s) |
5th (s) |
6th (s) |
7th (s) |
8th (s) |
Mean ±S.D. (s) |
|
F7 |
221 |
219 |
198 |
225 |
210 |
229 |
233 |
203 |
217.25±12.475 |
|
F12 |
260 |
278 |
260 |
280 |
310 |
265 |
282 |
289 |
278±16.826 |
S.D. :- standard deviation
Table 12: Average film weight, thickness, folding endurance and mocoadhesive time values of different formulations
|
Formulations |
Film weight* (mg) |
Film thickness* (mm) |
Folding endurance* |
Mucoadhesive time* |
|
F7 |
230±40 |
0.63±0.138 |
114.37±7.130 |
217.25±12.475 |
|
F12 |
63.75±13.02 |
0.17±0.037 |
289.87±5.166 |
278±16.826 |
*Each data represent mean ± standard deviation.
Photographs of prepared mucoadhesive buccal patches



IMPORTANCE OF THE WORK
Working under placebo conditions definitely nullify the wastage of so called potent drugs which have their greater importance. On considering the research and industrial level, on the part of economy it will surely be considered as economy efficient work as it saves thousands of dollers spend on the purchase of drugs which was used with the trials of formulations or with novel drug delivery system.
Buccal lining is supposed to be more advantageous for drug delivery as bypass of thegastrointestinal tract and hepatic portal system, increasing the bioavailability of orally administered drugs that otherwise undergo hepatic first-pass metabolism.In addition the drug is protected from degradation due to pH and digestive enzymes of the middle gastrointestinal tract. Improved patient compliance due to the elimination of associated pain with injections, administration of drugs in unconscious or incapacitated patients, convenience of administration as compared to injections or oral medications have been observed. So, the prepared buccal patches shows the increased ease of drug administration.
FUTURE ASPECTS OF THE WORK
*In mucoadhesive placebo buccal patches we can use any potent drug which fulfill the criteria for buccal patch as drug delivery system.
*We can perform the dissolution of medicated mucoadhesive buccal patch for drug release profile studies.
*We can further perform the in-vivo studies for the prepared mucoadhesive buccal patches.
*We can perform the stability test for the prepared mucoadhesive buccal patches.
CONCLUSION
A new mucoadhesive placebo buccal patch configuration has been developed i.e. use of water as solvent with polymer Methyle cellulose and Polyvinylpyrollidone. An optimization of mucoadhesive buccal patch has been performed according to concentration of polymer and on the basis of use of different solvents in developing mucoadhesive buccal patches i.e. using methanol, acetic acid and water as solvent in different buccal patches.
After observing all the physical properties of prepared mucoadhesive buccal patches I conclude that in comparison to acetic acid and methanol, water has proved to be a better solvent in preparing mucoadhesive buccal patches with Methyle cellulose as polymer and it shows promising results.
REFERENCES
1. Aungst, B.J., Rogers, N.J., and Shefter, E., Comparison of nasal, rectal, buccal, sublingual and intramuscular insulin efficacy and the effects of a bile salt absorption promoter, The J. Pharmacol. Exp. Ther., 244:23-27, 1988.
2. Aungst, B.J. and Rogers, N.J., Site dependence of absorption-promoting actions of Laureth-9, Na salicylate, Na2EDTA, and Aprotinin on rectal, nasal, and buccal insulin delivery, Pharm. Res., 5:305-308, 1988.
3. Lee, W.E., Permeation enhancers for the nasal delivery of protein and peptide therapeutics, Bio Pharm, 3:22-25, 1990.
4. Tengamnuay, P. and Mitra, A.K., Bile salt-fatty acid mixed micelles as nasal absorption promoters of peptides. I. Effects of ionic strength. adjuvant composition, and lipid structure on the nasal absorption of [D-Arg2]Kyotorphin, Pharm. Res., 7:127-133, 1990.
5. Shao, Z. and Mitra, A.K., Nasal membrane and intracellular protein and enzyme release by bile salts and bile salt-fatty acid mixed micelles: correlation with facilitated drug transport, Pharm. Res., 9:1992, 1992.
6. Shao, Z. and Mitra, A.K., Bile salt fatty acid mixed micelles as nasal absorption promoters. III. Effects on nasal transport and enzymatic degradation of acyclovir prodrugs, Pharm. Res., 11:243-250, 1994.
7. Soyani, A.P. and Chien, Y.W., Systemic delivery of peptides and proteins across absorptive mucosae, Crit. Rev. Therap. Drug Carrier Systems, 13:85-184, 1996.
8. Adjei, A., Sundberg, D., Miller, J., and Chun, A., Bioavailability of leuprolide acetate following nasal inhalation delivery to rats and healthy humans, Pharm. Res., 9:244-249, 1992.
9. Shimamoto, T., Pharmaceutical aspects. Nasal and depot formulations of leuprolide, J. Androl., 8:S14-S16, 1987.
10. Dal Negra, R., Turco, P., Pomari, C., and Trevisan, F., Calcitonin nasal spray in patienmts with chronic asthma: a double-blind crossover study vs placebo, Int. J. Clin. Pharmacol. Ther. Toxicol., 29:144-146, 1991.
11. Plosker, G.L. and McTavish, D., Intranasal salcatonin (salmon calcitonin). A review of its pharmacological properties and role in the management of postmenopausal osteoporosis, Drugs Aging, 8:378-400, 1996.
12. Reginster, J.Y. and Lecart, M.P., Efficacy and safety of drugs for Paget's disease of bone, Bone, 17:485S-488S, 1995.
13. Rathbone, M.J. and Hadgraft, J., Absorption of drugs from the human oral cavity, Int. J. Pharm., 74:9-24, 1991.
14. de Vries, M.E., Bodde, H.E., Verhoef, J.C., and Junginger, H.E., Developments in buccal drug delivery, Crit. Rev. Ther. Drug Carr. Sys., 8:271-303, 1991.
15. Squier, C.A., The permeability of oral mucosa, Crit. Rev. Oral Biol. Med., 2:13-32, 1991.
16. Bodde, H.E., De Vries, M.E., and Junginger, H.E., Mucoadhesive polymers for the buccal delivery of peptides, structure-adhesiveness relationships, J. Control. Rel., 13:225-231, 1990.
17. Gandhi, R.E. and Robinson, J.R., Bioadhesion in drug delivery, Ind. J. Pharm. Sci., 50:145-152, 1988.
18. Harris, D. and Robinson, J.R., Drug delivery via the mucous membranes of the oral cavity, J. Pharm. Sci., 81:1-10, 1992. Reproduced with permission of the American Pharmaceutical Association.
19. Wertz, P.W. and Squier, C.A., Cellular and molecular basis of barrier function in oral epithelium, Crit. Rev. Ther. Drug Carr. Sys., 8:237-269, 1991.
20. Squier, C.A., Cox, P., and Wertz, P.W., Lipid content and water permeability of skin and oral mucosa, The J. Invest. Dermat., 96:123-126, 1991.
21. Squier, C.A. and Wertz, P.W. Structure and function of the oral mucosa and implications for drug delivery, in eds. M.J. Rathbone, Oral Mucosal Drug Delivery, Marcel Dekker, Inc., New York, New York, 1-26, 1996.
22. Galey, W.R., Lonsdale, H.K., and Nacht, S., The in vitro permeability of skin and buccal mucosa to selected drugs and tritiated water, J. Invest. Dermat., 67:713-717, 1976.
23. Gandhi, R.B. and Robinson, J.R., Oral cavity as a site for bioadhesive drug delivery, Adv. Drug Del. Rev., 13:43-74, 1994.
24. Squier, C.A. and Hall, B.K., The permeability of mammalian non-keratinized oral epithelia to horseraddish peroxidase applied in vivo and in vitro, Arch. Oral Biol., 29:45-50, 1984.
25. Ishida, M., Nambu, N., and Nagai, T., Mucosal dosage form of lidocaine for toothache using hydroxypropyl cellulose and carbopol, Chem. Pharm. Bull., 30:980-984, 1982.
26. Collins, A.E.M., Deasy, P.B., Mac Carthy, D.J., and Shanley, D.B., Evaluation of a controlled release compact containing tetracycline hydrochloride bonded to tooth for the treatment of periodontal disease, Int. J. Pharm., 51:103-114, 1989.
27. Elkayam, R., Friedman, M., Stabholz, A., Soskolne, A.w., Sela, M.N., and Golub, L., Sustained release device containing minocycline for local treatment of periodontal disease, J. Control. Rel., 7:231-236, 1988.
28. Samaranayake, L. and Ferguson, M., Delivery of antifungal agents to the oral cavity, Adv. Drug Del. Rev., 13:161-179, 1994.
29. Nagai, T., Adhesive topical drug delivery system, J. Control. Rel., 2:121-134, 1985.
30. Nagai, T. and Machida, Y., Mucosal adhesive dosage forms, Pharm. Int., 196-200, 1985.
31. Aungst, B.J. and Rogers, N.J., Comparison of the effects of various transmucosal absorption promoters on buccal insulin delivery, Int. J. Pharm., 53:227-235, 1989.
32. Siegel, I.A. and Gordon, H.P., Surfactant-induced increase of permeability of rat oral mucosa to non-electolytes in vivo, Arch. Oral Biol., 30:43-47, 1985.
33. Shojaei, A.H. and Li, X., In vitro permeation of acyclovir through porcine buccal mucosa, Proceedings of International Symposium on Controlled Release of Bioactive Materials, 23:507-508, 1996.
34. Shojaei, A.H. and Li, X., Determination of transport route of acyclovir across buccal mucosa, Proceed. Int. Symp. Control. Rel. Bioact. Mater., 24:427-428, 1997.
35. Manganaro, A.M. and Wertz, P.W., The effects of permeabilizers on the in vitro penetration of propranolol through porcine buccal epithelium, Mil. Med., 161:669-672, 1996.
36. Gandhi, R. and Robinson, J., Mechanisms of penetration enhancement for transbuccal delivery of salicylic acid, Int. J. Pharm., 85:129-140, 1992.
37. Hoogstraate, A.J., Verhoef, J.C., Tuk, B., Pijpers, A., van leengoed, L.A.M.G., Vheijden, J.H.M., Junjinger, H.E., and Bodde, H.E., Buccal delivery of fluorescein isothiocyanate-dextran 4400 and the peptide drug buserelin with glycodeoxycholate as an absorption enhancer in pigs, J. Control. Rel., 41:77-84, 1996.
38. Wolany, G.J.M., Munzer, J., Rummelt, A., and Merkle, H.P., Buccal absorption of Sandostatin (octreotide) in conscious beagle dogs, Proceed. Intern. Symp. Control. Rel. Bioact. Mater., 17:224-225, 1990.
39. Nakane, S., Kakumoto, M., Yulimatsu, K., and Chien, Y.W., Oramucosal delivery of LHRH: Pharmacokinetic studies of controlled and enhanced transmucosal permeation, Pharm. Dev. Tech., 1:251-259, 1996.
40. Steward, A., Bayley, D.L., and Howes, C., The effect of enhancers on the buccal absorption of hybrid (BDBB) alpha-interferom, Int. J. Pharm., 104:145-149, 1994.
41. Senel, S., Hoogstraate, A.J., Spies, F., Verhoef, J.C., Bos-van Geest, A., Junginger, H.E., and Bodde, H.E., Enhancement of in vitro permeability of porcine buccal mucosa by bile salts: kinetic and histological studies, J. Control. Rel., 32:45-56, 1994.
42. Hoogstraate, A.J., Senel, S., Cullander, C., Verhoef, J., Junginger, H.E., and Bodde, H.E., Effects of bile salts on transport rates and routes of FTIC-labelled compounds across porcine buccal epithelium in vitro, J. Control. Rel., 40:211-221, 1996.
43. Oh, C.K. and Ritschel, W.A., Biopharmaceutic aspects of buccal absorption of insulin, Meth. Find Exp. Clin. Pharmacol., 12:205-212, 1990.
44. Kurosaki, Y., Hisaichi, S., Hong, L., Nakayama, T., and Kimura, T., Enhanced permeability of keratinized oral-mucosa to salicylic acid with 1-dodecylacycloheptan-2-one (Azone). In vitro studies in hamster cheek pouch, Int. J. Pharm., 49:47-55, 1989.
45. Kurosaki, Y., Hisaichi, S., Nakayama, T., and Kimura, T., Enhancing effect of 1-dodecylazacycloheptan-2-one (Azone) on the absorption of salicyclic acid from keratinized oral mucosa and the duration of enhancement in vivo, Int. J. Pharm., 51:47-54, 1989.
46. Siegel, I.A. and Gordon, H.P., Effects of surfactants on the permeability of canine oral mucosa in vitro, Tox. Lett., 26:153-157, 1985.
47. Kurosaki, Y., Hisaichi, S., Hamada, C., Nakayama, T., and Kimura, T., Effects of surfactants on the absorption of salicylic acid from hamster cheek pouch as a model of keratinized oral mucosa, Int. J. Pharm., 47:13-19, 1988.
48. Coutel-Egros, A., Maitani, Y., Veillard, M., Machida, Y., and Nagai, T., Combined effects of pH, cosolvent and penetration enhancers on the in vitro buccal absorption of propranolol through excised hamster cheek pouch, Int. J. Pharm., 84:117-128, 1992.
49. Coutel-Egros, A., Maitani, Y., Veillard, M., Machida, Y., and Nagai, T., Combined effects of pH, cosolvent and penetration enhancers on the in vitro buccal absorption of propranolol through excised hamster cheek pouch, Int. J. Pharm., 84:117-128, 1992.
50. Ishida, M., Machida, Y., Nambu, N., and Nagai, T., New mucosal dosage form of insulin, Chem. Pharm. Bull., 29:810-816, 1981.
51. Ch'ng, H.S., Park, H., Kelly, P., and Robinson, J.R., Bioadhesive polymers as platforms for oral controlled drug delivery II: Synthesis and evaluation of some swelling, water-insoluble bioadhesive polymers, J. Pharm. Sci., 74:399-405, 1985.
52. Sanzgiri, Y.D., Topp, E.M., Benedetti, L., and Stella, V.J., Evaluation of mucoadhesive properties of hyaluronic caid benzyl esters, Int. J. Pharm., 107:91-97, 1994.
53. Lehr, C.M., Bouwstra, J.A., Schact, E.H., and Junginger, H.E., In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers, Int. J. Pharm., 78:43-48, 1992
54. Park, K. and Robinson, J.R., Bioadhesive polymers as platforms for oral-controlled drug delivery: method to study bioadhesion, Int. J. Pharm., 19:107-127, 1984.
55. Nagai, T. and Machida, Y., Buccal delivery systems using hydrogels, Adv. Drug Del. Rev., 11:179-191, 1993.
56. Nagai and Machida, Y., Buccal delivery systems , Adv. Drug Del. Rev., 11:187-194, 1993.
57. Parikh Bhavik et. al. Design and evaluation of buccal patches of valsartan IJPI’s Journal of Pharmaceutics and Cosmetology, IJPI’s Journal of Pharmaceutics and Cosmetology; 2011; (1); 50-55.
58. Rohit Chaudhary et. al. Formulation, Development and In-Vitro Evaluation of Mucoadhesive Buccal Patches Of Methotrexate International Journal of Pharma Sciences and Research (IJPSR), 1, (9), 2010, 357-365.
59. Khairnar G.A. et. al. Development of buccal drug delivery system based on mucoadhesive polymers, International Journal of PharmTech Research; 2010, 2, (1), 719-735.
60. Myung Kwan Chun et. al Preparation of buccal patch composed of carbopol, poloxamer and hydroxypropyl methylcellulose, Archives of life science; 2010, 26, 973-978.
61. Surya Adhikarinet et. al. Formulation and evalution of buccal patch delivery of Atenolol, AAPS Pharmatech, 2010, 11, (3), 1038-1044.
62. Rana Abu Huwaij et. al. Formulation and in-vitro evaluation of Xanthum gum or Corbopol 934 based Mucoadhesive patches, loaded with Nicotine, AAPS Pharmatech, 2010, 11, (3), 1-7.
63. Gazzi Shanker et. al. Formulation and Evaluation of buccal drug delivery of Tizanidine Hydrochloride Tablets, AAPS Pharmatech, 2010, 10, (2), 530-539.
64. Deelip Derle et. al. Formulation and evaluation of buccoadhesive bi-layer tablet of propranolol hydrochloride, International Journal of Pharmacy and Pharmaceutical Sciences, 2009 1, 1, 206-212.
65. Subhash V. Deshmane et. al. Chitosan based sustained release mucoadhesive buccal patches containing verapamil hcl, International Journal of Pharmacy and Pharmaceutical Sciences, 2009 1, 1, 216-219.
66. Chandra Sekhar Kolli et. al. Development of Mucoadhesive Patches for Buccal Administration of Prochlorperazine: Evaluation of In Vitro Release and Mechanical Properties, International Journal of Pharmaceutical Sciences and Nanotechnology,2009 1, 1, 64-70.
67. Supriya Sidhaye et. al. Mucoadhessive bilayered patches for administration of Sumatriptan Succinate, AAPS Pharmatech, 2008, 9, (3), 909-916.
68. Y. Vamshi Vishnu et. al. Development of Mucoadhesive Patches for Buccal Administration of Carvedilol, Current Drug Delivery, 2007, 4, 27-39.
69. Vishnu M. Patel et. al. Design and characterization of chitosan-containing mucoadhesive buccal patches of propranolol hydrochloride, Acta Pharm. 2007, 57, 61–72.
70. Vishnu Patel et.al. Effect of hydrophilic polymers on bucciadhesive eudragit paches of propranolol hydrochloride using factorial design, AAPS Pharmatech, 2007, 8, (3), E119-E126.
71. Marta Corbonits et. al. A comparison of a Novel Testosterone Bioadhesive Buccal System, Striant, with a Testosterone Adhesive Patch in Hypogonadal Males , The Journal of Clinical Endocrinology and Metabolism, 2004, 89, (5), 2039-2043.
72. L. Panigrahi et. al. Design and characterization of mucoadhesive buccal patches of salbutamol sulphate, Acta Pol Pharm., 2004, 61, (5), 351-360.
73. Deirdre faye Vaughan, Pharmacokinetics of albuterol and butorphanol administered intravenously and via a buccal patch, 2003.
74. Noha adel nafee et. al. Design and characterization of mucoadhesive buccal patches containing cetylpyridinium chloride, Acta Pol Pharm., 2003, 53, 199-212.
75. Amir H. Shojaei, Buccal Mucosa As A Route For Systemic Drug Delivery: A Review, J Pharm Pharmaceut Sci, 1998, 1, (1), 15-30.
76. C. Li et. al. Evaluation of a mucoadhesive buccal patch for delivery of peptides: in vitro screening of bioadhesion, Drug Dev Ind Pharm, 1998, 24, (10), 919-926.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









