About Authors:
Shakya Pragati*, Khwaja Sadiya,
Asst. Professor, Integral University,
Lucknow,
U.P, India
1.ABSTRACT
Transdermal delivery of medications was foreshadowed in earlier eras by the use of certain plasters and ointments. The mustard plaster, applied as a home remedy for severe chest congestion, may be considered an example. (This author remembers his mother making mustard plasters for members of the family.) Powdered mustard seed (Brassica nigra) was mixed with warm water, and the resulting paste was spread on a strip of flannel, which was applied to the patient’s chest with a cloth binding wrapped around the body to hold the plaster in place. The moisture and body warmth activated an enzyme (myrosin) in the mustard that hydrolyzed a glycoside (sinigrin), causing the release of the pungent active ingredient allyl isothio-cyanate (CH2=CHCH2NCS) (7). This substance possesses the qualifications listed above for transdermal absorption. A transdermal patch or skin patch is a medicated adhesive patch that is placed on the skin to deliver a specific dose of medication through the skin and into the bloodstream.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1235
There are four types of transdermal drug delivery system:
1. Membrane permeation Controlled systems
2. Matrix diffusion-controlled system
3. Adhesive dispersion- type system
4. Microreservoir type or microsealed dissolution controlled systems.
The basic components of transdermal patches are Polymer matrix or matrices , drug , Permeation enhancers and other excipients. The mechanism of permeation can involve the passage through the epidermis itself known as transepidermal absorption. Transderm-Scop , Nitro dur ,Transderm-Nitro are some of the marketed formulations of transdermal patches.
2. INTRODUCTION
Transdermal drug delivery system has been in existence for a long time. In the past, the most commonly applied systems were topically applied creams and ointments for dermatological disorders.The occurrence of systemic side-effects with some of these formulations is indicative of absorption through the skin. A number of drugs have been applied to the skin for systemic treatment. In a broad sense, the term transdermal delivery system includes all topically administered drug formulations intended to deliver the active ingredient into the general circulation. Transdermal therapeutic systems have been designed to provide controlled continuous delivery of drugs via the skin to the systemic circulation. Moreover, it over comes various side effects like painful delivery of the drugs and the first pass metabolism of the drug occurred by other means of drug delivery systems. So, this Transdermal Drug Delivery System has been a great field of interest in the recent time. Many drugs which can be injected directly into the blood stream via skin have been formulated. The main advantages of this system are that there is controlled release of the drug and the medication is painless. The drug is mainly delivered to the skin with the help of a transdermal patch which adheres to the skin. A Transdermal Patch has several components like liners, adherents, drug reservoirs, drug release membrane etc. which play a vital role in the release of the drug via skin. Various types of patches along with various methods of applications have been discovered to delivery the drug from the transdermal patch. Because of its great advantages, it has become one of the highly research field among the various drug delivery system. Here, a general view over the transdermal patch has been discussed along with its advantages, disadvantages, methods of applying, care taken while applying, types and applications of transdermal patch and recent advances along with recent patents and market products.
Definition:
A transdermal patch or skin patch is a medicated adhesive patch that is placed on the skin to deliver a specific dose of medication through the skin and into the bloodstream.
 The first commercially available prescription patch was approved by the United States Food and Drug Administration in December 1979, which administered scopolamine for motion sickness.
The first commercially available prescription patch was approved by the United States Food and Drug Administration in December 1979, which administered scopolamine for motion sickness.
Transdermal drug delivery system are topicaly administered medicaments in the form of patches that deliver drugs for systemic effects at a predetermined and controlled rate. A transdermal drug delivery device, which may be of an active or a passive design, is a device which provides an alternative route for administering medication. These devices allow for pharmaceuticals to be delivered across the skin barrier. In theory, transdermal patches work very simply. A drug is applied in a relatively high dosage to the inside of a patch, which is worn on the skin for an extended period of time. Through a diffusion process, the drug enters the bloodstream directly through the skin. Since there is high concentration on the patch and low concentration in the blood, the drug will keep diffusing into the blood for a long period of time, maintaining the constant concentration of drug in the blood flow.
This approach to drug delivery offers many advantages over traditional methods. As a substitute for the oral route, transdermal drug delivery enables the avoidance of gastrointestinal absorption, with its associated pitfalls of enzymatic and pH associated deactivation. This method also allows for reduced pharmacological dosaging due to the shortened metabolization pathway of the transdermal route versus the gastrointestinal pathway. The patch also permits constant dosing rather than the peaks and valleys in medication level associated with orally administered medications. Multi-day therapy with a single application, rapid notification of medication in the event of emergency, as well as the capacity to terminate drug effects rapidly via patch removal, are all further advantages of this route.
However this system has its own limitations in which the drug that require high blood levels cannot be administered and may even cause irritation or sensitization of the skin.the adhesives may not adhere well to all types of skin and may be uncomfortable to wear. Along with these limitations the high cost of the product is also a major drawback for the wide acceptance of this product. (5)
3.TYPES OF TRANSDERMAL DRUG DELIVERY SYSTEMS
[adsense:468x15:2204050025]
3.1 Membrane permeation Controlled systems:
In this type of system,the drug reservoir is totally encapsulated in a shallow compartment moulded from a drug impermeable metallic plastic laminate and a rate controlling polymeric membrane e.g. Ethylene vinyl acetate with defined drug permeability. The drug molecules are permitted to release only through the rate-controlling membrane. In the drug reservoir compartment ,the drug solids are either dispersed in a solid-polymer matrix or suspended in a viscous liquid medium to form a paste like suspension.A thin layer of adhesive polymer is applied to the external surface of the rate-controlling membrane to achive an intimate contact of the transdermal system and the skin surface.
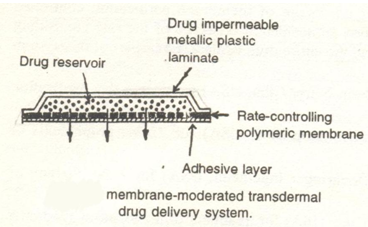
Example: Transderm-Nitro , Transderm-Scop , Catapress , Estraderm .(4)
3.2 Matrix diffusion-controlled system:
In this approach, the drug reservoir is prepared by homogenously dispersing drug particles in a hydrophilic or lipophilic polymer matrix.The resultant medicated polymer is then moulded into a medicated disc with a defined surface area and controlled thickness.This drug reservoir containing polymer disc is then pasted on to an occlusive base plate in a compartment fabricated from a drug impermeable plastic backing. The adhesive polymer is then spread along the circumference to form a strip of adhesive rim around the medicated disc.(4)
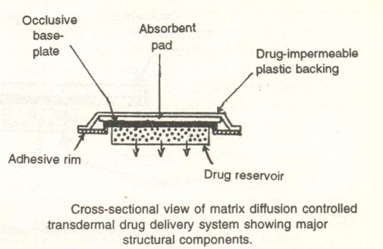
Example: Nitro-Dur System .
3.3 Adhesive dispersion- type system:
This is a simplified form of the membrane permeation-controlled system . The drug reservoir is formulated by directly dispersing the drug in an adhesive polymer eg. polyisobutylene and then spreading the medicated adhesive , by solvent casting or hot melt onto a flat sheet of drug impermeable metallic plastic backing to form a thin drug reservoir layer. On the top of the drug reservoir layer, thin layers of non- medicated, rate-controlling adhesive polymer of a specific permeability are applied to produce an adhesive diffusion - controlled delivery system.
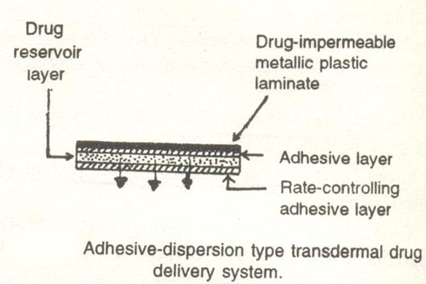
Example: Deponit , Frandol Tape . (4)
3.4 Microreservoir type or microsealed dissolution controlled systems:
Here, the drug reservoir is formed by first suspending the drug solids in an aqueous solution of a water soluble liquid polymer and then dispersing the drug suspension homogenously in a lipophilic polymer by high shear mechanical force to form a large number of microreservoirs. These are unleachable microscopic spheres of drug reservoirs . This thermodynamically unstable dispersion is stabilized quickly by immediate addition of cross linking polymers like Gluteraldehyde the polymer which produces a medicated polmer disc with a constant surface area and a fixed thickness. A transdermal therapeutic system is produced by positioning the medicated disc at the centre and surrounding it with an adhesive rim and then it is spread on to the occlusive base plate with adhesive foam pad.
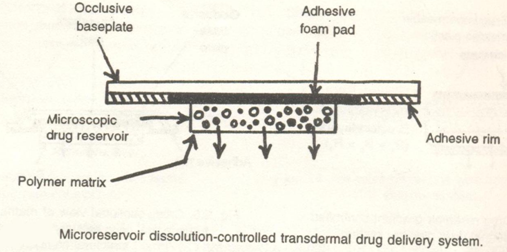
Example: Nitro-Disc System . (4)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
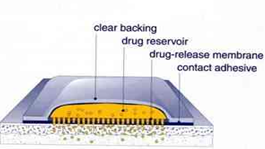
4.BASIC COMPONENTS OF TRANSDERMAL DRUG DELIVERY SYSTEM
The components of transdermal devices include:
4.1. Polymer matrix or matrices.
4.2. The drug
4.3. Permeation enhancers
4.4. Other excipients
4.1.Polymer Matrix:
The Polymer controls the release of the drug from the device. The polymer should be stable, non-reactive with the drug, easily manufactured into the desired product and inexpensive. The polymer and its degradation products must be non-toxic or non-antagonistic to the host.(9)
Possible useful polymers for transdermal devices are:
a) Natural Polymers: Example: Cellulose derivatives, Zein, Gelatin, Shellac, Waxes, Proteins, Gums and their derivatives, Natural rubber, Starch .
b)Synthetic Elastomers : e.g. Polybutadieine, Hydrin rubber, Polysiloxane, Silicone rubber, Nitrile, Acrylonitrile, Butyl rubber, Styrenebutadieine rubber, Neoprene etc.
c) Synthetic Polymers: e.g. Polyvinyl alcohol, Polyvinyl chloride, Polyethylene, Polypropylene, Polyacrylate, Polyamide, Polyurea, Polyvinylpyrrolidone, Polymethylmethacrylate, Epoxy etc.
4.2.Drug:
For successfully developing a transdermal drug delivery system, the drug should be chosen with great care. The following are some of the desirable properties of a drug for transdermal delivery.(21-22)
Physicochemical properties:
1. The drug should have a molecular weight less than approximately 1000 daltons.
2. The drug should have affinity for both – lipophilic and hydrophilic phases. Extreme partitioning characteristics are not conducive to successful drug delivery via the skin.
3. The drug should have low melting point.
Along with these properties the drug should be potent, having short half life and be non irritating.
4.3 Permeation Enhancers: These are compounds which promote skin permeability by altering the skin as a barrier to the flux of a desired penetrant.(24)
These may conveniently be classified under the following main headings:
a) Solvents : These compounds increase penetration possibly by swallowing the polar pathway and/or by fluidizing lipids. Examples include water alcohols – methanol and ethanol; alkyl methyl sulfoxides – dimethyl sulfoxide, alkyl homologs of methyl sulfoxide dimethyl acetamide and dimethyl formamide ; pyrrolidones – 2 pyrrolidone, N-methyl, 2-purrolidone; laurocapram (Azone), miscellaneous solvents – propylene glycol, glycerol, silicone fluids, isopropyl palmitate.
b) Surfactants: These compounds are proposed to enhance polar pathway transport, especially of hydrophilic drugs.The ability of a surfactant to alter penetration is a function of the polar head group and the hydrocarbon chain length.
Anionic Surfactants: e.g. Dioctyl sulphosuccinate, Sodium lauryl sulphate, Decodecylmethyl sulphoxide etc.
Nonionic Surfactants: e.g. Pluronic F127, Pluronic F68, etc.
Bile Salts: e.g. Sodium ms taurocholate, Sodium deoxycholate, Sodium tauroglycocholate.
Biary system: These systems apparently open up the heterogeneous multilaminate pathway as well as the continuous pathways.e.g. Propylene glycol-oleic acid and 1, 4-butane diol-linoleic acid.
c) Miscellaneous chemicals: These include urea, a hydrating and keratolytic agent; N, N-dimethyl-m-toluamide; calcium thioglycolate; anticholinergic agents.
Some potential permeation enhancers have recently been described but the available data on their effectiveness sparse. These include eucalyptol, di-o-methyl-ß-cyclodextrin and soyabean casein.
The rate of drug transport across the skin (stratum corneum) follows Ficks law of diffusion and can be explained by the following equation:-
J= K.D ./\C
h
where J is the steady state flux across the stratum corneum.
K is the partition coefficient of the drug between skin and the formulation medium.
D is the diffusion constant or coefficient of the drug molecule in the stratum corneum.
/\C is the concentration difference across the stratum corneum.
h is thickness of the stratum corneum.
4.4.Other Excipients :
a) Adhesives: The fastening of all transdermal devices to the skin has so far been done by usinga pressure sensitive adhesive which can be positioned on the face of the device or in the back of the device and extending peripherally. Both adhesive systems should fulfill the following criteria (18)
(i)Should adhere to the skin aggressively, should be easily removed.
(ii)Should not leave an unwashable residue on the skin.
(iii) Should not irritate or sensitize the skin.
The face adhesive system should also fulfill the following criteria.
(i)Physical and chemical compatibility with the drug, excipients and enhancers of the device of which it is a part.
(ii) Permeation of drug should not be affected.
(iii) The delivery of simple or blended permeation enhancers should not be affected. eg. Polyisobutylenes, acrylics and silicones.
b) Backing membrane:
Backing membranes are flexible and they provide a good bond to the drug reservoir, prevent drug from leaving the dosage form through the top, and accept printing. It is impermeable substance that protects the product during use on the skin e.g. metallic plastic laminate, plastic backing with absorbent pad and occlusive base plate (aluminium foil), adhesive foam pad (flexible polyurethane) with occlusive base plate (aluminium foil disc) etc.(18)
5. MECHANISM OF PERMEATION
Percutaneous absorption involves passive diffusion of substances through the skin. The mechanism of permeation can involve the passage through the epidermis itself known as transepidermal absorption.
Permeation by the transepidermal route first involves partitioning into the stratum corneum. Diffusion then takes place across this tissue . Most substances diffuse across the stratum corneum via the intercellular lipoidal route . When a permeating drug exists at the stratum corneum, it enters the wet cell mass of the epidermis and since the epidermis has no direct blood supply, the drug is forced to diffuse across it to reach the vasculature immediately beneath . The viable epidermis is considered as a single field of diffusion in models. It is permeable field that functions as a viscid watery regime to most penetrants . The epidermal cell membranes are tightly joined and there is little to no intercellular space for ions and polar non electrolyte molecules to diffusionally squeeze through. Thus, permeation requires frequent crossings of cell membranes .
Permeation through the dermal region represents a final hurdle to systemic entry. Permeation through the dermis is through the interlocking channels of the ground substance. Diffusion through the dermis occurs through the gaps between the collagen fibres which are far too wide to filter large molecules.
6.RATIONALE FOR DRUG CANDIDATE USED IN TRANSDERMAL SYSTEM
1) Transdermal patch is used when the patient has intolerable side effects including constipation and who is unable to take the oral medication and is requesting for an alternative method of drug delivery.
2) It is also used when the pain control might be improved by reliable administration .
3) Transdermal patches are used for the drugs which have to be administered for long period of time or which causes adverse effects to the non-target tissues.
4) The use of the transdermal patch is not suitable when the cure of acute pain is required and where the requirement of the dose is equal to or less than 30mg/24hrs.
5) Transdermal delivery is an important option for delivering drugs with poor oral bioavailability and those that undergo extensive first-pass hepatic metabolism.
6) It is also very useful delivery option for drugs with short half-lives or inconvenient dosing regimens and for the drugs with narrow therapeutic window.
7) Transdermal patches are convenient, user friendly, and painless and permit self administration. They offer multiday dosing, which improves patient compliance with the drug regimen.
8) Administration of drug via transdermal patch produces a steady drug serum concentration without significant differences in peak and trough serum concentrations during the dosing interval . For some drugs this is associated with fewer systemic side effects.
9) It avoids the need for invasive parentral administration .
10) It is an alternative route of administration if patients cannot tolerate oral dosage forms or if the drug causes gastrointestinal upset .
11) It is suitable for the patients who are nauseous or unconscious.
12) Drug therapy can be terminated rapidly.
13) Commonly used dosage forms in patches were creams, lotions, ointments, gels .
7.BIOLOGICAL PARAMETERS
The biological parameters should also be considered during drug selection in the preparation of transdermal patches. Biological factors should be considered such as skin irritation, site of application of the patch , example: Scopolamine patch for motion sickness is applied backside of the ear and the Transderm-Nitro is applied on the chest. When a pharmacologically active material has to be presented to the skin, an occlusive or allergic response is significant, limits have to be determined for the acceptability of the undesired effect.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
8.THE FUTURE OF TRANSDERMAL THERAPY
Ten years ago, the nicotine patch had revolutionized smoking cessation; patients were being treated with nitroglycerin for angina, clonidine for hypertension, scopolamine for motion sickness, and estradiol for estrogen deficiency, all through patches. At that time, biotech medicinals were still being developed. During the past decade biotech products have come into their own, but transdermals have essentially remained static. The number of drugs formulated in patches has hardly increased, and there has been little change in the composition of the patch systems. Modifications have been mostly limited to refinements of the materials used . One reason for this undoubtedly is the fact that only certain specialized firms can manufacture transdermal patches. Companies prefer to have full control of their projects, and to enjoy the higher profits on products developed and manufactured in house. Another reason is that only a limited number of drugs fit the molecular weight, lipophilicity, and potency requirements for transdermal absorption.
Molecular Absorption Enhancement:
Considerable research has been done on absorption enhancers, compounds that promote the passage of drugs through the stratum corneum. Terpene derivatives as well as certain phenols seem to improve transdermal absorption. For example, linalool, alpha terpineol, and carvacrol were studied in conjunction with haloperidol (a commonly prescribed neuroleptic drug). All three enhanced haloperidol absorption, but only linalool increased it to a therapeutic level . Limonene, menthone, and eugenol were found to enhance transdermal absorption of tamoxifen . Phloretin, a polyphenol, enhanced the absorption of lignocaine . In general, absorption enhancement research has been done with excised animal skin (pig or rabbit) or human skin obtained from cadavers or plastic surgery procedures.
In contrast, an interesting clinical trial was reported from Australia where estradiol was formulated as a metered-dose aerosol, using padimate O [a para-aminobenzoic acid (PABA) derivative used as a sunscreen agent] as the penetration enhancer. The volunteer subjects were four healthy, postmenopausal women. The aerosol was applied to the ventral forearm in three sprays, each delivering one milligram of estradiol. Each spray covered 10 cm2 of skin. After administering the spray, the skin was not touched for two minutes, but then normal activity, including washing and dressing, was resumed. The drug was applied in this way for nine successive days. Plasma estradiol/estrone ratios obtained for the topical aerosol were consistent with those produced by a topical gel and a transdermal patch, showing that a clinically relevant dose of estradiol was delivered. Using the Draize skin irritation test, no irritation was observed.
This dosage form appears to be a practical alternative to the patch, unless inadvertent inhalation of the spray turns out to be a problem.
Absorption Enhancement by Energy Input:
The above are potential adjuvants to the existing “passive” transdermal systems. Also under study is the possibility of active transfer of drugs through the skin by the action of electrical or other forms of energy. The most research has been devoted to iontophoresis; sonophoresis and electroporation have been less well studied.
Iontophoresis is a method of transferring substances across the skin by applying an electrical potential difference. It promotes the transfer of charged ionic drugs and possibly high molecular weight substances such as peptides. Electric current is applied through two electrodes, placed on the patient’s skin. The first, or donor, electrode (cathode) delivers the negatively charged therapeutic agent (e.g., an organic acid), whereas the second, or receptor, electrode (anode) serves to close the circuit. This setup is named cathodal iontophoresis. For positively charged drugs (e.g., amines or peptides), the cell arrangement is reversed (anodal iontophoresis). The silver (anode) and silver chloride (cathode) electrode system—utilized in both types of iontophoresis––is favored largely because it does not affect the drug solution to the extent that other electrode systems can .
Current commercial applications of iontophoresis include intradermal administration of lidocaine as a local anesthetic and dexamethasone for local inflammation. The devices used are typically bench-top systems with patches connected to a power supply through cables; however, innovations in electronic circuit and battery technology may make small, integrated patch-like systems practicable .
A small number of human trials have been conducted as proof of concept. Thus, fentanyl was tested in twelve healthy volunteers who were protected from its opioid effects by naltrexone administration. Analysis of plasma levels of fentanyl revealed transdermal absorption. Luteinizing hormone-releasing hormone , a decapeptide having a molecular weight of about 1200 Dalton , was tested in eight healthy male volunteers, demonstrating that pulsatile delivery of this hormone is feasible .
Electroporation is a technique that delivers high voltage pulses of micro-to-millisecond duration to the skin, causing transient changes in cell membranes or lipid bilayers . It is hypothesized that pretreatment of the skin in this way would enable the passage of large, polar molecules such as heparin and peptides.
Low frequency ultrasound treatment, or sonopheresis, was reported to enhance absorption of mannitol, which was used as a model for highly hydrophilic drugs .
A Potential Breakthrough? :
The literature cited in this pick-and-choose historical article on both the molecular and energetic enhancement of trans-dermal penetration is of academic interest, but has not resulted in new products. However, a breakthrough might have occurred recently. At a recent meeting of the American Society of Plastic Surgeons, a firm announced the market introduction of its new ActiPatch Therapy––a dermal patch with an embedded microchip that delivers weeks of continuous therapy. The patch is described as the miniaturized equivalent of the pulsed electromagnetic energy machines long used by physicians to reduce swelling, relieve pain and enhance healing of surgical incisions, accidental wounds and bedsores . Although this patch does not deliver drug therapy, the fact that a microchip can act as a continuous source of energy may be applicable in the future to iontophoresis. Thus, we may see major advances in the currently stagnant field of transdermal drug delivery.
9.RECENT RESEARCH DONE IN THE FIELD
Many research works have been and are few are going on in this field. Few of the latest research done in the field of transdermal patches are stated below:
9.1 Pain-free diabetic monitoring using transdermal patches:
 The first prototype patch measures about 1cm2 and is made using polymers and thin metallic films. The 5×5 sampling array can be clearly seen, as well as their metallic interconnections. When the seal is compromised, the interstitial fluid, and the biomolecules contained therein, becomes accessible on the skin surface. Utilizing micro-heating elements integrated into the structural layer of the patch closest to the skin surface, a high-temperature heat pulse can be applied locally, breaching the stratum corneum. During this ablation process, the skin surface experiences temperatures of 130°C for 30ms duration. The temperature diminishes rapidly from the skin surface and neither the living tissue nor the nerve endings are affected. This painless and bloodless process results in disruption of a 40–50μm diameter region of the dead skin layer, approximately the size of a hair follicle, allowing the interstitial fluid to interact with the patch's electrode sites.
The first prototype patch measures about 1cm2 and is made using polymers and thin metallic films. The 5×5 sampling array can be clearly seen, as well as their metallic interconnections. When the seal is compromised, the interstitial fluid, and the biomolecules contained therein, becomes accessible on the skin surface. Utilizing micro-heating elements integrated into the structural layer of the patch closest to the skin surface, a high-temperature heat pulse can be applied locally, breaching the stratum corneum. During this ablation process, the skin surface experiences temperatures of 130°C for 30ms duration. The temperature diminishes rapidly from the skin surface and neither the living tissue nor the nerve endings are affected. This painless and bloodless process results in disruption of a 40–50μm diameter region of the dead skin layer, approximately the size of a hair follicle, allowing the interstitial fluid to interact with the patch's electrode sites.
9.2 Testosterone Transdermal Patch System in Young Women with Spontaneous Premature Ovarian Failure:
In premenopausal women, the daily testosterone production is approximately 300 µg, of which approximately half is derived from the ovaries and half from the adrenal glands. Young women with spontaneous premature ovarian failure (sPOF) may have lower androgen levels, compared with normal ovulatory women. Testosterone transdermal patch (TTP) was designed to deliver the normal ovarian production rate of testosterone. The addition of TTP to cyclic E2/MPA therapy in women with sPOF produced mean free testosterone levels that approximate the upper limit of normal.
9.3 Transdermal Patch of Oxybutynin used in overactive Bladder:
The product is a transdermal patch containing Oxybutynin HCl and is approved in US under the brand name of Oxytrol and in Europe under the brand name of Kentera. OXYTROL is a thin, flexible and clear patch that is applied to the abdomen, hip or buttock twice weekly and provides continuous and consistent delivery of oxybutynin over a three to four day interval. OXYTROL offers OAB patient’s continuous effective bladder control with some of the side effects, such as dry mouth and constipation encountered with and oral formulation. In most patients these side effects however are not a troublesome.
9.4 Transdermal Patch (Ortho Evra™)
 The patch is 4.5 square centimeters in size and has three layers: the inner release liner which should be removed before application, a layer containing hormones, and an outer polyester protective layer. The patch contains 6 milligram of progestin, Norelgestromin 0.75 milligram of Ethinyle Estradiol. The patch is applied on the skin through which the hormones are absorbed in order to provide continuous flow of hormones during menstrual cycle. The patch is marketed by Ortho McNeil Pharmaceutical with the brand name Ortho Evra.
The patch is 4.5 square centimeters in size and has three layers: the inner release liner which should be removed before application, a layer containing hormones, and an outer polyester protective layer. The patch contains 6 milligram of progestin, Norelgestromin 0.75 milligram of Ethinyle Estradiol. The patch is applied on the skin through which the hormones are absorbed in order to provide continuous flow of hormones during menstrual cycle. The patch is marketed by Ortho McNeil Pharmaceutical with the brand name Ortho Evra.
9.5 Rotigotine transdermal patch:
The rotigotine transdermal patch is used for symptom control in Parkinson’s disease. The patches are effective in reducing the symptoms of early Parkinson’s disease, and in reducing “off” time in advanced Parkinson’s disease. It is available in market under the brand name of NeuproR.

NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
10. MARKETED FORMULATIONS
10.1) Transdermal Patches containing Nitroglycerine: Transderm-Nitro (Alza), Nitro dur (Key Pharmaceuticals), Nitro disc (Searle,USA), Deponit (Schwarz Pharma), Minitran (3M Pharmaceuticals).
10.2) Transdermal Patch containing Scopolamine: Transderm-Scop (Alza).
10.3) Transdermal Patch containing Clonidine: Catapres TTS (Alza).
10.4) Transdermal Patch containing Estradiol: Climara (3M Pharmaceuticals), Estraderm (Alza).
10.5) Transdermal Patch containing Nicotine: Nicoderm (Alza).
10.6) Transdermal Patch containing Testosterone: Testoderm (Alza).
10.7) Transdermal Patch containing Insulin: U-Strip (Dermisonics)
11. MOST RECENT PATENTS
Below are listed some of the most recent patents made in the field of transdermal patch.
Patent No.
Date
Title
Information
11.1 US741530633
Aug. 19, 2008
Transdermal Delivery System for Anti-Emetic Medication:
The present invention provides a transdermal delivery system for hydrophilic anti-emetic agents and methods of using thereof. The system includes an anti-emetic hydrophilic adhesive composition of a hydrophilic polymer and hydrophilic anti-emetic agent, a patch containing at least one hydrophilic layer of the composition, and an apparatus that generates hydrophilic micro-channels in skin of a subject using the patch or composition
11.2 US741374834
Aug. 19, 2008
Transdermal Buprenorphine to treat Pain in Sickle Cell Crisis:
A specific dosage regimen of buprenorphine achieves pain relief from painful episodes due to sickle cell disease. The dosage regimen comprises administering to a patient in need of pain relief from sickle cell disease at least one BTDS transdermal patch.
11.3 US738778935
Jul. 17, 2008
Transdermal Delivery of Non-Steroidal Anti Inflammatory Drugs:
The present invention provides a transdermal drug delivery system which comprises: a therapeutically effective amount of a non-steroidal anti-inflammatory drug; at least one dermal penetration enhancer, which is a safe skin-tolerant ester sunscreen ester; and at least one volatile liquid. The invention also provides a method for administering at least one systemic or locally acting non-steroidal anti-inflammatory drug to an animal
11.4 US739812136
Jul. 8, 2008
Iontophoresis Device:
An iontophoresis device useful for administering an ionic drug by iontophoresis has an iontophoresis electrode section and a ground electrode section
Both of which are to be connected to a power source. The iontophoresis device includes elements (members) of both of the electrode sections are all formed of membrane bodies, and includes ion exchange membranes different in ion selectivity, one being selective to ions of the same species as charged ions of the ionic drug and the other to ions different in species from the charged ions of the ionic drug that are arranged in the iontophoresis electrode section, and at least an ion exchange membrane selective to ions opposite to the charged ions of the ionic drug is arranged in the ground electrode section. The iontophoresis device can administer the ionic drug stably over a long period of time at high transport efficiency.
11.5 US739511137
Jul. 1, 2008
Transdermal Delivery System for Water Insoluble Drug:
The present invention provides a system for transdermal delivery of water insoluble drugs and methods using the same. The system includes a pharmaceutical composition of a water insoluble drug and a carrier molecule that enhances the solubility of the drug in aqueous solution, a medical patch containing the same and an apparatus that generates hydrophilic micro-channels in an area of skin of a subject using the composition or patch.
12. REFERENCES
1) Jain, N.K., Controlled and Novel Drug Delivery,(2005) 100,CBS Publishers & Distributors,New Delhi.
2) Mathiowitz, Edith, Encyclopedia of Controlled Drug Delivery, Volume I ,360, Wiley India Private limited.
3) Remington, The Science and Practice of Pharmacy, Volume I ,(Edition 21) 948-949.
4) Brahmandkar , D.M., Biopharmaceutics and Pharmacokinetics , (2005) 365-368,Vallabh Prakashan, Delhi.
5) Kandavilli S, Nair V, Panchagnula R. Polymers in transdermal drug delivery systems, Pharmaceutical Technology 2002, 62-78. Available from: pharmtech.com. Accessed on 15 Jan,2008.
6) Guy RH. Current status and future prospects of transdermal drug delivery, Pharm Res 1996, 13, 1765-1769.
7) Guy RH, Hadgraft J, Bucks DA. Transdermal drug delivery and cutaneous metabolism, Xenobiotica 1987, 7, 325-343.
8) Chein YW. Transdermal Controlled Systemic Medication. New York and Basel, Marcel Dekker Inc. 1987; 159 – 176.
9) Keith AD. Polymer matrix considerations for transdermal devices, Drug Dev. Ind. Pharm 1983, 9, 605.
10) Baker RW, Heller J. Material selection for transdermal delivery systems; In: Hadgraft J, Guys RH, editors. Transdermal Drug Delivery: Development Issues and Research Initiatives. New York, Marcel Dekker Inc. 1989; 293-311.
11) Guyot M, Fawaz F. Design and in vitro evaluation of adhesive matrix for transdermal delivery of propranolol , Int J Pharm 2000, 204, 171-182.
12) Gabiga H, Cal K, Janicki S. Effect of penetration enhancers on isosorbide dinitrate penetration through rat skin from a transdermal therapeutic system, Int J Pharm 2000, 199, 1-6.
13) Minghetti P, Cilurzo F, Casiragh A, Molla FA, Montanari L. Dermal patches for controlled release of miconazole: Influence of drug concentration on the technical characteristics, Drug Dev Ind Pharm 1999, 25, 679-684.
14) Tsai CJ, Hu LR, Fang JY, Lin HH. Chitosan hydrogel as a base for transdermal delivery of berberine and its evaluation in rat skin, Biol. Pharm. Bull 1999, 22, 397-401.
15) Bromberg L. Cross linked polyethylene glycol networks as reservoirs for protein delivery, J Apply Poly Sci 1996, 59, 459-466.
16) Verma PRP, Iyer SS. Transdermal delivery of propranolol using mixed grades of eudragit: Design and in vitro and in vivo evaluation, Drug Dev Ind Pharm 2000, 26, 471-476.
17) Ubaidulla U, Reddy MV, Ruckmani K, Ahmad FJ, Khar RK. Transdermal therapeutic system of carvedilol: Effect of hydrophilic and hydrophobic matrix on in vitro and in vivo characteristics, AAPS Pharm SciTech 2007, 8(1), Article 2.
18) Gannu R, Vamshi Vishnu Y, Kishan V, Madhusudan Rao Y. Development of nitrendipine transdermal patches: In vitro and ex vivo characterization, Current Drug Delivery 2007, 4, 69-76.
19) Gale R, Spitze LA. Permeability of camphor in ethylene vinyl acetate copolymers. In proceedings: Eighth International Symposium on Controlled Release of Bioactive Materials. Minneapolis, MN, Controlled Release Society. 1981; 183.
20) Boretos JW, Detmer DE , Donachy JH. Segmented polyurethane: a polyether polymer II. Two year experience, J Biomed Mat Res 1971, 5, 373.
21) Chung SJ. Future drug delivery research in South Korea, J Controlled Release 1999, 62, 73-79.
22) Izumoto T, Aioi A, Uenoyana S, Kariyama K, Azuma M. Relationship between the transference of drug from a transdermal patch and physicochemical properties, Chem Pharm Bull (Tokyo) 1992, 40, 456-458.
23) Gordon RA, Peterson TA. Four myths about transdermal drug delivery, Drug Delivery Technology 2003, 3, 1-7.
24) Williams AC, Barry BW. Penetration enhancers, Advanced drug delivery reviews 2004, 56, 603-618.
25) Karande P, Jain A, Ergun K, Kispersky V, Mitragotri S. Design principles of chemical penetration enhancers for transdermal drug delivery, Proceedings of the national academy of sciences of the United States of America 2005,102, 4688-4693.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









