 About Authors:
About Authors:
Piyush Gupta*
Department of Pharmaceutics,
Regional College of Pharmacy,
Jaipur, Rajasthan
ABSTRACT:-
The term MICROSPHERE is defined as a spherical particle with size varying from 50nm to 2µm, containing a core substance. Microspheres are, in strict sense, spherical empty particles.However, the terms microspheres and microencapsulationare used synonymously. In addition, some related terms are “beads” are used alternatively. Spheres and spherical particles are also usedfor a large size and rigid morphology. The microspheres are characteristically free flowing powders consisting of proteins orsynthetic polymers, which are biodegradable in nature, andideally having a particle size less than 200 micrometer. Novel Drug Delivery Systems are developed to address the challengesof drug development such as Bioavailability, Permeability & Poor solubility. These demand changes in the conventional use of Excipients, the growth of the Biotechnology industry,including Stem cell therapy,Vaccines & Genetic products, also necessitates different drug delivery requirements, there by demonstrating the acceptance of these excipients by the US food & drug administration or other agencies in the major markets.For materials in which Toxicity is a possible concern, formulators can give information about the excipients regulatory acceptance& allowable amount by consulting with excipients manufactures& toxicology experts.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1266
INTRODUCTION
Between 1940s and 1960s, the concept of Micro encapsulation technology began as an alternative means of delivering drugs. In continued quest for the more refined system, in 1980s Polymer/Membrane technology came to be known at forefront. Further, the process of targeting and site specific delivery with absolute accuracy can be achieved by attaching bioactive molecule to liposome, bioerodable polymer, implants, monoclonal antibodies and various particulate carriers (e.g. Nano particles and Microspheres etc).The term Microsphere is defined as a spherical particle with size varying with diameters in the micrometer range (typically 1μm to 1000μm (1mm), containing a core substance.The microspheres are characteristically free flowing powders consisting of proteins or synthetic polymers, which are biodegradable in nature, and ideally having a particle size less than 200 micrometer. Microsphere have been extensively studied for use as drug delivery systems, where they have been shown to protect sensitive macromolecules from enzymatic and acid degradation, and allow controlled release and tissue targeting of the formulated drug.
Drug carriers one can name soluble polymers, microparticles made of insoluble or biodegradable natural and synthetic polymers, microcapsules, cells, cell ghosts, lipoproteins, liposomes, and micelles. The carriers can be made slowly degradable, stimuli-reactive (e.g. pH or temperature-sensitive), and even targeted (e.g. by conjugating them with specific antibodies against certain characteristic components of the area of interest).[18]
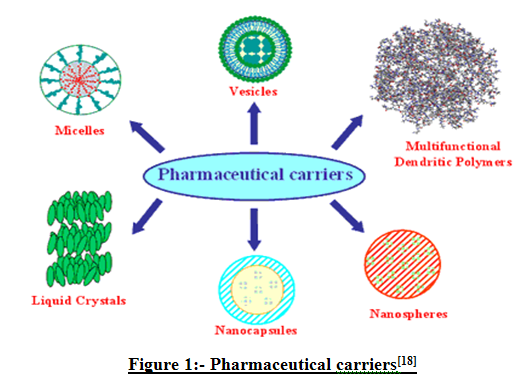
Figure 1:-Pharmaceutical carriers[18]
The Novel Drug Delivery System started the alternative means of delivering the drug in the form of microspheres. A unique pharmaceutical delivery system comprising a physiologically acceptable nonaqueous liquid such as an edible oil and a thickening agent such as colloidal silicon dioxide which together form a semi-solid having the consistency of a pudding is provided. Microspheres prepared with gelatin as the polymer have been found to be highly mucoadhesive and have been used for the controlled release of many drugs. Microspheres prepared from admixtures of gelatin and crosslinked chitosan demonstrated some advantage over that prepared from gelatin alone in terms of better controlled release rate of cemetidine. Nanoparticles (including nanospheres and nanocapsules of size 10-200nm) are in the solid state and are either amorphous or crystalline. They are able to absorb and/or encapsulate a drug, thus protecting it against chemical and enzymatic degradation. Novel drug delivery system development is largely based on promoting the therapeutic effects of a drug and minimizing its toxic effects by increasing the amount and persistence of a drug in the vicinity of a target cell and reducing the drug exposure of nontarget cells.[18]
CLASSIFICATION
There are different types of Microspheres.
1. Glass microspheres
· Hollow glass microspheres
· Solid glass microspheres
2.Polymer microspheres
· Polyethylene microspheres
· Polystyrene microspheres
· Fluorescent microspheres
3.Starch microspheres
· Cross-linked starch microspheres
4.Ceramic microspheres
5.Albumin microspheres
6.Gelatin microspheres
7.Dextran microspheres
8.Poly lactide and polyglycolide microspheres
9.Poly anhydride microspheres
10.Poly phosphane microspheres
11.Chitosan microspheres
12.Lipid cross linked chitosan microspheres
13.Carrageenan microspheres
14.Alginate microspheres
15.Poly (alkyl cynoacrylate) microspheres
16.Poly acrolein microspheres
[adsense:468x15:2204050025]
ADVANTAGES
(1) Improved antigenicity by adjuvant action.
(2) Modulation of antigen release.
(3) Stabilization of antigen.
(4) Microspheres are very small particles that can be loaded easily.
PREREQUISITES
The material utilized for the preparation of microspheres should ideally fulfill the following prerequisites:-
(1) Longer duration of action
(2) Control of content release
(3) Increase of content of therapeutic efficiency
(4) Protection of drug
(5) Reduction of toxicity
(6) Biocompatibility
(7) Sterilizability
(8) Relative stability
(9) Water solubility or dispersability
(10)Bioresorbability
(11)Targetability
(12)Polyvalent
GOVERNING FACTORS
In the preparation of microspheres ,the governing factors are:-
1. The particle size requirements
2. The drug or protein should not be affected by the process.
3. Reproducibility of the release profile and the method.
4. No stability problems.
5. No toxic products produced as the final product.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
FORMULATION
MATERIALS:-A number of different substances both biodegradable & non biodegradable has been investigated for the preparations of microspheres. These materials include the polymers of natural and synthetic origin and also modified natural substances.
1. SYNTHETIC POLYMERS
(1) Non-biodegradable polymers
(1) PMMA (Polymethylmethacrylate)
(2) Acrolein
(3) Glycidyl methaacrylate
(4) Epoxy polymers
(2)Biodegradable polymers
(1)Lactides and glycosides and their copolymers
(2) Polyalkyl cyano acrylates
(3) Polyanhydrides
2. NATURAL MATERIALS
(1) Proteins
(1) Albumin
(2) Gelatin
(3) Collagen
(2) Carbohydrates
(1)Starch
(2)Agrose
(3) Carrageenen
(4)Chitosan
3. CHEMICALLY MODIFIED CARBOHYDRATES
(1) DEAE cellulose
(2) Poly(acryl)dextran
(3) Poly(acryl)starch
METHODS:-
1.Single Emulsion Technique:-The natural polymers are dissolved or dispersed in aqueous or non aqueous medium followed by dispersion in the non aqueous medium e.g. oil. Than cross linking of the dispersed globules is done by the means chemical cross linkers glutaraldehyde, formaldehyde, teraphthaloyl chloride etc. After centrifugation, washing and separation the microspheres can be obtained.
2. Double Emulsion Technique:-In this technique multiple emulsions of o/w or w/o is formed. The aqueous protein solution is dispersed in a lipopolic organic continuous phase. The primary emulsion thus formed is subjected to homogenization before the addition to aqueous solution of poly vinyl alcohol. This results in the formation of multiple emulsions. This emulsion is then subjected to solvent removal or extraction. This results in the formation of microspheres.
3. Polymerization Technique:-In this technique, a dispersion is made by using active material and the aqueous solution of NaOH with surfactant is made. This results in the miceller solution of polymer in aqueous medium. After heating the above system polymerization occurs which on settling forms microspheres.
4. Phase Separation Technique:-In this technique, the polymer is first dissolved in a suitable solvent and then the drug is dispersed by making its aqueous solution. Than the phase separation is induced by the techniques like addition of incompatible polymer or change of ph etc. As a result polymer rich globules are formed which get harden after some time. On separation the microspheres are formed.
5. Spray Drying and Spray Congealing:- In this technique the polymer is first dissolved in a suitable volatile organic solvent like acetone, dichloromethane etc. the drug in the solid form is than dispersed in the polymer solution. This dispersion is than atomized in a stream of hot air. The atomization leads to the formation of the small droplets from which the solvent evaporates instantaneously leading the formation of the microsphere.
6. Solvent Extraction Technique:- The solvent extraction technique depends on the removal of the organic phase by extraction of the organic solvent. The organic solvent used is isopropyl alcohol and the organic phase is removed by extraction with water. The therapeutic benefit of microencapsulated drugs and vaccines brought forth the need to prepare such particles in larger quantities and in sufficient quality suitable for clinical trials and commercialisation. Our findings will be outlined according to the four major substeps of microsphere preparation by solvent extraction/evaporation, namely,
(1)Incorporation of the bioactive compound.
(2)Formation of the microdroplets.
(3)Solvent removal.
(4)Harvesting and drying the particles.[2] [3] [4]
EVALUATION
1. Morphological examination:-The morphology of microspheres was examined by Scanning Electron Microscopy (SEM, JSM-T220A, JEOL, Tokyo, Japan). Samples of microspheres were dusted onto double-sided tape on an aluminum stub and coated with gold using a cold sputter coater to a thickness of 400Å and then imaged using a 25kv electron beam.
2. Production yield, drug content, and loading efficiency:- The percentage of production yield was calculated from the weight of dried microspheres (W1) recovered from each of 3 batches and the sum of the initial dry weight of starting materials (W2) as the following equation:-
Percentage of Production Yield = W1 / W2 × 100
The amount of drug encapsulation in the microspheres was determined using the formula E= Qp / Qt×100, where E is the percentage of encapsulation of microspheres; Qp is the product of drug content per g of microspheres and Qt is the yield of microspheres in g.[5]
The drug loading in microspheres was estimated using the formula, L=Qm / Wm×100, where L is the percentage of loading of microspheres, Wm is the weight of the microspheres; Qm is the quantity of the drug present in Wm of microspheres.[5]
3. Particle size measurement:-The most widely used technique for determining the particle size and shape is Scanning Electron Microscopy, Thermal Electron Microscopy. The particle size can be determined by using light microscopy while confocal floresence microscopy is used for the structural characterization. In this technique, the field viewed through the microscope can be projected on a screen or photographed for latter measurement. Particles may also be counted with electronic scanners to avoid the strain of visual observation. For measuring very small particle size, an electron microscope or a scanning electron microscope may be used. The latter is also capable of proving an estimate of the particle depth.[3]
4. Determination of bulk density and angle of repose:-The density of microspheres is determined by using multivolume pychometer, by using helium. Accurate weight of microspheres (Wm) was transferred into a 100mL graduated cylinder to obtain the apparent volumes (V) of between 50 and 100 mL. The bulk density was calculated in gram per milliliter by the following formula:-
Bulk Density = Wm / V
The angle of repose was measured from a heap carefully built up by dropping the microsphere samples through a glass funnel to the horizontal plate of a powder characteristic tester (PP-N, Hosokawa powder tester, Kawaramachi Chuo-ku, Osaka, Japan). The results were averaged from 3 determinations.
5. Zeta potential study:-The zeta potential of microspheres dispersed in 0.0005M phosphate buffer of pH 6.8 was determined by a zeta meter (ZM3UG, Zeta meter, Zeta Meter Co, Staunton, VA). The directional movement of 200 microspheres from each formulation was observed and averaged from 3 determinations.
6. Adhesion property:-The adhesive property was determined by an adapted method described by Vyas et al. 2Å freshly cut piece, 5cm long, of pig intestine obtained from a local abattoir within 1 hour of killing the animal was cleaned by washing with isotonic saline solution. An accurate weight of microspheres was placed on mucosal surface, which was attached over a polyethylene plate that fixed in an angle of 40° relative to the horizontal plane and phosphate buffer of pH 6.8 warmed at 37°C was peristaltically pumped at a rate of 5 mL/min over the tissue. The duration for complete washing of microspheres from pig intestine was recorded and averaged from 5 determinations.
7. Swelling property:-The swelling of microspheres was conducted in phosphate buffer of pH 6.8. Their diameters were periodically measured by using a laser particle size distribution analyzer until they were decreased by erosion and dissolution. The percentage of swelling was determined at different time intervals by the difference between diameter of microspheres at time t (Dt) and initial time (t = 0 [D0]) as calculated from the following equation and averaged from 3 determinations:-
Percentage of Swelling = Dt - D0 × 100
8. Infrared absorption study:-The IR spectra of propranolol HCl and additives in the spray-dried microspheres were examined using the potassium bromide disc method by an infrared spectrophotometer (1760X, PerkinElmer, Wellesley, MA) in the range of 4000 to 400 cm−1.
9. Isoelectric point determination:-The isoelecrric point of microspheres is determined by using microelectrophoresis, which measures the mobility of microspheres.
10. Chemical analysis:- The surface chemistry of microspheres is determined by using the electron spectroscopy.
11. Surface carboxylic acid residue:- This is measured by using radioactive glycine.
APPLICATIONS
Microspheresvary widely in quality, sphericity, uniformity, particle size and particle size distribution. The appropriate microsphere needs to be chosen for each unique application.
1.Assay:-Coated microspheres provide measuring tool in biology and drug research.
2.Buoyancy:- Hollow microspheres are used to decrease material density inPlastics. (Glass & Polymer microspheres)
3.Ceramics:- Used to create porous ceramics used for filters (microspheres melt out during firing). (Polyethylene microspheres)
4.Cosmetics:-Opaque microspheres used to hide wrinkles and give color,clear microspheres provide “smooth ball bearing” texture during application. (Polyethylene microspheres)
5.Drug Delivery:- Miniature time release drug capsule. (Polymer microspheres)
6.Electronic paper:-Dual Functional microspheres used in Gyricon electronicpaper. (Polyethylene microspheres)
7.Personal Care:-Added to Scrubs as an exfoliating agent. (Polyethylene microspheres)
8.Spacers:- Used in LCD (Liquid Crystal Display) screens to provide a precision spacing between glass panels. (Glass microspheres)
9.Standards:- Monodispere microspheres are used to calibrate particle sieves and particle counting apparatus.
10.Retroreflective:- Added on top of paint used on roads and signs to increase night visibility of road stripes and sings.(Glass microspheres)
11.Thickening Agent:- Added to paints and epoxies to modify viscosity andBuoyancy. (Glass & Polymer microspheres)
12.Immune system:-In the drug delivery by using microspheres immune system, the drug is delivered by the use of macrophages. The microspheres with particle size less than 10 micro meters are directly taken up by the antigen presenting cell, while the greater ones first undergo degradation andthen release the drug.
13.Drug targeting by using the microspheres.
14.Microspheres in vaccine delivery.
15.Unique advantage of Opaque Polyethylene microspheres in cosmetics is that maximum hiding power is achieved with one invisible and feather-light layer -revolutionizing make-up products.
POSSIBLE DRUGS
1. Acetazolamide
2. Cefuroxime sodium
3. Diltiazem hydrochloride
4. Azithromycin
5. Refampicin(lipid microspheres)
6. Amoxicilline
7. Diclofenac sodium
CONCLUSION
Microspheres are, in strict sense, spherical empty particles. However, the terms microspheres and microencapsulation are used synonymously. In addition, some related terms are “beads” are used alternatively. Spheres and spherical particles are also used for a large size and rigid morphology. The microsphers are characteristically free flowing powders consisting of proteins or synthetic polymers, which are biodegradable in nature and ideally having a particle size less than 200 micrometer. Solid biodegradable microspheres incorporating a drug dispersed or dissolved throughout particle matrix have the potential for the controlled release of the drug. These carriers received much attention not only for prolonged release but also for the targeting of the anticancer drugs to the turnover.
REFERENCES
1. Bhalerao S.S., Lalla J.K., Rane M.S., “Study of processing parameters influencing the properties of diltiazem hydrochloride”, J. Microencap. 2001;18: Page no.- 299-307.
2. Bhalerao S.S., Lalla J.K., Rane M.S., “A study of electrokinetic and stability property of suspension of diltiazem hydrochloride microcapsule”, Indian Drugs 2001;38: Page no.- 464-467.
3. Chowdary K.P., Ramesh K.V., “Studies on microencapsulation of diltiazem”, J. Pharm. Sci. 1993;55: Page no.- 52-54.
4. Reddy M.N., Shriwaikar A.A. Rosin, “a polymer for microencapsulation of diltiazem hydrochloride for sustained release by emulsion-solvent evaporation technique”, Indian J. Pharm. Sci. 2000;62: Page no.- 308-310.
5. Saravanan M., Dhanraj M.D., Sridhar S.K., Ramachandran S., Sam S.K., Rao S.G., “Preparation, characterization and in vitro release kinetics of ibuprofen polystyrene microspheres”, Indian J. Pharm. Sci. 2004;66: Page no.- 287-292.
6. Pandey V.P., Manavalan R., Sundarrajan T., Ganesh K.S., ‘Formulation and release characteristics of sustained release diltiazem hydrochloride tablets”, Indian J. Pharm. Sci. 2003; 65: Page no.- 44-48.
7. Ishikawa T., Watanabe Y., Takayama K., Endo H., Matsumoto M., “Effect of hydroxypropylmethylcellulose (HPMC) on the release profiles and bioavailability of a poorly water-soluble drug from tablets prepared using macrogol and HPMC”, Int. J. Pharm. 2000;202: Page no.- 173-178.
8. Higuchi T., “Mechanism of sustained-action medication -theoretical analysis of rate of release of solid drugs dispersed in solid matrices”, J. Pharm. Sci. 1963;52: Page no.- 1145-1149.
9. Wagner J.G., “Interpretation of percent dissolved-time plots derived from in vitro testing of conventional tablets and capsules”, J. Pharm. Sci. 1969;58: Page no.- 1253-1257.
10. S. Freiberg and X. X. Zhu, “International Journal of Pharmaceutics”, Volume 282, Issues 1-2, 10 September 2004, Page no.- 1-18.
11. M. A. Bayomi, S. A. Al-Suwayeh, A. M. El-Helw and A. F. Mesnad, Pharmaceutica Acta Helvetiae, Volume 73, Issue 4, December 1998, Page no.- 187-192.
12. Jin-Chul Kim, Dae Ho Kim, Min Chul Kwon, Hae Gon Chung, Seung-Jun Lee, Jong-Duk Kim, Hyeon Yong Lee, “Journal of Dispersion Science and Technology”, Volume 27, Issue 2, April 2006, Page no.- 165-169.
13. Amita Bagal, Ravi Dahiya, Vance Tsai, Peter A. Adamson, “Archives of Facial Plastic Surgery”, Jul/Aug 2007, Vol 9, No. 4, Page no.- 275-280.
14. Kumaresh S. Soppimath, Anandrao R. Kulkarni, Walter E. Rudzinski, Tejraj M. Aminabhavi, Drug Metabolism Reviews, Volume 33, Issue 2, April 2001, Page no.- 149-160.
15. T. Hekmatara, G. Regdon, P. Sipos, I. Er?s and K. Pintye- Hódi, “Journal of Thermal Analysis and Calorimetry”, Volume 86, No.- 2, November 2006, Page no.- 287-290.
16. M.N. Reddy and A .A. Shirwalkar, “Indian Journal of Pharmaceutical Sciences”, April 2000, Volume 62, Issue 4, Page no.- 308-310.
17. Reza Arshadya, “Journal of Controlled Release”, Volume 14, Issue 2, October 1990, Page no.- 111-131.
18. Costas Kaparissides, Sofia Alexandridou, Katerina Kotti and Sotira Chaitidou, “A Zojono journal of Nanotechnology Online, Recent Advances in Novel Drug Delivery Systems”, July, 2005, Page no.- 121-131.
19. Lidia Elfstrand , Ann-Charlotte Eliasson, Monica Jönsson , Mats Reslow, Marie Wahlgren, Starch–Stärke, Jun 2006, Volume 58, Issue 8, Page no.- 381-390.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









