 About Authors:
About Authors:
Roopesh Sachan*1, Prof. Satyanand Tyagi2, Tarun Parashar1, Soniya1, Patel Chirag J*3,Patel Pinkesh3, Rishikesh Gupta4
1*Department of Pharmaceutics, Himalayan Institute of Pharmacy and Research, Rajawala, Dehradun, Uttarakhand, India-248007.
2President & Founder, Tyagi Pharmacy Association (TPA) & Scientific Writer (Pharmacy), Chattarpur, New Delhi, India-110074.
3Department of Pharmaceutics, Maharishi Arvind Institute of Pharmacy, Mansarovar, Jaipur, Rajasthan, India-302020.
4Institute of Pharmacy, Bundelkhand University, Jhansi, Uttar Pradesh, India-284128.
*roopeshsachan@gmail.com, +91-9557469989, 9236167104
ABSTRACT:
Nanoparticle drug-delivery systems are the popular ones as are able to increase the selectivity and stability of therapeutic agents. However reticuloendothelial system (RES) uptake, drug leakage, immunogenicity, hemolytic toxicity, cytotoxicity, hydrophobicity restrict the use of these nanostructures. These shortcomings are overcome by surface engineering the dendrimer such as Polyester dendrimer, Citric acid dendrimer, Arginine dendrimer, Glycodendrimers, PEGylated dendrimers, etc.The field of Dendrimers has recently emerged as the most commercially viable technology of this century because of its wide-ranging potential applications in many fields such as: healthcare, electronics, photonics, biotechnology, engineering products, pharmaceuticals, drug delivery, catalysis, electronic devices, environmental issues and nanotechnologies. Dendrimer as a drug delivery agent is a promising, safe and selective drug delivery option.
It’s highly selective nature for targeting the desired tissue is the most essential property and holds a promising future for the treatment of several disorders. Its other properties like very small size, polyvalency, monodispersity, stability make it an appropriate carrier for delivering drugs with precision and selectivity.
Dendrimers are being used as drug delivery systems for various drugs like anticancer drugs (methotrexate), drug for prevention of HIV, enhancing bioavailability of pilocarpine for ocular drug delivery. Dendrimers help in achieving increased bioavailability, sustained, controlled as well as targeted release of drug. There is reduction in the amount of drug and systemic toxicity while the therapeutic efficacy increases. This approach as a drug delivery system certainly promises a reliable, safe, selective and precise method of drug delivery.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1583
INTRODUCTION
The word “Dendrimer” originated from two words, the Greek word dendron, meaning tree, and meros, meaning part [1]. Dendrimer is a nanoparticle (10-9) and so has advantages over microparticles or others due to its small size, easy uptake by cells (through endocytosis). They are branched macromolecules have a central core unit having a high degree of molecular uniformity, narrow molecular weight, distribution, specific size and shape characteristics, and a highly- functionalized, terminal surface [2].
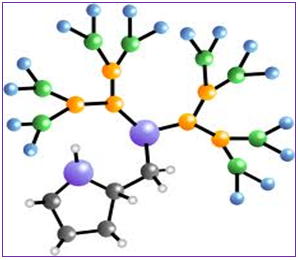
Figure 1: Structure of dendrimer
The manufacturing process is a series of repetitive steps generating shells, starting with a central initiator core. Each subsequent shell represents a new "generation" of polymer with a larger molecular diameter, twice the number of reactive surface sites, and approximately double the molecular weight of the preceding generation [3].
Due to their multivalent and monodisperse character, dendrimers have stimulated wide interest in the field of chemistry and biology, especially in applications like drug delivery, gene therapy and chemotherapy. Dendrimers have cellular uptake through endocytosis and thus brings drug “bound” to dendrimers into the cell [4].
[adsense:468x15:2204050025]
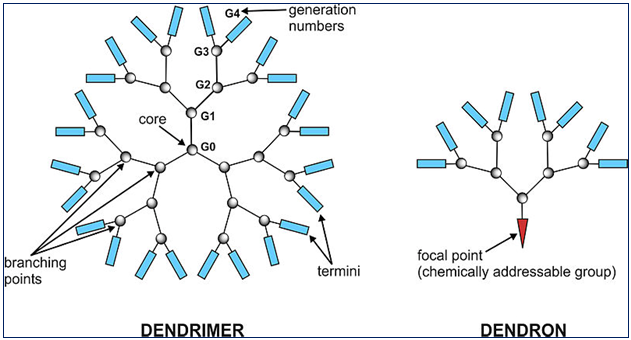
Figure 2: Dendrimer and Dendron
ADVANTAGES OF DENDRIMERS
1. In target drug delivery: Dendrimers are suitable for targeting solid tumours due to increased permeability, limited drainage in tumour vasculature which will lead to accumulation of macromolecules in tumour (enhanced permeation rate). There is also reduction in amount of drug used via targeted delivery (attaching site specific ligands at surface or magnetic guidance) and thus reduction in systemic toxicity.
2. Drugs can be easily made to remain within layers of skin and not penetrate in systemic circulation.
3. Medication to the affected part inside a patient's body directly.
4. Controlled and sustained release of drugs can be obtained.
5. Bypassing the gastric medium and hence the eschewing the variation due to effect of gastric secretions.
6. Increase in therapeutic efficacy, decrease in side effects: decreased clearance of drug via altered distribution of drug in organs at site of localization and transportation due to controlled and sustained release of the drug.
7. Relatively high drug loading [3, 5].
COMPONENTS OF A DENDRIMER STRUCTURE [1, 3, 6, 7]
1. Generation of Dendrimer
It is the hyperbranching when going from the centre of the dendrimer towards the periphery, resulting in homostructural layers between the focal points (branching points). The number of focal points when going from the core towards the dendrimer surface is the generation number. That is a dendrimer having five focal points when going from the centre to the periphery is denoted as the 5th generation dendrimer. Here, we abbreviate this term to simply a G5-dendrimer, e.g. a 5th generation polypropylene imine is abbreviated to a “G5-PPI-” dendrimer, The core part of the dendrimer is sometimes denoted generation “zero”, or in the terminology presented here “G0”. The core structure thus presents no focal points, as hydrogen substituents are not considered focal points. Intermediates during the dendrimer synthesis are sometimes denoted half-generations; a well-known example is the carboxylic acid-terminated PAMAM dendrimers.
2. Shell
The dendrimer shell is the homo-structural spatial segment between the focal points, the “generation space”. The “outer shell” is the space between the last outer branching point and the surface. The “inner shells” are generally referred to as the dendrimer interior.
3. Pincer
In dendrimers, the outer shell consists of a varying number of pincers created by the last focal point before reaching the dendrimer surface.
In PPI and PAMAM dendrimers the number of pincers is half the number of surface groups (because in these dendrimers the chain divides into two chains in each focal point).
4. End-group
It is also generally referred to as the “terminal group” or the “surface group” of the dendrimer. Dendrimers having amine end-groups are termed “amino-terminated dendrimers”.
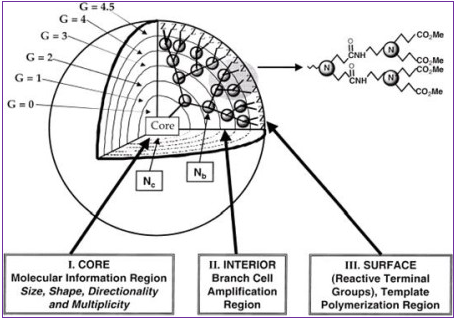
Figure 3: Three dimensional projection of dendrimer core-shell architecture for G=4.5 PAMAM dendrimer with principal architectural components (I) core, (II) interior & (III) surface
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
TYPES OF DENDRIMERS [1, 8-11]
1. Pamam Dendrimer
Poly (amidoamine) dendrimers (PAMAM) are synthesized by the divergent method starting from ammonia or ethylenediamine initiator core reagents. Products up to generation 10(7) (a molecular weight of over 9, 30,000 g/mol) have been obtained (by comparison, the molecular weight of human hemoglobin is approximately 65,000 g/mol).
PAMAM dendrimers are commercially available, usually as methanol solutions. Starburst dendrimers is applied as a trademark name for a sub-class of PAMAM dendrimers based on a tris-aminoethylene-imine core.
The name refers to the star like pattern observed when looking at the structure of the high-generation dendrimers of this type in two-dimensions.
2. Pamamos Dendrimer
Radially layered poly (amidoamine-organosilicon) dendrimers (PAMAMOS) are inverted unimolecular micelles that consist of hydrophilic, nucleophilic polyamidoamine (PAMAM) interiors and hydrophobic organosilicon (OS) exteriors. These dendrimers are exceptionally useful precursors for the preparation of honeycomb-like networks with nanoscopic PAMAM and OS domains.
3. PPI Dendrimer
PPI-dendrimers stand for “Poly (Propylene Imine)” describing the propylamine spacer moieties in the oldest known dendrimer type developed initially by Vögtle. These dendrimers are generally poly-alkyl amines having primary amines as end groups, the dendrimer interior consists of numerous of tertiary tris-propylene amines. PPI dendrimers are commercially available up to G5, and has found widespread applications in material science as well as in biology. As an alternative name to PPI, POPAM is sometimes used to describe this class of dendrimers. POPAM stands for Poly (Propylene Amine), which closely resembles the PPI abbreviation. In addition, these dendrimers are also sometimes denoted “DAB-dendrimers” where DAB refers to the core structure, which is usually based on Diamino butane.
4. Tecto Dendrimer
These are composed of a core dendrimer, surrounded by dendrimers of several steps (each type design) to perform a function necessary for a smart therapeutic nanodevice. Different compounds perform varied functions ranging from diseased cell recognition, diagnosis of disease state drug delivery, reporting location to reporting outcomes of therapy.
5. Multiple Antigen Peptide Dendrimers
It is a dendron-like molecular construct based upon a polylysine skeleton. Lysine with its alkyl amino side-chain serves as a good monomer for the introduction of numerous of branching points. This type of dendrimer was introduced by J. P. Tam in 1988, has predominantly found its use in biological applications, e.g. vaccine and diagnostic research.
6. Fréchet Type Dendrimers
It is a more recent type of dendrimer developed by Hawker and Fréchet based on poly-benzyl ether hyper branched skeleton. These dendrimers usually have carboxylic acid groups as surface groups, serving as a good anchoring point for further surface functionalisation, and as polar surface groups to increase the solubility of this hydrophobic dendrimer type in polar solvents or aqueous media.
7. Multilingual Dendrimers
In these dendrimers, the surface contains multiple copies of a particular functional group.
8. Chiral Dendrimers
The chirality in these dendrimers are based upon the construction of a constitutionally different but chemically similar branches to chiral core.
9. Hybrid Dendrimers Linear Polymers
These are hybrids (block or graft polymers) of dendritic and linear polymers.
10. Amphiphilic Dendrimers
They are built with two segregated sites of chain end, one half is electron donating and the other half is electron withdrawing.
11. Micellar Dendrimers
These are unimolecular micelles of water soluble hyper branched polyphenylenes.
METHOD OF PREPARATION
1. Divergent Method:
Divergent dendrimer synthesis is a technique that effectively grows the dendrimer structure from the initiator core to the periphery in a stepwise fashion by iterative addition of monomer units.
Specifically, it is initiated by coupling of a monomer unit to a multifunctional initiator core where the dendrimer generation increases by successive addition of the building blocks to the surface of the parent dendrimer. Tomalia and co-workers used this strategy to couple N-(2-aminoethyl) acryl amide monomers to an ammonia core to develop PAMAM?--NH2 dendrimers. Each branching unit is synthesized in a two step sequence starting with exhaustive Michael addition of the acrylate ester to the ammonia core followed by amidation with excess EDA. The first step produces a half-generation, and the addition of the diamine yields the full generation[1, 12].
2. Convergent Method:
The convergent approach to dendrimer synthesis was developed to address the deficiencies of the divergent method. Convergent synthesis begins with the dendrimer surface units coupled to additional building blocks to form the branching structure, thus constructing dendrons from the periphery toward the central focal point. Each dendron is then coupled through its focal point to a multifunctional core to produce the complete dendrimer. Unlike divergent synthesis, convergent reactions are simple to purify since the desired dendrons are substantially different from the reaction byproducts, thus eliminating the need for highly efficient reactions. While the number of synthetic steps is similar for both convergent and divergent techniques, the convergent approach has fewer non-ideal growth events, which leads to improved mono-dispersity of the final dendrimers [1, 13].
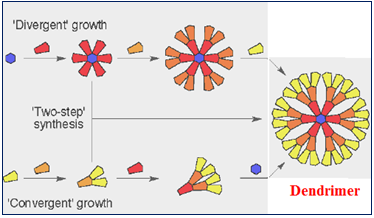
Figure 4: Preparations of Dendrimers by divergent and convergent method
3. Double Exponential growth: Monomers are prepared from a single starting for divergent and convergent growth. Resulted two products reacted to give orthogonally protected trimer, which can be used to repeat the growth again [5].
AEFFECT OF VARIOUS FACTORS ON THE PROPERTIES OF DENDRIMERS
1. Effect of pH
Amino-terminated PPI and PAMAM dendrimers have basic surface groups as well as a basic interior. For these types of dendrimers with interiors containing tertiary amines, the low pH region generally leads to extended conformations due to electrostatic repulsion between the positively charged ammonium groups. Applying molecular dynamics to predict the structural behaviour of PAMAM dendrimers as a function of pH show that the dendrimer has an extended conformation, based on a highly ordered structure at low pH (pH<4). At this pH, the interior is getting increasingly “hollow” as the generation number increases as a result of repulsion between the positively charged amines both at the dendrimer surface and the tertiary amines in the interior. At neutral pH, back-folding occurs which may be a consequence of hydrogen bonding between the uncharged tertiary amines in the interior and the positively charged surface amines. At higher pH (pH>10) the dendrimer contract as the charge of the molecule becomes neutral, acquiring a more spherical (globular) structure, where the repulsive forces between the dendrimer arms and between the surface groups reaches a minimum. At this pH, the conformation has a higher degree of back-folding as a consequence of the weak “inter-dendron” repulsive forces (Fig. 4).
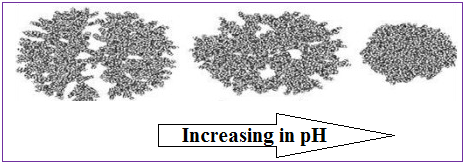
Figure 5: Three-dimensional structure of a G6-PAMAM dendrimer, under different pH.
When looking at the pH-dependent conformational changes of PPI dendrimers having acidic (carboxylic acid) end-groups, the picture is somewhat different compared to what is observed for their amino-terminated counterparts (Figure 5). Small angle neutron scattering (SANS) and NMR measurements of self-diffusion coefficients at different pH values show that at pH 2 the dendrimer core has the most extended conformation due to the electrostatic repulsion between the positively charged protonated tertiary amines, leading to a large radius of the core, whereas the dendrimer reaches its minimum radius at pH 6, where the amount of positively charged amines equals the amount of negatively charged carboxylic groups (isoelectric point) resulting in a “dense core” conformation more subjective to back-folding. Thus, at pH 6 some degree of back-folding occurs as a result of attractive interactions between the negatively charged surface carboxy-groups and the positively charged tertiary amines in the inner shells of the dendrimer. At pH 11 the electrostatic repulsion between the negative charged forces the surface groups apart to give a more extended conformation with a highly expanded surface area (Fig. 5) [14, 15].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
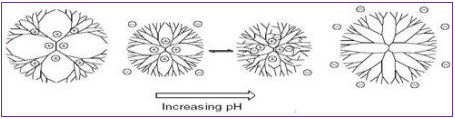
Figure 6: Two-dimensional depiction of conformational changes upon different pH of a carboxy-terminated PPI-dendrimer.
2. Effect of Solvent
The ability of the solvent to solvate the dendrimer structure is a very important parameter when investigating the conformational state of a dendrimer. Dendrimers of all generations generally experience a larger extent of back-folding with decreasing solvent quality, i.e. decreasing solvation. However, being more flexible, the low generation dendrimers show the highest tendency towards back-folding as a result of poor solvation compared to the higher generation dendrimers. NMR studies performed on PPI dendrimers conclude that a nonpolar solvent like benzene, poorly solvates the dendrons favoring intramolecular interactions between the dendrimer segments and back-folding.
However, a weakly acidic solvent like chloroform can act as a hydrogen donor for the interior amines in a basic dendrimer like PPI, leading to an extended conformation of the dendrimer because of extensive hydrogen bonding between the solvent and the dendrimer amines. Both experimental as well as theoretical studies on amino-terminated PPI and PAMAM dendrimers (polar dendrimers) show the tendency that nonpolar aprotic (poor) solvents induce higher molecular densities in the core region as a result of back-folding, whereas polar (good) solvents solvate the dendrimer arms and induce a higher molecular density on the dendrimer surface. Back-folding of the polar surface groups may expose the more hydrophobic dendrimer parts to the surroundings leading to a decreased surface polarity of the back-folded dendrimer [16].
3. Effect of Salt
High ionic strength (high concentration of salts) has a strong effect on charged PPI dendrimers and favors a contracted conformation of dendrimers, with a high degree of back-folding somewhat similar to what is observed upon increasing pH or poor salvation. At low salt conditions, the repulsive forces between the charged dendrimer segments results in an extended conformation in order to minimize charge repulsion in the structure (Fig. 6) [1, 14].
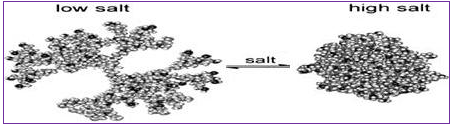
Figure 7: The three-dimensional conformational change of a PPI dendrimer upon increasing ionic strength
4. Effect of Concentration
In dendrimers with flexible structures the conformation is not only affected by small molecules like solvents, salts or protons, but may also be sensitive to larger objects, such as other dendrimers or surfaces which can have a great affect on the molecular density and conformation of the dendrimer.
Small angle X-ray scattering (SAXS) experiments performed on PPI dendrimers (G4, G5) in a polar solvent like methanol show that the molecular conformation of dendrimers upon increasing concentration becomes increasingly contracted. This molecular contraction may minimize the repulsive forces between the dendrimer molecules and increase the ability of the dendrimers to exhibit a more tight intermolecular packing [1].
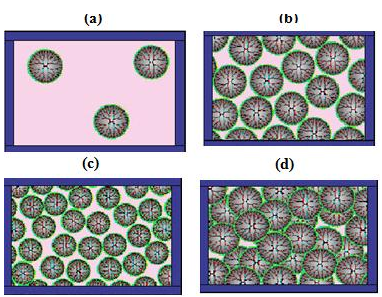
Figure 8: Dendrimer at different concentrations (a) Dilute (b) Contact (c) Collapse (d) Interpenetrate
CHARACTERIZATION OF DENDRIMERS
The development of mass spectroscopic techniques such as MALDI and electrospray mass spectrometry has allowed the absolute determination of dendrimer perfection. Mass spectrometric results on dendrimers demonstrate the extreme sensitivity of the technique and the uniformity of the molecular mass. Scattering techniques measure the radius of gyration (Rg) of dendrimers, which is an average of the spatial distribution of all of the units. Transmission electron microscopy (TEM) has been used to image individual dendritic molecules, usually the larger generations. Recently atomic force microscopy (AFM) has also been used to image dendritic molecules.
Following methods can be used for characterization of dendritic polymers.
1. Spectroscopy and spectrometry methods like Nuclear Magnetic Resonance (NMR), Infra-red (IR) and Raman, Ultra-violet-visible (UV-VIS), Fluorescence, Chirality, Optical rotation, Circular dichroism (CD), X-ray diffraction, and Mass spectrometry
2. Scattering techniques like Small angle X-ray scattering (SAXS), Small angle neutron scattering (SANS), and Laser light scattering (LLS)
3. Electrical techniques like Electron paramagnetic resonance (EPR), Electrochemistry, and Electrophoresis
4. Microscopy like Transmission electron microscopy, Scanning electron microscopy and atomic force microscopy
5. Rheology, physical properties like intrinsic viscosity, Differential Scanning Calorimetry (DSC), and Dielectric spectroscopy (DS)
6. Miscellaneous like X-ray Photoelectron Spectroscopy (XPS), measurements of dipole moments, titrimetry, etc [1, 5].
APPLICATIONS OF DENDRIMERS IN DRUG DELIVERY
1. Various routes for dendrimer drug delivery: Oral, parenteral, intra-ocular, nasal
2. Gene therapy, immunodiagnostics: Dendrimers can act as vectors, in gene therapy. PAMAM dendrimers have been tested as genetic material carriers. Numerous reports have been published describing the use of amino-terminated PAMAM or PPI dendrimers as non-viral gene transfer agents, enhancing the transfection of DNA by endocytosis and, ultimately, into the cell nucleus [5, 12].
3. Dendrimer in ocular drug delivery: To enhance pilocarpine bioavailability.
4. Dendrimers in pulmonary drug delivery: For Enoxaparin (40% increase in relative bioavailability by G2 and G3 generation positively charged PAMAM dendrimers).
5. Dendrimer in transdermal drug delivery: Improvement in solubility and plasma circulation time. PAMAM dendrimer complex with NSAIDs as permeation enhancers [17].
6. Dendrimers for controlled release drug delivery: Anticancer drugs like methotrexate, adriamycin.
7. Some of the methods to initiate the release include light, removal of protecting groups, and antibodies. Dendrimers have attracted attention as possible drug carriers because of their unique properties namely their well defined three-dimensional structure, the availability of many functional surface groups, their low polydispersity and their ability to mimic. Drug molecules can be loaded both in the interior of the dendrimers as well as attached to the surface groups. Dendrimers can function as drug carriers either by encapsulating drugs within the dendritic structure, or by inter-acting with drugs at their terminal functional groups via electrostatic or covalent bonds (prodrug)[5, 18].
8. Dendrimers in targeted drug delivery- folic acid PAMAM dendrimers modified with carboxymethyl PEG5000 surface chains.
9. Dendrimers as Nano-Drugs: Poly (lysine) dendrimers modified with sulfonated naphthyl groups have been found to be useful as antiviral drugs against the herpes simplex virus can potentially prevent/reduce transmission of HIV and other sexually transmitted diseases.
10. Dendrimers in photodynamic therapy: The photosensitizer 5-aminolevulinic acid has been attached to the surface of dendrimers and studied as an agent for PDT of tumorigenic keratinocytes .Photosensitive dyes have been incorporated into dendrimers and utilized in PDT devices. This cancer treatment involves the administration of a light- activated photosensitizing drug that selectively concentrates in diseased tissue [5, 19].
CONCLUSION
A large number of drugs being developed today are facing problems of poor solubility, bioavailability and permeability. Dendrimers can work as a useful tool for optimizing drug delivery of such problematic drugs. Also the problem of biocompatibility and toxicity can overcome by careful surface engineering. Dendrimers, due to its superior architecture; high level of branching, multivalency, globular architecture and molecular weight, prove to be a novel and reliable method of drug delivery. The review clearly illustrates the different aspects of dendrimers as novel drug delivery system and there will be accretion in the dendrimers seen as drug delivery systems with the advent of more and more dendrimers used for it. Recent successes in simplifying and optimizing the synthesis of dendrimers provide a large variety of structures with reduced cost of their production.
ACKNOWLEDGEMENT
The corresponding author, Roopesh Sachan is highly thankful to his Parents and Teachers for their moral support and encouragement. Last but not the least, support of all my friends and the one above all of us, the omnipresent God, for answering my prayers for giving me the strength to plod on despite my constitution wanting to give up and throw in the towel, thank you so much Dear Lord.
REFERENCES
1. Senthil KM, Valarmathi S, Priyanka B, Prudhvi DS, Raja A, “Dendrimers: A novel drug delivery system. Journal of Pharmaceutical Science and Technology”, 2012, 4 (7), 972-984.
2. Sonke S, Tomalia DA, “Dendrimers in biomedical applications reflections on the Field”, Advanced Drug Delivery Reviews, 2005, 57, 2126-2129.
3. Jain NK, Khopade AJ, “Dendrimers as potential delivery systems for bioactives”, Advances in controlled and novel drug delivery, 2001, 361-380.
4. Tomalia D.A, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J and Smith P, “A New Class of Polymers: Starburst-Dendritic Macromolecules”, Polym. J., 1985, 17 (1), 117-132.
5. Mishra I. “Dendrimer: A novel drug delivery system. Journal of Drug Delivery & Therapeutics”, 2011, 1 (2): 70-74.
6. Boris D, Rubinstein M, “A self-consistent mean field model of a starburst dendrimer: dense core vs. dense shell”, Macromolecules, 1996, 29, 7251-7260.
7. Jevprasesphant R, Penny J, Jalal R, Attwood D, McKeown N.B and D’Emanuele A, “The influence of surface modification on the cytotoxicity of PAMAM dendrimers”, Int. J. Pharm., 2003, 252, 263-266.
8. Pushkar S, Philip A, Pathak K and Pathak D, “Dendrimers: Nanotechnology Derived Novel Polymers in Drug Delivery”, Indian J. Pharm. Educ. Res., 2006, 40 (3), 153-158.
9. Hawker C, Fréchet JMJ, “A new convergent approach to monodisperse dendritic molecule” J. Chem. Soc. Chem. Commun., 1990, 15, 1010-1012.
10. Frechet JMJ, Tomalia DA, “Introduction to the Dendritic state”, Dendrimers and other Dendritic Polymers, John Wiley & Sons Ltd, 2001, 24-23.
11. Hawker C, Wooley KL, Fréchet JMJ, J. “Dendrimers and Related Polymers”, Chem. Soc. Perkin. Trans., 1993, 1, 1287-1289.
12. Sonke S, Tomalia DA, “Dendrimers in biomedical applications reflections on the Field”, Advanced Drug Delivery Reviews, 2005, 57, 2106-2129.
13. Barbara K, Maria B, “Review Dendrimers: properties and applications”, Acta Biochimica Polonica, 2001, 48 (1), 199-208.
14. Wang DJ, Imae T, “fluorescence emission from Dendrimer & its pH dependence”, J. Am. Chem. Soc., 2004, 126 (41), 13204-13205.
15. Gupta U, Agashe H, Jain NK, “Polypropylene imine dendrimer mediated solubility enhancement: effect of pH and functional groups of hydrophobes”, J. Pharm. Sci., 2007, 10 (3), 358-67.
16. Chai M, Niu Y, Youngs WJ, Rinaldi PL, “Structure and conformation of DAB dendrimers in solution via multidimensional NMR techniques”, J. Am. Chem. Soc., 2001, 123, 4670-4678.
17. Peeyush K, “Dendrimer: a novel polymer for drug delivery”, JITPS, 2010, 1(6), 252-269.
18. Gillies ER and Fréchet JMJ, “Dendrimers and dendritic polymers in drug delivery” Drug Discovery Today, 2005, 10, 35-43.
19. Nachiket SD, Shashikant RP, Musmade DS, Gaware VM, Mangesh BH, Santosh RB, Dattatrya AN, “Convergent synthesis: A strategy to synthesize compounds of biological interest”, Scholars Research Library, 2010, 2 (1), 318-328.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









