 ABOUT AUTHORS:
ABOUT AUTHORS:
Anjali* , Lakshmi Goswami, Dr. Preeti Kothiyal
Shri Guru Ram Rai Institute of Technology and Sciences
Patel nagar, Dehradun
anjali23mar@gmail.com
ABSTRACT
In field of nanotechnology nonmaterial are at the leading edge. Nanotechnology has received a lot of attention with enthusiasm because of its future potential. Their unique size-dependent properties make these materials greater and indispensable in many areas of human activity. Particulate systems like nanoparticles have been used as a physical approach to alter and improve the pharmacokinetic and pharmacodynamic properties of various types of drug molecules. Polymeric nanoparticles have been extensively studied as particulate carriers in the pharmaceutical and medical fields, because they show promise as drug delivery systems as a result of their controlled and sustained release properties, subcellular size, biocompatibility with tissue and cells. This technology offers the advantage of protecting drugs from degradation; reduce the number of doses required. This brief review tries to summarise the most recent developments in the field of applied nanomaterials, nanoparticles, solid lipid nanoparticles, nanocrystals, nanosuspensions, nanoemulsions, in particular their application in biology and medicine, and discusses their commercialisation prospect.
[adsense:336x280:8701650588]
Reference ID: PHARMATUTOR-ART-1949
INTRODUCTION
Nanotechnology is science of matter and material that deal with the particle size in nanometers. The word’ nano’ is derived from latin word, which means dwarf (1nm=10-9m). Nanomedicine deals with comprehensive monitoring, control, construction, repair, defense and improve human biological system at molecular level using engineered nanostructures and nanodevices.1 where the drug is dissolved, entrapped, encapsulated or attached to a nanoparticle matrix. Depending upon the method of preparation nanoparticles, nanospheres or nanocapsules can be obtained. Pharmaceutical nanotechnology has provided more fine tuned diagnosis and focused treatment of disease at a molecular level.2 NPs are promising vehicles for drug delivery by easy manipulation to prepare carriers with the objective of delivering the drugs to specific target, such an advantage improves the drug safety. Polymer-based nanoparticles effectively carry drugs, proteins, and DNA to target cells and organs. Their nanometer-size promotes effective permeation through cell membranes and stability in the blood stream. Polymers are very convenient materials for the manufacture of countless and varied molecular designs that can be integrated into unique nanoparticle constructs with many potential medical applications.3
ADVANTAGES OF NANOTECHNOLOGY

Polymers used in preparation of nanoparticles
The polymers should be compatible with the body in the terms of adaptability (non-toxicity) and (non-antigenicity) and should be biodegradable and biocompatible.
Natural polymers: The most commonly used natural polymers in preparation of polymeric nanoparticles are.
* Sodium alginate
* Albumin
* Chitosan
* Gelatin
There are many synthetic polymers like 6,7,8,9,10
* Poly malic acid
* Poly(N-vinyl pyrrolidone)
* Poly(methyl methacrylate)
* Poly(vinyl alcohol)
* Poly(acrylic acid)
* Poly acrylamide
* Poly(ethylene glycol)
* Poly(methacrylic acid)
* Polylactides(PLA)
[adsense:468x15:2204050025]
Methods for preparation of nanoparticles from dispersion of preformed polymer
Dispersion of drug in preformed polymers is a common technique used to prepare biodegradable nanoparticles from poly (lactic acid) (PLA), poly (D, L-glycolide) (PLG), poly (D, L-lactide-co-glycolide) (PLGA) and poly (cyanoacrylate) (PCA). These can be accomplished by different methods described below.
a) Solvent evaporation
b) Nanoprecipitation
c) Emulsification/solvent diffusion
d) Salting out
e) Dialysis
f) Supercritical fluid technology (SCF)
Methods for preparation of nanoparticles from polymerization of monomers
a) Emulsion
b) Mini emulsion
c) Micro emulsion
d) Interfacial polymerization
e) Controlled/Living radical polymerization(C/LRP)
1. Solvent evaporation
Solvent evaporation was the first method developed to prepare NPs. In this method, polymer solutions are prepared in volatile solvents and emulsions are formulated. The emulsion is converted into a nanoparticle suspension on evaporation of the solvent for the polymer, which is allowed to diffuse through the continuous phase of the emulsion. In the conventional methods, two main strategies are being used for the formation of emulsions, the preparation of single-emulsions, e.g., oil-in-water (o/w) or double-emulsions, e.g., (water-in-oil)-in-water, (w/o)/w. These methods utilize high-speed homogenization or ultrasonication, followed by evaporation of the solvent, either by continuous magnetic stirring at room temperature or under reduced pressure. Afterwards, the solidified nanoparticles can be collected by ultracentrifugation and washed with distilled water to remove additives such as surfactants. Finally, the product is lyophilized11,12. Song et al.13 prepared nanoparticles of PLGA with a typical particle size of 60–200nm by employing dichloromethane and acetone (8:2, v/v) as the solvent system and PVA as the stabilizing agent. Particle size was found to be influenced by the type and concentrations of stabilizer, homogenizer speed and polymer concentration.

FIG 1: Schematic representation of the solvent-evaporation technique.
2. Nanoprecipitation
Nanoprecipitation is also called solvent displacement method. It involves the precipitation of a preformed polymer from an organic solution and the diffusion of the organic solvent in the aqueous medium in the presence or absence of a surfactant14-18. The polymer generally PLA, is dissolved in a water-miscible solvent of intermediate polarity, leading to the precipitation of nanospheres. This phase is injected into a stirred aqueous solution containing a stabilizer as a surfactant. Polymer deposition on the interface between the water and the organic solvent, caused by fast diffusion of the solvent, leads to the instantaneous formation of a colloidal suspension19. To facilitate the formation of colloidal polymer particles during the first step of the procedure, phase separation is performed with a totally miscible solvent that is also a non solvent of the polymer20. The solvent displacement technique allows the preparation of nanocapsules when a small volume of nontoxic oil is incorporated in the organic phase. Considering the oil-based central cavities of the nanocapsules, high loading efficiencies are generally reported for lipophilic drugs when nanocapsules are prepared. The usefulness of this simple technique is limited to water-miscible solvents, in which the diffusion rate is enough to produce spontaneous emulsification. Then, even though some water-miscible solvents produce certain instability when mixed in water, spontaneous emulsification is not observed if the coalescence rate of the formed droplets is sufficiently high21. Although, acetone/dichloromethane (ICH, class 2) are used to dissolve and increase the entrapment of drugs, the dichloromethane increases the mean particle size22 and is considered toxic. This method is basically applicable to lipophilic drugs because of the miscibility of the solvent with the aqueous phase, and it is not an efficient means to encapsulate water-soluble drugs. This method has been applied to various polymeric materials such as PLGA, PLA, PCL,23,24 and poly (methyl vinyl ether-comaleic anhydride) (PVM/MA)25,26. This technique was well adapted for the incorporation of cyclosporin A, because entrapment efficiencies as high as 98% were obtained27. Highly loaded nanoparticulate systems based on amphiphilic h-cyclodextrins to facilitate the parenteral administration of the poorly soluble antifungal drugs Bifonazole and Clotrimazole were prepared according to the solvent displacement method 28

Fig 2: Nanoparticles by nanoprecipitation method
3. Emulsification/solvent diffusion (ESD)
This is a modified version of solvent evaporation method29. The encapsulating polymer is dissolved in a partially water soluble solvent such as propylene carbonate and saturated with water to ensure the initial thermodynamic equilibrium of both liquids. In fact, to produce the precipitation of the polymer and the consequent formation of nanoparticles, it is necessary to promote the diffusion of the solvent of the dispersed phase by dilution with an excess of water when the organic solvent is partly miscible with water or with another organic solvent in the opposite case. Subsequently, the polymer-water saturated solvent phase is emulsified in an aqueous solution containing stabilizer, leading to solvent diffusion to the external phase and the formation of nanospheres or nanocapsules, according to the oil-to-polymer ratio. Finally, the solvent is eliminated by evaporation or filtration, according to its boiling point. This technique presents several advantages, such as high encapsulation efficiencies (generally >70%), no need for homogenization, high batch-to-batch reproducibility, ease of scale-up, simplicity, and narrow size distribution. Disadvantages are the high volumes of water to be eliminated from the suspension and the leakage of water-soluble drug into the saturated-aqueous external phase during emulsification, reducing encapsulation efficiency. As with some of the other techniques, this one is efficient in encapsulating lipophilic drugs. Several drug-loaded nanoparticles were produced by the ESD technique, including mesotetra(hydroxyphenyl)porphyrin-loaded PLGA (p-THPP) nanoparticles30,31, doxorubicin-loaded PLGA nanoparticles32, plasmid DNA-loaded PLA nanoparticles33, coumarin-loaded PLA nanoparticles 34, indocyanine 35, cyclosporine (Cy-A)-loaded gelatin and cyclosporin (Cy-A)-loaded sodium glycolate nanoparticles 36.
4. Salting-out procedure
In this method the use of potentially toxic solvents is avoided. Here only acetone is used and it can be easily removed in the final step by cross-flow filtration. The preparation method consists of adding, under mechanical stirring, an electrolyte saturated solution containing a hydrocolloid, generally poly (vinyl alcohol) as a stabilizing and viscosity increasing agent to an acetone solution of polymer. After the preparation of an oil-in-water emulsion, sufficient water or an aqueous solution of PEG is added to allow complete diffusion of acetone into the aqueous phase, thus inducing the formation of nanospheres.37
5. Dialysis
Dialysis offers a simple and effective method for the preparation of small, narrow-distributed PN38,39. Polymer is dissolved in an organic solvent and placed inside a dialysis tube with proper molecular weight cut off. Dialysis is performed against a non-solvent miscible with the former miscible. The displacement of the solvent inside the membrane is followed by the progressive aggregation of polymer due to a loss of solubility and the formation of homogeneous suspensions of nanoparticles. The mechanism of NP formation by dialysis method is not fully understood at present. A number of polymer and copolymer nanoparticles 40,49 were obtained by this technique. Poly(benzyl-l-glutamate)-b-poly(ethylene oxide), Poly(lactide)-b-poly(ethylene oxide) nanoparticles were prepared using DMF as the solvent. The solvent used in the preparation of the polymer solution affects the morphology and particle size distribution of the nanoparticles. Chronopoulou et al.50 reported a novel osmosis based method for the preparation of various natural and synthetic NP.
6. Supercritical fluid technology
The need to develop environmentally safer methods for the production of NP has motivated research on the utility of supercritical fluids as more environmental friendly solvents, with the potential to produce NPs with high purity and without any trace of organic solvent51. Supercritical fluid and dense gas technology are expected to offer an interesting and effective technique of particle production, avoiding most of the drawbacks of the traditional methods.
Two principles have been developed for the production of nanoparticles using supercritical fluids:
1. Rapid expansion of supercritical solution (RESS)
2. Rapid expansion of supercritical solution into liquid solvent (RESOLV).
Rapid expansion of supercritical solution
In traditional RESS, the solute is dissolved in a supercritical fluid to form a solution, followed by the rapid expansion of the solution across an orifice or a capillary nozzle into ambient air. The high degree of super saturation, accompanied by the rapid pressure reduction in the expansion, results in homogenous nucleation and thereby, the formation of well-dispersed particles. Results from mechanistic studies of different model solutes for the RESS process indicate that both nanometer and micrometer-sized particles are present in the expansion jet 52. A few studies were carried out on the production of NPs using RESS. Poly (perfluoropolyetherdiamide) droplets produced from the rapid expansion of CO2 solutions. The RESS experimental apparatus consists of three major units: a high-pressure stainless steel mixing cell, a syringe pump, and a pre-expansion unit. A solution of polymer in CO2 is prepared at ambient temperature. The supercritical solution is now allowed to expand through the nozzle, at ambient pressure. The concentration and degree of saturation of the polymer have a considerable effect on the particle size and morphology of the particles for RESS 53-54.
Fig 3: Rapid expansion of supercritical solution
Rapid expansion of supercritical solution into liquid solvent
A simple, but significant modification to RESS involves expansion of the supercritical solution into a liquid solvent instead of ambient air, termed as RESOL. Meziani et al 55 reported the preparation of Poly (heptadecafluorodecyl acrylate) nanoparticles having an average size of less than 50 nm. Even though in RESS technique no organic solvents used for the formation of NPs, the prime products obtained using this technique are microscaled rather than nanoscaled, which is the main drawback of RESS. In order to overcome this drawback a new supercritical fluid technology known as RESOLV has been developed. In RESOLV the liquid solvent apparently suppresses the particle growth in the expansion jet, thus making it possible to obtain primarily nanosized particles 56.

Fig 4: Rapid expansion of supercritical solution into liquid solvent
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Preparation of nanoparticles by polymerization of a monomer
To attain the desired properties for a particular application, suitable polymer nanoparticles must be designed, which can be done during the polymerization of monomers. Processes for the production of NPs through the polymerization of monomers are discussed below.57
1. Emulsification polymerization: Incase of conventional emulsification polymerization the continuous phase is aqueous (o/w emulsion), where as in inverse emulsification polymerization the continuous phase is organic (w/o emulsion). In both cases the monomer is emulsified in non-solvent phase with surfactant molecules, leading to the formation of monomer-swollen micelles and stabilized monomer droplets. The polymerization reaction takes place in the presence of a chemical or physical initiator. The drug to be associated to the nanospheres may be present during polymerization or can be added to preformed nanospheres, so that the drug can be either incorporated in to the matrix or simply adsorbed at the surface of the nanospheres.58
2. Mini-emulsion polymerization
A typical formulation used in mini- emulsion polymerization consists of water, monomer mixture, co-stabilizer, surfactant, and initiator. The key difference between emulsion polymerization and mini-emulsion polymerization is the utilization of a low molecular mass compound as the co-stabilizer and also the use of a high-shear device (ultrasound, etc.). Mini-emulsions are critically stabilized, require a high-shear to reach a steady state and have an interfacial tension much greater than zero.59-60.
3. Micro-emulsion polymerization
Micro-emulsion polymerization is a new and effective approach for preparing nanosized polymer particles and has attracted significant attention. Although emulsion and micro-emulsion polymerization appear similar because both methods can produce colloidal polymer particles of high molar mass, they are entirely different when compared kinetically. Both particle size and the average number of chains per particle are considerably smaller in micro-emulsion polymerization. In micro-emulsion polymerization, an initiator, typically water-soluble, is added to the aqueous phase of a thermodynamically stable micro-emulsion containing swollen micelles. The polymerization starts from this thermodynamically stable, spontaneously formed state and relies on high quantities of surfactant systems, which possess an interfacial tension at the oil/water interface close to zero. Furthermore, the particles are completely covered with surfactant because of the utilization of a high amount of surfactant. Initially, polymer chains are formed onlyin some droplets, as the initiation cannot be attained simultaneously in all microdroplets. Later, the osmotic and elastic influence of the chains destabilize the fragile micro- emulsions and typically lead to an increase in the particle size, the formation of empty micelles, and secondary nucleation. Very small latexes, 5–50nm in size, coexist with a majority of empty micelles in the final product. The types of initiator and concentration, surfactant, monomer and reaction temperature are some of the critical factors affecting the micro-emulsion polymerization kinetics and the properties of NP 61.
4. Interfacial polymerization
It is one of the well-established methods used for the preparation of polymer nanoparticles 62. It involves step polymerization of two reactive monomers or agents, which are dissolved respectively in two phases (i.e., continuous- and dispersed-phase), and the reaction takes place at the interface of the two liquids. Nanometer-sized hollow polymer particles were synthesized by employing interfacial cross-linking reactions as polyaddition and polycondensation 100-102 or radical polymerization63. Oil-containing nanocapsules were obtained by the polymerization of monomers at the oil/water interface of a very fine oil-in-water micro- emulsion. The organic solvent, which was completely miscible with water, served as a monomer vehicle and the interfacial polymerization of the monomer was believed to occur at the surface of the oil droplets that formed during emulsification64. To promote nanocapsule formation, the use of aprotic solvents, such as acetone and acetonitrile was recommended. Protic solvents, such as ethanol, n-butanol and isopropanol, were found to induce the formation of nanospheres in addition to nanocapsules. Alternatively, water-containing nanocapsules can be obtained by the interfacial polymerization of monomers in water-in-oil micro-emulsions. In these systems, the polymer formed locally at the water-oil interface and precipitated to produce the nanocapsule shell 65.
5. Controlled/living radical polymerization (c/lrp)
The primary limitations of radical polymerization include the lack of control over the molar mass, the molar mass distribution, the end functionalities and the macromolecular architecture. The limitations are caused by the unavoidable fast radical–radical termination reactions. The recent emergence of many so-called controlled or ‘living’ radical polymerization (C/LRP) processes has opened a new area using an old polymerization technique66. The most important factors contributing to this trend of the C/LRP process are increased environmental concern and a sharp growth of pharmaceutical and medical applications for hydrophilic polymers. These factors have given rise to “green chemistry” and created a demand for environmentally and chemically benign solvents such as water and supercritical carbon dioxide. Industrial radical polymerization is widely performed in aqueous dispersed systems and specifically in emulsion polymerization. The primary goal was to control the characteristics of the polymer in terms of molar mass, molar mass distribution, architecture and function. Implementation of C/LRP in the industrially important aqueous dispersed systems, resulting in the formation of polymeric nanoparticles with precise particle size and size distribution control, is crucial for future commercial success of C/LRP. Among the available controlled/living radical polymerization methods successful and extensively studied methods are 1) nitroxide-mediated polymerization (NMP) atom transfer radical polymerization (ATRP) reversible addition and fragmentation transfer chain polymerization (RAFT)67-68. The nature and concentration of the control agent, monomer, initiator and emulsion type (apart from temperature) are vital in determining the size of NPs. Of these, the nature of the control agent is critical in determining the particle size of the final product.
Different types of Nanoparticles used for drug delivery 69-71
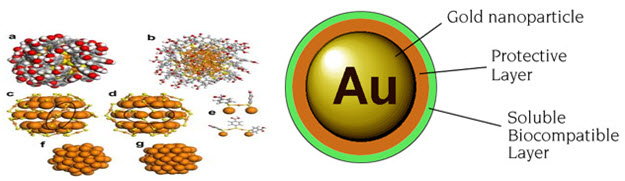
Gold Nanoparticles : Gold nanoparticles can provide effective carriers for biomolecules such as DNA, RNA, proteins and drugs, protecting these materials from degradation and transporting them across the cell-membrane barrier without effective toxicity.
Magnetic Nanoparticles (MNPs) : These are a class of engineered particulate materials of < 100nm that can be manipulated under the influence of an external magnetic field. MNPs commonly composed ofmagnetic elements such as iron, cobalt and their oxides like magnetite, maghemite, cobalt ferrite, and chromium dioxide. Applications of MNPs include targeted drug delivery, gene delivery cell separation and cell labeling.

Ceramic Nanoparticles : Nanoparticles of silica, titanium, alumina etc. are normally called as ceramic nanoparticles. The advantages of ceramic nanoparticles are preparation is very simple and they are unaffected by change in pH or temperature.

Protein Nanoparticles : Protein nanoparticles are biodegradable, non-antigenic, metabolizable and can also be easily amenable for surface modification and covalent attachment of drugs and ligands. The proteins used for the preparation of nanoparticles are albumin, gelatin, gliadin and legumin.
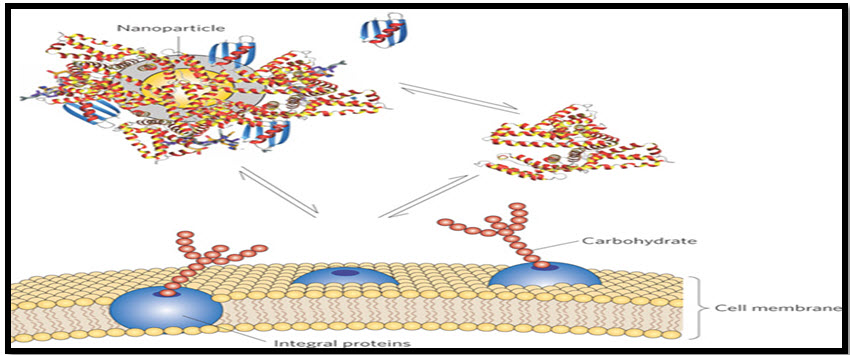
Solid Lipid Nanoparticles (SLNs): These are a new generation of submicron-sized lipid emulsions where the liquid lipid (oil) has been substituted by a solid lipid. SLNs offer unique properties such as small size, large surface area, high drug loading and the interaction of phases at the interfaces, and are attractive for their potential to improve performance of pharmaceuticals, Nutraceuticals and other materials.
Nanogels: Nanogels are cross-linked nanoscale particles made of flexible hydrophilic polymers. These are soluble in water. Nanogels possess large surface area, tuneable size and a network to allow incorporation of molecules. These are used to incorporate drugs, DNA/RNA and inorganic molecules such as quantum dots. These are also used for pH dependent release.

Nanoshells: A nanoshell comprises of a spherical core made from silica or other similar materials, surrounded by a coating of few nanometers thickness. The coatings comprise a metal such as gold or silver. In cancer applications, antibodies or other biomolecules are attached to the gold surface to target at tumor site.
Dendrimers: Dendrimers are unimolecular, monodisperse, micellar nanostructures with a well defined regularly branched symmetrical structure and a high density of functional end groups. Dendrimers contain three regions core, branches and surface. The first and most widely studied dendrimers are poly (amidoamino) (PAMAM) dendrimer. The advantage of dendrimers is that they are similar in size to many proteins and biomolecules like insulin, cytochrome C and haemoglobin. These are effective against bacterial and viral infection. Dendrimers hybridized with chitosan have useful antibacterial properties as well as potentially acting as drug delivery agents.
Carbon nanotubes: Carbon nanotubes are hexagonal networks of carbon atoms, 1nm in diameter and 1-100nm in length. Two types of nanotubes are present i.e., single-walled nanotubes, and multi-walled nanotubes. The advantages of nanotubes are ultra-light weight, high mechanical strength, and high surface area. Due to their size and shape, carbon nanotubes can enter living cells without causing cell death or obvious damage. Carbon nanotubes have the ability to transport drug molecules, protein and nucleotides. Therapeutic applications of carbon nanotubes including boron neutron capture therapy (BNCT), inducing immunoresponse, gene and Si RNA delivery.

Carbon nanohorns: Carbon nanohorns have a structure similar to carbon nanotubes except they are closed at one end, forming a cone shaped cap, or horn.

Nanodiamonds: Also called diamond nanoparticles, used to immobilize proteins and deliver drug molecules. Fluorescent nanodiamonds can enter cells, and may have applications in cell tracking and imagining.
Cyclodextrin Nanosponges: Cyclodextrin nanosponges are complex networks of cross-linked cyclodextrins and formed into a roughly spherical structure, about the size of a protein, with channels and pores inside.
Drug carrying Implantable Thin Films: These are nanoscale thin films that can be precisely controlled to release chemical agents by applying an electrostatic field. The advantages are ease of preparation, versatility, and capability of incorporating high loading of biomolecules into films. The film can be implanted in the body and can carry discrete packets of drugs that can be released separately, which could be particularly useful for chemotherapy.
Quantum dots: Quantum dots (QDs) are semiconducting materials consisting of a semiconductor core (CdSe), coated by a shell (e.g., ZnS). These are used as diagnostic tools, detection and analysis of biomolecules, immunoassays, DNA hybridization, and development of non-viral vectors for gene therapy, transport vehicles for DNA, protein, drugs or cells.
Some of the selected drugs as nano drug delivery systems nanoparticles 72-79
|
|
|||
|
Sl. No. |
Name of the drug |
Purpose |
|
|
1 |
Clonazepam |
To determine the drug loading capacity & drug release |
|
|
2 |
Morphine |
To study antinociceptive activity and blood brain delivery (by nasal route) |
|
|
3 |
Adriamycin |
To enhance effective delivery of Adriamycin |
|
|
4 |
Dexamethasone |
To increase the amount of drug release with respect to pure drug |
|
|
5 |
Tamoxifen |
To increase the local concentration of tamoxifen in estrogen receptor positive breast cancer cells |
|
|
6 |
Cyclosporin A |
To form stable suspension of submicron particles of Cyclosporin A |
|
|
7 |
Amoxicillin |
To evaluate the effectiveness of amoxicillin in eradiating Helicobacter pyroli |
|
|
8 |
Ketoprofen |
To outline the effects of interactions between a model drug and various acrylic polymers |
|
|
9 |
Praziquantel |
To study the effect of formulation variables on size distribution |
|
|
10 |
Glycyrrhetic acid |
Encapsulation efficiency of GLA is increased. |
|
|
11 |
Aspirin |
Capable of releasing the drug in a slow sustained manner. |
|
|
12 |
Indomethacin |
To enhance sustained releasecof IMC and delay clearance of IMC without significant effect on metabolism of IMC itself |
|
|
13 |
Docetaxel |
For effective delivery of drug to solid tumors |
|
|
14 |
Estradiol |
To increase oral bioavailability of Estradiol. (oral) |
|
|
15 |
Cyproterone |
To improve skin penetration of the poorly absorbed drug Cyproterone (topical) |
|
|
16 |
Curcumin |
For coating curcumin onto a metal stent by electrophoretic deposition thereby avoiding problem with restenosis after percutaneous coronary intervention |
|
|
17 |
Ropivacaine |
To decrease the systemic toxicity of ropivacaine |
|
|
18 |
salbutamol suyate |
The achieved size and shape of spray dried nanosized particles is suitable for the respiratory deposition in lungs (pulmonary inhalation) |
|
|
19 |
Didanosine |
For sustained release of Didanosine |
|
|
20 |
Lamivudine |
Increased bioavailability of lamivudine is observed when tested in AIDS patients. |
|
|
21 |
Simvastatin nanocarriers |
To enhance effective delivery of poorly water soluble drug simvastatin. (oral) |
|
|
22 |
Doxorubicin |
To improve oral bioavailability of Doxorubicin |
|
|
23 |
Amphotericin B |
To improve oral bioavailability and to show reduced nephrotoxicity compared to intravenous fungizone. (oral) |
|
|
24 |
Rifampicin |
To formulate Rifampicin for aerosol delivery in a dry powder, which is suited for shelf stability, effective dispersibility and extended release with local lung and systemic drug delivery (pulmonary). |
|
|
25 |
Naringenin |
To enhance hepatoprotective effect in-vivo on oral administration. (oral) |
|
|
26 |
Curcumin |
To enhance the transport of curcumin to brain and to enhance the delivery system to cross the BBB. (intravenous) |
|
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RECENT DEVELOPMENTS
Tissue engineering
Natural bone surface is quite often contains features that are about 100 nm across. If the surface of an artificial bone implant were left smooth, the body would try to reject it. Because of that smooth surface is likely to cause production of a fibrous tissue covering the surface of the implant. This layer reduces the bone-implant contact, which may result in loosening of the implant and further inflammation. It was demonstrated that by creating nano-sized features on the surface of the hip or knee prosthesis one could reduce the chances of rejection as well as to stimulate the production of osteoblasts. The osteoblasts are the cells responsible for the growth of the bone matrix and are found on the advancing surface of the developing bone. The effect was demonstrated with polymeric, ceramic and, more recently, metal materials. More than 90% of the human bone cells from suspension adhered to the nanostructured metal surface but only 50% in the control sample. In the end this findings would allow to design a more durable and longer lasting hip or knee replacements and to reduce the chances of the implant getting loose. Titanium is a well-known bone repairing material widely used in orthopaedics and dentistry. It has a high fracture resistance, ductility and weight to strength ratio. Unfortunately, it suffers from the lack of bioactivity, as it does not support sell adhesion and growth well. Apatite coatings are known to be bioactive and to bond to the bone. Hence, several techniques were used in the past to produce an apatite coating on titanium. Those coatings suffer from thickness non-uniformity, poor adhesion and low mechanical strength. In addition, a stable porous structure is required to support the nutrients transport through the cell growth. It was shown that using a biomimetic approach – a slow growth of nanostructured apatite film from the simulated body fluid – resulted in the formation of a strongly adherent, uniform nanoporous layer. The layer was found to be built of 60 nm crystallites, and possess a stable nanoporous structure and bioactivity. A real bone is a nanocomposite material, composed of hydroxyapatite crystallites in the organic matrix, which is mainly composed of collagen. Thanks to that, the bone is mechanically tough and, at the same time, plastic, so it can recover from a mechanical damage. The actual nanoscale mechanism leading to this useful combination of properties is still debated. An artificial hybrid material was prepared from 15–18 nm ceramic nanoparticles and poly (methyl methacrylate) copolymer. Using tribology approach, a viscoelastic behaviour (healing) of the human teeth was demonstrated. An investigated hybrid material, deposited as a coating on the tooth surface, improved scratch resistance as well as possessed a healing behaviour similar to that of the tooth.80

Cancer therapy
Photodynamic cancer therapy is based on the destruction of the cancer cells by laser generated atomic oxygen, which is cytotoxic. A greater quantity of a special dye that is used to generate the atomic oxygen is taken in by the cancer cells when compared with a healthy tissue. Hence, only the cancer cells are destroyed then exposed to a laser radiation. Unfortunately, the remaining dye molecules migrate to the skin and the eyes and make the patient very sensitive to the daylight exposure. This effect can last for up to six weeks. To avoid this side effect, the hydrophobic version of the dye molecule was enclosed inside a porous nanoparticle. The dye stayed trapped inside the Ormosil nanoparticle and did not spread to the other parts of the body. At the same time, its oxygen generating ability has not been affected and the pore size of about 1 nm freely allowed for the oxygen to diffuse out.

Multicolor optical coding for biological assays
The ever increasing research in proteomics and genomic generates escalating number of sequence data and requires development of high throughput screening technologies. Realistically, various array technologies that are currently used in parallel analysis are likely to reach saturation when a number of array elements exceed several millions. A three-dimensional approach, based on optical "bar coding" of polymer particles in solution, is limited only by the number of unique tags one can reliably produce and detect. Single quantum dots of compound semiconductors were successfully used as a replacement of organic dyes in various bio-tagging applications. This idea has been taken one step further by combining differently sized and hence having different fluorescent colours quantum dots, and combining them in polymeric microbeads. A precise control of quantum dot ratios has been achieved. The selection of nanoparticles used in those experiments had 6 different colours as well as 10 intensities. It is enough to encode over 1 million combinations. The uniformity and reproducibility of beads was high letting for the bead identification accuracies of 99.99%.
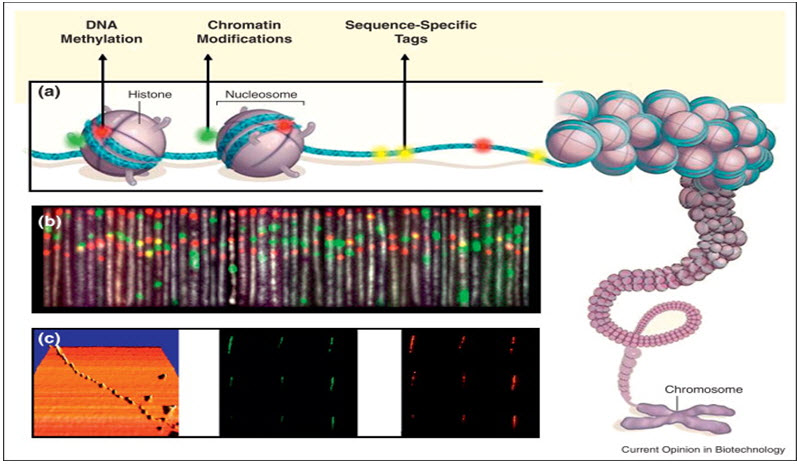
Manipulation of cells and biomolecules
Functionalised magnetic nanoparticles have found many applications including cell separation and probing; these and other applications are discussed in a recent review. Most of the magnetic particles studied so far are spherical, which somewhat limits the possibilities to make these nanoparticles multifunctional. Alternative cylindrically shaped nanoparticles can be created by employing metal electrodeposition into nanoporous alumina template. Depending on the properties of the template, nanocylinder radius can be selected in the range of 5 to 500 nm while their length can be as big as 60 μm. By sequentially depositing various thicknesses of different metals, the structure and the magnetic properties of individual cylinders can be tuned widely. As surface chemistry for functionalisation of metal surfaces is well developed, different ligands can be selectively attached to different segments. For example, porphyrinswith thiol or carboxyl linkers were simultaneously attached to the gold or nickel segments respectively. Thus, it is possible to produce magnetic nanowires with spatially segregated fluorescent parts. In addition, because of the large aspect ratios, the residual magnetisation of these nanowires can be high. Hence, weaker magnetic field can be used to drive them. It has been shown that a self-assembly of magnetic nanowires in suspension can be controlled by weak external magnetic fields. This would potentially allow controlling cell assembly in different shapes and forms. Moreover, an external magnetic field can be combined with a lithographically defined magnetic pattern ("magnetic trapping").
Protein detection
Proteins are the important part of the cell's language, machinery and structure, and understanding their functionalities is extremely important for further progress in human well being. Gold nanoparticles are widely used in immunohistochemistry to identify protein-protein interaction. However, the multiple simultaneous detection capabilities of this technique are fairly limited. Surfaceenhanced Raman scattering spectroscopy is a well-established technique for detection and identification of single dye molecules. By combining both methods in a single nanoparticle probe one can drastically improve the multiplexing capabilities of protein probes. The group of Prof. Mirkin has designed a sophisticated multifunctionalprobe that is built around a 13 nm gold nanoparticle. The nanoparticles are coated with hydrophilic oligonucleotides containing a Raman dye at one end and terminally capped with a small molecule recognition element (e.g. biotin). Moreover, this molecule is catalytically active and will be coated with silver in the solution of Ag(I) and hydroquinone. After the probe is attached to a small molecule or an antigen it is designed to detect, the substrate is exposed to silver and hydroquinone solution. A silverplating is happening close to the Raman dye, which allows for dye signature detection with a standard Raman microscope. Apart from being able to recognise small molecules this probe can be modified to contain antibodies on th









