INTRODUCTION:
An online pharmacy, internet pharmacy, mail-order pharmacy or E-Pharmacy is a pharmacy that operates over the internet and sends the orders to customers through mail or shipping companies. An E-Pharmacy model is required to have two operating components for dispensing prescription medicines.
1) Technology:
• Web-based or mobile-based application for consumers to upload the scanned copy of their prescriptions and place requests for Rx based medicines.
• Every order is verified and checked by a team of registered pharmacists.
• The registered pharmacists to forward the validated prescriptions to the pharmacy store from where the medicines are dispensed.
• The web or mobile-based platform to be governed under the IT Act 2000 and only act as a platform to facilitate connection between consumer and pharmacy store (brick and mortar).
2) Pharmacy Retail Store:
• The licensed pharmacists of the store to check for the validity of the prescriptions; failing which the medicines would not be dispensed.
• The medicines should be dispensed from a licensed premise in a sealed tamper proof pack to the patient or patient’s relative (Patient’s agent).
• There should be proper invoice with batch number of the medicines dispensed, expiry date, name and address of the pharmacy with signature of the registered pharmacist/(s)
• The pharmacy store to be operated under the oversight of the Drugs and Cosmetics Act & Rules and need to comply with all the requirements of the act, as it does for its normal business.
An e-Pharmacy model would help with better purchasing margins, better inventory management, increased reach, reduced prices and greater provision of value-added services to the consumers.
Regulatory authorities and Government of India on E-Pharmacies:
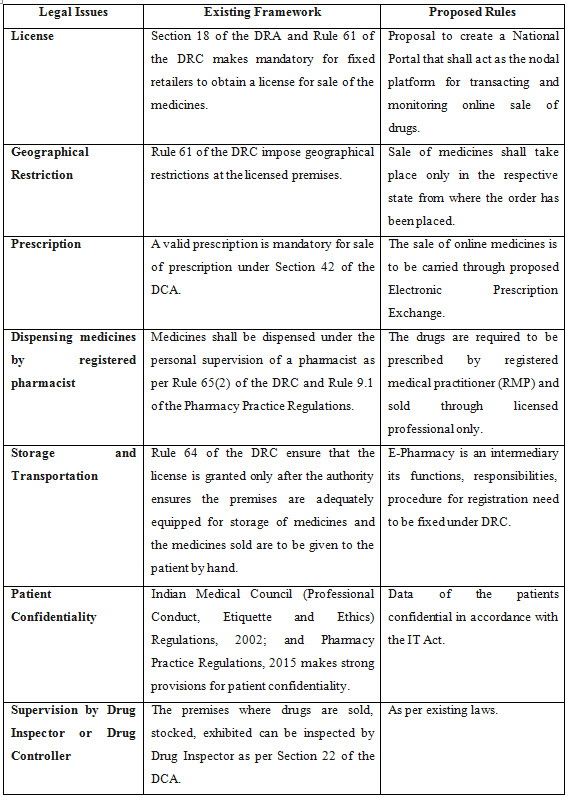
When All India Organization of Chemists and Druggists (AIOCD), an association of brick and mortar retail chemists called on all its 8 lacs members across India to observe a day-long nationwide strike on September 27, 2018, it attracted the attention of Indian government to issue a strong draft rule on the working of E-Pharmacy in India. The drug controller General of India (DCGI) Mr. Eswara Reddy said that “Under the rules it has been proposed that those who want to do the online pharmacy will have to register with the Central Drugs Standard Control Organisation”. Thus, Part VI - ''Sale of Drugs by E-Pharmacy'' has been proposed to be added to the Drugs and Cosmetics, Rules 1945.
On September 2018, the Union Health Ministry of India released the draft rules on sale of drugs by E-Pharmacies in order to regulate online sale of medicines PAN India. This interim draft ended all the debate against E-Pharmacies. It also stated that, no person will distribute or sell, stock, exhibit or offer for sale of drugs through E-Pharmacy unless registered. Some of the important measures proposed by draft rules include:
1. Registration of E-pharmacies: No person is allowed to sell drugs online without obtaining registration for the same.
2. E-pharmacy portal: All orders for E-pharmacies shall be placed only through E-pharmacy portal. Therefore, it is mandatory for every E-pharmacy to have an E-pharmacy portal.
3. Protection of privacy: E-pharmacies should keep all customer information confidential including prescription related information and adhere to applicable information technology laws in India. Further, E-pharmacy portal should be established in India and the data generated should be localized.
4. Sale through cash/credit memo: The supply of drugs by E-pharmacies shall be made only against a cash or credit memo and such memos should be maintained as record.
5. Measures to tackle counterfeit drugs, unauthorized sale and expired products:
• Details like name, address and license number of the licensee who is dispensing the drugs, should be mentioned on the memo.
• Serial number and date of the memo should be mentioned on the memo.
• Details of the drug including name, quantity, batch number, expiry date, manufacturer name should be mentioned on the memo.
• Details of E-pharmacy including name, address and signature/ digital signature of registered pharmacist in-charge should be mentioned on the memo.
6. Prohibition of certain Drugs: E-pharmacies are prohibited from selling drugs covered under the categories of the narcotic and psychotropic substance as referred to in the Narcotic Drugs and Psychotropic Substances Act, 1985 , tranquilizers and the drugs as specified in Schedule X of the Rules.
7. Periodic Inspection of E-pharmacy: The premises from where the E-pharmacy business is conducted shall be inspected, every two years, by the concerned authorities.
8. Details of Drugs and Patients on the E-pharmacy portal: The details of the drugs dispensed including the patient details shall be maintained on the e-pharmacy portal.
9. Verification by registered pharmacist: Every prescription received on E-pharmacy portal should be verified by a registered pharmacist on behalf of the E-pharmacy and details of the patient, registered medical practitioner shall be checked.
10. Prevention of unauthorized sale: All E-pharmacies shall be required to maintain and update information regarding the drugs availability, types of drugs offered for sale, supply channels or vendor lists, details of registered pharmacists, registered medical practitioner etc.
Current Indian laws pertaining to offline and E-Pharmacies:
Currently, Indian Pharmacy law do not distinguish between online and offline pharmacy. Here is the set of rules which is defined in the Indian pharmacy law:
• All the Rx medicine (those medicine/s which can be dispensed against a valid prescription only) sale in India is mandated only in accordance with the Drugs and Cosmetics Act, 1940 (DCA) and Drugs and Cosmetics, Rules 1945 (DCR).
• Any pharmacy retail store / shop must obtain license under Section 18 of the Drugs and Cosmetics Act and Rule 61 of the Drugs and Cosmetics, Rules 1945 to carry sale of prescribed medicines only against a valid prescription of a registered medical practitioner (RMP) as defined in the Section 42 of the Pharmacy Act, 1948.
• Only a registered Pharmacist whose licence has been issued by the particular Indian state drug authority to dispense medicines under Rule 65(2) of the Drugs and Cosmetics, Rules 1945 and Rule 9.1 of the Pharmacy Practice Regulations, 2015.
In nut shell, E-Pharmacy in India comes under the pursuit of Drugs and Cosmetics Act, 1940 and Information Technology Act, 2000.
Current market scenario of E-Pharmacy in India:
India currently has more than 8,50,000 independent pharmacy retail stores, that fulfills only 60% of the total medicine demand PAN India. However, offline pharmacies retail stores (brick and mortal retail pharmacies) are currently responsible for 99% of the medicine sales per year, with E-Pharmacy contributing to only 1% of the total medicine sale in India. Now, sweeping into Tier 2 and Tier 3 cities, E-Pharmacy is making an improvement in the health care accessibility in both rural and urban areas.
Due to increase in the rate of chronic diseases like diabetes, high blood pressure, obesity, asthma etc. in Indians, E-Pharmacy is mushrooming in the E-Commerce segment. Several Indian government programmes in health care segment like Make in India, Digital India, Jan Aushadhi (for promotion of generic medicines), Tele-medicine and E-Healthcare are helping rural India to improve their health in a quality manner. This is the reason, E-Pharmacy is expected to grow at a CAGR of over 20%, crossing the US$ 3 Billion mark by 2024.
On 23rd February 2019, the Department for Promotion of Industry and Internal Trade published the Draft National e-Commerce Policy. It will pave an opportunity for domestic E-Commerce industry in India, with stiff competition among global E-commerce giants like Amazon and Flipkart for lion’s share in the market. One of the segments of E-commerce which is having tremendous potential in the E-commerce segment is E-Pharmacy. Today, the E-Pharmacy have market potential of more than a Billion dollar with giant key players like 1mg, Netmeds, Medlife, Pharm Easy etc.
Suggested recommendation regarding E-Pharmacies in India
Indian Internet Pharmacies Association (IIPA) or Digital Health Platforms (DHP):
IIPA now renamed as DHP is an association of online pharmacy and health space entrepreneurs in India. Currently, Mr. Prashant Tandon is the President of DHP (Digital Health Platform). As per Digital Health Platforms following are the set of rules which association members like 1 mg, M-Chemist, Netmeds, Pharm Easy to name a few and other online pharmacies key players follows for sale of the Rx medicines online:
• No sale without a valid prescription issued by RMP (registered medical practitioner).
• No sale of Schedule X drugs (narcotic and psychotropic substances-based drugs).
• Final packing in a tamper-proof cover under the personal supervision of registered Pharmacist of the pharmacy.
• Valid invoice bill for every order or sale of a medicine.
• Facilitate medicine recall in case directed by the Indian Government.
The IIPA also is working actively with the Indian Government to bring in changes to the regulations including the use of AADHAAR UID number to be linked to prescriptions for ensuring no misuse of medicine online.
Advantages of E-Pharmacy in India:
• Patients or consumers can easily order online medicines from their mobile phones or computers which will help especially elderly/old patients or sick who cannot move or go outside to find a offline pharmacy. Same applies to a group of nuclear family, working couples of tier 1 cities who have no time to search for an offline pharmacy.
• E-Pharmacies allows access to rural areas where there is limited presence of retail pharmacy.
• E-Pharmacies offers health information to consumers about particular medicines like drug interactions, side-effects, medicine reminders etc. which enables the consumer pr patient in improving compliance.
• All online transactions on E-Pharmacy can be tracked with complete details of the medicines, batch number, retail pharmacy name and address, prescribing doctor, name and address of the patient which can reduce drug abuse and self-medication in the patients.
• E-Pharmacy will assist in back-tracing the channel / manufacturer / supplier of the counterfeit (fake) medicines making the health-care market more transparent and authentic.
• All orders on E-Pharmacy are documented with records of the prescriptions with ease in tracking all orders via order ID.
• Every order has a valid invoice GST bill and tax to the Government is paid in full.
• Delivery of cold chain medicines (2-8 degree celsius storage medicines) by E-Pharmacies in cold chain boxes maintains the quality of insulin, hormones and other blood related products up to longer duration of time.
Conclusion:
The Indian Judiciary and regulatory authorities on E-pharmacies should take into consideration the measures introduced by various other developed countries like USA and EU with common logo mark, online tracking system, E-Prescription for certain drugs for handling problems related to online sale of medicine in India.
References:
1) https://health.economictimes.indiatimes.com/news/policy/will-regulations-on-e-pharmacies-affect-the-brick-and-mortar-stores/70036824
2) https://www.internationaltradecomplianceupdate.com/2019/03/22/india-releases-draft-national-e-commerce-policy/
3) https://www.researchandmarkets.com/reports/4537360/india-e-pharmacy-market-opportunity-outlook-2024
4) https://www.omicsonline.org/open-access/epharmacies-regulation-in-india-bringing-new-dimensions-to-pharmasector-2167-7689-1000175.php?aid=80341
5) https://qz.com/india/1494451/delhi-high-court-bans-e-pharmacies-like-netmeds-medlife-1mg/
6) http://ficci.in/spdocument/20746/E-Pharmacy-in-India-Last-Mile-Access-to-Medicines_v5.pdf
7) https://en.wikipedia.org/wiki/Online_pharmacy
- Sunny Sinha, Anand Kotiyal
1Mg Technologies Private Limited,
Gurugram, Haryana









