About Authors:
Rawat Smriti*, Bisht Seema, Kothi Yal Preeti
Shri Guru Ram Rai Institute of Technology & Sciences
Dehradun, Uttarakhand, India.
*smritirawat000@gmail.com
Abstract
Many human biological processes are regulated by circadian rhythms, which follow 24-h cycles and involve the neuroendocrine and immune systems. There is strong evidence for an impact of circadian rhythms in the symptoms of Rheumatoid arthritis, and a number of pro-inflammatory cytokines that exhibit a peculiar rhythmicity in particular serum TNF- α and serum IL-6, and together with other relevant immunological parameters display an elevation in the early morning hours. The increase in nocturnal anti-inflammatory cortisol secretion is insufficient to suppress ongoing inflammation, resulting in the morning symptoms of joint stiffness, pain, and functional disability are characterized.With chronotherapeutics becoming ever more popular, more consideration is being given to the timing of therapy for a number of chronic diseases. The recent surge of interest in the field of chronobiology has highlighted the importance of optimising timing of treatment administration. In this article we discuss the chronobiology of Rheumatoid arthritis and the availability of a new programmed-release.
REFERENCE ID: PHARMATUTOR-ART-1924
INTRODUCTION
Most humans have a relatively regular activity pattern (sleep, labor, and meal etc.). This activity can be roughly classified into the rest phase and active phase, and body temperature, heart rate, blood pressure, and the dominance of the sympathetic and parasympathetic nerves differ in each phase. These variations display daily rhythms, which are known as circadian rhythms. Circadian rhythms are self-sustained endogenous oscillations that occur with a periodicity of approximately 24 hours and are regulated by the circadian clock.[1]The term ‘‘circadian’’ was coined by Franz Halberg from the Latin circa, meaning about, and dies, meaning day [2]. The environmental light/dark cycle is the most salient cue influencing these circadian rhythms[3]. These circadian rhythms are also associated with the risk or frequency of disease occurrence. For example, asthma attacks get worse between midnight and early morning and are seldom observed in the daytime. In addition, the risks of spontaneous acute dissection and rupture of the thoracic aorta, myocardial ischemia, ischemic stroke, and subarachnoid hemorrhage are higher during the active phase than during the rest phase. The variations in heart rate, blood pressure, and blood flow, etc., induced by the wake-sleep transition are considered to affect the risk of such problems occurring[4]. Interestingly, pain such as toothache, migraine, and rheumatoid arthritis pain is more acute in the early morning.
Chronotherapeutics, have been developed in close connection with emerging pulsatile delivery views. In this respect, it is well established that the symptoms of many pathologies, as well as the pharmacokinetic and pharmacodynamic profiles of most drugs, are subject to circadian variation patterns. To introduce the concept of chronotherapeutics, it is important to define the following concepts.
Chronotherapy: -[5] Co-ordination of biological rhythms and medical treatment is called chronotherapy.
Chronobiology is thus that discipline which deals with difference in physiology of an individual according to time of day, month or year or even period in one’s life.[6] Endocrine system provides examples illustrating chronobiology, e.g., a range of hormones including cortisol, catecholamines are secreted in morning, whereas hormones like melatonin (MLT), adrenocorticotropic hormone finds their maxima in the evening or during sleep. Priming of these hormones at various durations of the day leads to alteration in body’s physiological functions at various times of the day.[7, 8]
Pharmaceutics is an area of biomedical and pharmaceutical sciences that deals with the design and evaluation of pharmaceutical dosage forms (or drug delivery systems) to assure their safety, effectiveness, quality, and reliability.
Chronopharmaceutical drug delivery systems (ChrDDSs) should embody time-controlled and site-specific drug delivery systems [9]. The delivery of medications – in the right concentration, to the right targeted tissues, at the right time – to meet biological-rhythm. This technique is thus beneficial to treat diseases like asthma, Rheumatoid arthritis (RA), cardiovascular diseases since they show chronobiological behavior. This methodology also proves beneficial for pathophysiological states where night time dosing is required.[10, 11]
BIOLOGICAL RHYTHMS [12]
These are the biological process that shows cyclic variation over time. There are three types of Rhythms generally present in the human body, which was shown in Table 1.
A. Circadian Rhythms: “Circa” means about and “dies” means day.
B. Ultradian Rhythms: Oscillation of shorter duration is termed as Ultradian. (More than one cycle/day)
C. Infradian Rhythms: Oscillations those are longer than 24 h. (less than one cycle/day)
DISEASES AND CHRONOTHERAPEUTICS:
The potential benefits of chronotherapeutics have been demonstrated in the management of a number of diseases. In particular there is a great deal of interest in how chronotherapy can particularly benefit patients suffering from allergic rhinitis, rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, asthma, cancer, cardiovascular diseases, and peptic ulcer disease.
Arthritis
Arthritis (derived from Greek word artho: joint, itis: inflammation), is a condition involving damage to joints of the body. Arthritis may broadly be classified as under:
a) Rheumatoid arthritis (RA)
b) Septic arthritis
c) Juvenile arthritis
d) Ankylosing spondilytis
RA is a disease in which body’s own immune system starts to attack body’s tissue. Immune complex composed of Ig M (Ig: immunoglobulin) activate complement and release cytokines which are chemotactic for neutrophils. These inflammatory cells secrete lysosomal enzymes which damage cartilage and bones, while prostaglandins produced in the process cause vasodilatation and pain.Common symptoms includes varied level of pain, swelling, joint stiffness and sometimes a constant ache around the joints.[20, 21]
Theory regarding pathogenesis of RA
According to theory T-cells may be important in initiating the disease, but chronic inflammation is self perpetuated by macrophages and fibroblasts. Absence of activated
T-Phenotypes in chronic RA and presence of activated macrophage and fibroblast phenotypes lay substance to this theory. At site of inflammation fibroblast of affected cartilage secretes:
i. Cytokines: IL-6, IL-8 (IL: Interleukin)
ii. Prostaglandins
iii. Protease enzyme
Protease and prostaglandins act directly to erode and destroy bones and cartilage thus producing inflammation and other symptoms of arthritis[22, 23]
Circadian rhythm of nocturnal hormones in rheumatoid arthritis:
From decades it had been known that pathological symptoms in RA follow circadian rhythm, with priming of symptoms in early morning, abatement during the noon and then starts increasing from late evening. Serum concentration and release cytokine is triggered by melatonin and other hormones from hypothalamus, and follow a strict 24 h cycle. IL-6 is the most important of all cytokines responsible for pathological symptoms pertaining to RA. Other pro- inflammatory hormones manifesting RA include TNF, IL-1, IL-8, IL-12 and IL-17.20.Positive genetic relationship between melatonin and RA had been reported by the researchers that melatonin (MLT) levels increased progressively from 8 PM to early morning with a peak at midnight. From researches it had been concluded that MLT concentration stimulate the production of interferon γ, IL-1, IL-2, IL-6 etc. in mononuclear cells of blood, moreover MLT enhances production of inflammatory cytokine from human monocytes. Excess concentration of MLT had been reported in synovial fluids of patients having RA, moreover binding sites for MLT were also found to be present in synovial macrophage. It was found that concentration of various cytokine primes during night and early morning just after the stage when MLT serum levels are higher whereas plasma cortisol level the lowest, thus it is concluded that MLT up regulates cytokine production and immune functions, thus leading to joint inflammation, joint stiffness etc. [24-27] Cortisol secretion and glucocorticoids receptor density has been reported to be altered in patients with RA [28, 29]. It was found that increased cortisol synthesis inhibits the rise in concentration of IL-6 in RA patients.
Circadian Rhythms in Rheumatoid Arthritis
In patients with RA, disease symptoms such as joint pain, morning stiffness and functional disability increase in the early morning, with abatement during the day and a smaller new increase in the early evening.[30]Circadian changes in the metabolism or nocturnal secretion of endogenous corticosteroids are responsible, in part, for the time- dependent changes observed in the inflammatiory response of RA.[31]
Herold and Günther [32] reported that plasma C-reactive protein (CRP) levels, an indicator of inflammatory responses, showed a circadian rhythm with a peak in the early morning and a trough in the evening in RA patients, which matches the rhythms of pain and stiffness. Proinflammatory cytokines, such as TNF- α and IL-6, are secreted from activated monocytes, and macrophages increase CRP levels in hepatocytes. There are clear circadian rhythms in the blood concentrations of these cytokines, with higher levels seen in the early morning in RA patients [33]. Since the circadian rhythms of CRP and cytokines are similar, it is considered that cytokine rhythms contribute to the rhythm of CRP levels. Among the pro-inflammatory cytokines involved in circadian changes, IL-6 is of particular relevance as its levels most closely follow the daily pattern of RA symptoms. [34, 35]
Circadian Rhythms inOsteoarthritis
The circadian rhythm of pain and stiffness in osteoarthritis differs from that of rheumatoid arthritis. Osteoarthritis is a degenerative disease of the joints and is the commonest of all joint diseases, affecting nearly everyone at least to some degree by age 70. The weight bearing joints of the hip, knee, back, toes a pain of osteoarthritis sufferers is typically less intense in the morning than in the afternoon. The successful treatment of osteoarthritis requires that medications be taken at the right time or evening. The temporal pattern of pain and stiffness in osteoarthritis sufferers differs between persons. Thus, an individualized chronotherapy of NSAIDs is necessary. The chronotherapy of osteoarthritis involves the administration of once-a-day forms of ketoprofen, indomethacin and other such medicines in relation to the time of day pain is worse. If pain is worse at night or early in afternoon, an evening once-a day NSAIDs schedule is recommended. If pain is worse in the afternoon or night, a once-a-day morning or noontime treatment schedule is best, providing the amount of side effects produced by the morning one, in particular, is minimal [36].
Circadian Rhythms inAnkylosing Spondylitis
Ankylosing spondylitis is characterized by swelling and discomfort of the joints of the back. In its occurrence it is an inherited disorder that is more common in men than women. Overall, back stiffness and pain were a problem throughout the 24 h, but pain intensity was rated 2 to 3 times higher and stiffness about 8 times greater between 06:00 and 09:00 am than between noon and 15:00 pm when each was least bothersome. The symptoms also exhibited a second less prominent peak between 19:00 and 21:00 pm. Marked seasonal variation in ankylosing spondylitis was also prominent. The onset of backache and stiffness was 12 times more frequent in winter than summer. Moreover, reoccurrence of back problems occurs 2 to 3 times more often in winter than summer [37, 38].
Chronotherapy for Rheumatoid Arthritis:
In the treatment of RA, non-steroidal anti-inflammatory drugs (NSAID) are used to decrease pain; steroids are used to reduce pain and inflammation; and diseases modifying antirheumatic drugs (DMARD) are used prior to the development of destructive changes in bones, joints, and organ tissues. In addition, biological DMARD can be used to target specific cytokines.
Use of NSAID’s:
Arthritis develops in many RA patients, thus NSAID such as indomethacin are often used as analgesics. Levi et al. reported on the use of chronotherapy involving indomethacin for osteoarthritis [39]. The patients with osteoarthritis took an Indomethacin sustained-release (ISR) oral preparation once a day at 8:00, noon. The evening dosing protocol showed higher safety than the morning dosing protocol. Moreover, he reported people to be much better tolerant with less complaint of side effects when drug was administered as a single daily dose in the evening than morning. In another study examining the role of treatment schedule on the therapeutic effect of the NSAID’s: flurbiprofen, it was found that to control the morning symptoms of RA, a daily NSAID’s dose must be taken in the evening or at bedtime.[40]
Use of Glucocorticoids:
Glucocorticoids have been used in RA therapy to treat symptoms such as joint stiffness and joint pain. Generally, glucocorticoids are administered in the morning according to the circadian rhythm of endogenous glucocorticoids. The duration of morning stiffness was markedly shorter in the night (10:00- 11:00) dosing than in the morning (6:00- 7:00) dosing. Buttgereit et al. developed a new modified-release formulation of prednisone that releases prednisone about 4 hours after ingestion [41]. When RA patients were randomly given a modified-release tablet at bedtime or an immediate-release prednisone tablet in the morning, the relative change in the duration of joint morning stiffness was significantly higher with the modified-release tablet than with the immediate-release tablet. Chronotherapy with a modified-release prednisone tablet is safe and effective as a treatment for RA therapy, even though it was thought that administering glucocorticoids at night would have negative effects upon the circadian rhythm of endogenous cortisol and reduce hypothalamic-pituitary-adrenal axis function. Therefore, chronotherapy using the modified- release prednisone tablet is considered to be a useful RA therapy.
Use of Methotrexate:
Methotrexate (MTX) is one of the most commonly used DMARD. It inhibits cytokine production by suppressing lymphocyte proliferation and TNF-αtranscriptional activity [42]. MTX induces a high American College of Rheumatology improvement response rate , inhibits joint inflammation and conveys a marked survival benefit [43]in RA patients although the exact mechanisms underlying its antirheumatic effects are not fully understood [44], MTX is used as an anchor drug in RA therapy. However, MTX also causes adverse effects, such as myelosuppression and interstitial pneumonitis because it is an anticancer agent. Therefore, it is necessary to design a safe and effective dosing protocol for MTX treatment.
Advantages of using Chronotherapy
1. It is more effective and less toxic when drugs are administered at selected time of the day.
2. Chronotherapeutics aids in delivering drug in concentration that may vary according to the body’s circadian rhythm.
3. It minimizes the side effect of glucocorticoids since the concentration of hormone used is very less.
Chronopharmaceutical Drug Delivery System:
Currently, the key technologies in chronopharmaceutics for oral delivery include ChronotopicTM technology, Diffucaps s, Egalet s, OROS s or ChronsetTM , the ContinTM, CodasTM, CeformTM, GeoClockTM, and PortTM systems, Three Dimensional PrintingTM (3DP), and the TIMERxTM technology.
The Chronotropic Technology:
It is basically composed of a drug-containing core provided with an outer release-controlling coating. Both single and multiple-unit dosage forms, such as tablets and capsules or minitablets and pellets, have been employed as the inner drug formulation. Cores either meant for an immediate or a prolonged liberation of the active ingredient has been proposed. However, the main focus has so far been on the accomplishment of a rapid and transient delayed release, which is generally considered the most challenging and appealing pulsatile delivery mode. The outer barrier consists of swellable hydrophilic polymers of different viscosity grade, typically hydroxypropylmethylcellulose (HPMC), by exploiting a variety of methods. When exposed to aqueous fluids, these polymers undergo a glassy-rubbery transition. In the hydrated state, they are subject to perme- ability increase, dissolution, and/or mechanical erosion phenomena, which delay the delivery of drugs from the core. The system has been shown to provide the pursued pulsatile release behavior in vitro as well as in vivo, with programmable lag phases followed by drug release according to the core characteristics. In principle, it is possible to finely modulate the lag time by relying on different coating materials and coating thickness values. Moreover, depending on such variables, diverse mechanisms have been hypothesized and, in some instances, demonstrated to be involved in the control of release. When proper modifications have been introduced into the system design, the Chronotopic technology yields oral time-dependent colon delivery as well.
In the Diffucaps technology [45]:
In this technology, a unit dosage form, such as a capsule for delivering drugs into the body in a circadian release fashion, is comprised of one or more populations of drug-containing particles (beads, pellets, granules, etc.). Each bead population exhibits a predesigned rapid or sustained release profile with or without a predetermined lag time of 3–5 hours. The active core of the dosage form may comprise an inert particle or an acidic or alkaline buffer crystal (e.g., cellulose ethers), which is coated with an API-containing film-forming formulation and preferably a water-soluble film-forming composition (e.g., hydroxypropylmethylcellulose, polyvinylpyrrolidone) to form a water-soluble/dispersible particle. The active core may be prepared by granulating and milling and/or by extrusion and spheronization of a polymer composition containing the API. Such a ChrDDS is designed to provide a plasma concentration–time profile that varies according to physiological need during the day, that is, mimicking the circadian rhythm and severity/manifestation of a cardiovascular disease, predicted based on pharmacokinetic and pharmacodynamic considerations and in vitro/in vivo correlations.
Chronset TM:
It is a proprietary OROSs delivery system that reproducibly delivers a bolus drug dose (W80% drug release within 15minutes) in a time- or site-specific manner to the gastro- intestinal tract (GIT). Using the Chronset technology, the drug formulation is completely protected from chemical and enzymatic degradation in the GIT before release, and the timing of release is unaffected by GIT contents. By specifically balancing the osmotic engine, the semipermeable membrane, and the other attributes of the system configuration, drug release onset times varying from 1 to 20 hours can be achieved. The design of Chronset, its operation mechanism, the control features, and in vivo performance in human volunteers are described.
The Egalet technology:
It offers a delayed- release form consisting of an impermeable shell with two lag plugs, enclosing a plug of active drug in the middle of the unit. After the inert plugs have eroded, the drug is released, thus a lag-time occurs. Time of release can then be modulated by the length and composition of the plugs. The shells are made of (slowly) biodegradable polymers (such as ethylcellulose) and include plastici- zers (such as cetostearyl alcohol), while the matrix of the plugs is comprise a mixture of pharmaceutical excipients including polymers like polyethylene oxide (PEO).
The CeformTM technology [46]:
It allows the production of uniformly sized and shaped microspheres of pharmaceutical compounds. This approach is based on ‘‘melt-spinning’’, which means subjecting solid feedstock (i.e., biodegradable polymer/bioactive agent combinations) to a combination of temperature, thermal gradients, mechanical forces, flow, and flow rates during processing. The microspheres obtained are almost perfectly spherical, having a diameter that is typically 150–180mm, and allow for high drug content. The microspheres can be used in a wide variety of dosage forms, including tablets, capsules, suspensions, effervescent tablets, and sachets. The microspheres may be coated for controlled release with an enteric coating or may be combined into a fast/slow release combination.
The CodasTM (Chronotherapeutic Oral Drug Absorption System) technology [47]:
There is a multiparticle system designed for bedtime drug dosing, incorporating a 4–5-hour delay in drug delivery. This delay is introduced by the level of nonenteric release-controlling polymer applied to drug-loaded beads. The release-controlling polymer is a combination of water-soluble and water- insoluble polymers. As water from the gastrointestinal tract comes into contact with the polymer-coated beads, the water-soluble polymer slowly dissolves and the drug diffuses through the resulting pores in the coating. The water-insoluble polymer continues to act as a barrier, maintaining the controlled release of verapamil [48]. The rate of release is essentially independent of pH, posture, and food.
The ContinTM technology:
Molecular coordination complexes are formed between a cellulose polymer and a nonpolar solid aliphatic alcohol optionally substituted with an aliphatic group by solvating the polymer with a volatile polar solvent and reacting the solvated cellulose polymer directly with the aliphatic alcohol, preferably as a melt. This constitutes the complex having utility as a matrix in controlled release formulations since it has a uniform porosity (semi permeable matrixes) that may be varied [49].
The GeoClockTM technology:
It is based on the Geomatrixt technology. In the Geomatrix technology, a multi layer tablet design was initially proposed for constant drug release. It consists of a drug-free barrier layer on one or both bases of an active core (hydrophilic matrix). The partial coating modulates the core hydration process and reduces the surface area available for drug release. During dissolution, the swellable barrier swells and gels, but is not eroded, thus acting as a modulating membrane during the release process. The erodible barrier, instead, was progressively removed by the dissolution medium, exposing in time an increasing extent of the planar surface(s) of the core to interaction with the outer environment and to drug release [50]. Quite recently, this technology has been used to develop Lodotrat, a prednisone-containing chronopharmaceutical formulation for rheumatoid arthritis management. With this new ChrDDS, the drug can be taken at bedtime, but the active substance only gets released in the early hours of the morning, the optimum time point to treat morning symptoms such as stiffness and pain due to the inhibition of inflammatory cytokines.
The PortTM (Programmable Oral Release Technologies) [51]:
It uses a uniquely coated, encapsulated system that can provide multiple programmed release of drug. The basic design of the Port technology tablet consists of a polymer core matrix coated with a semi permeable, rate-controlling polymer. Poorly soluble drugs can be coated with proprietary solubilization agents to ensure uniform controlled release from the dosage form. The basic design of the Port system of capsule consists of a hard gelatin capsule coated with a semi permeable, rate-controlling polymer. Inside the coated capsule is the osmotic energy source, which normally contains the therapeutic agent to be delivered. The capsule is sealed with a water-insoluble lipid separator plug. An immediate release dosage can be added above the plug to complete the dosing options.
Three Dimensional Printing (3DP) Technology:
It is used in the fabrication of complex oral dosage delivery pharmaceuticals based on solid free-form fabrication methods. It is possible to engineer devices with complicated internal geometries, varying densities, diffusivities, and chemicals [52]. Different types of complex oral drug delivery devices have been fabricated using the 3DP process: immediate–extended release tablets, pulse release, breakaway tablets, and dual pulsatory tablets. The enteric dual pulsatile tablets were constructed of one continuous enteric excipient phase into which diclofenac sodium was printed into two separated areas. These samples showed two pulses of release in vitro with a lag time between pulses of about 4 hours [53]. This technology is the basis of the TheriForm s technology[54]. The latter is a microfabrication process that works in a manner very similar to an ‘‘inkjet’’ printer. It is a fully integrated computer-aided development and manufacturing process. Products may be designed on a computer screen as three-dimensional models before actual implementation of their preparation process.
The TIMERxt technology [55]:
It is a very versatile hydrogel-based con- trolled release technology. The unique nature of TIMERx intermolecular physical chemistry was described in relation to the technology’s potential to provide any one of a number of different release profiles, ranging from zero order to chronotherapeutic release. The authors claimed that the ‘‘molecular engine’’ replaces the need for complex processing or novel excipients and allows desired drug release profiles to be ‘‘factory set’’ following a simple formulation development process [56]. Basically, this technology combines primarily xanthan and locust bean gums mixed with dextrose. The physical interaction between these components works to form a strong, binding gel in the presence of water. Drug release is controlled by the rate of water penetration from the gastrointestinal tract into the TIMERxt gum matrix, which expands to form a gel and subsequently releases the active drug substance. Other controlled release systems that may find future applications in chronotherapy include erodible polymers in different forms,programmable pulsatile release capsule devices ,guar gum-based matrix tablets [57], sigmoidal release systems [58,59], and self exploding micro- particles [60].
Future prospective
Other treatment methodologies which are under investigation and can prove to be a boon in cure of RA are:
1. Blocking the action of cytokine at site of target cells: Cytokines on attachment to target cells serve as inducers of chemotactic response for the neutrophils, which on proliferation lead to inflammatory response and hence onset of RA. Thus approaches preventing attachment of cytokines to the target cells may prove beneficial to cure RA.
2. Delivering a drug which blocks the receptors for melatonin at the time when its concentration is maximum.
Conclusion:
First and foremost aim of this article had been to make clinicians, biologists and pharmacists realize the importance of chronobiology and chronotherapeutics for safer and effective treatment of pathological states like RA. This article summarizes how by intelligently modifying the concentration of mediators, the diseased states can be modified /controlled. Thus the article tries to enlighten the path for new researchers and developers for developing a better, safer and therapeutically effective dosage form for treatment of RA. Moreover, The article also lays emphasis on the teamed effect as well as interdependence on each other of nocturnal hormones like MLT, inflammatory mediators like cytokine, glucocorticoids like cortisol which together leads to RA. In the era where big pharmaceutical giants strive to flood market with new solutions, use of these intelligent systems could not only offer better therapeutic results but also increases patient compliance in disease states like rheumatoid arthritis.
TABLE 1: SHOWING DIFFERENT TYPES OF RHYTHMS
|
S. No |
TYPE OF RHYTHMS |
EXAMPLE |
|
1. |
Circadian Rhythms |
Which lasts for about one day, like sleep-waking rhythm the body temperature |
|
2. |
Ultradian Rhythms |
Shorter than a day seconds (like heartbeat) |
|
3. |
Infradian Rhythms |
Longer than a day monthly rhythm-menstrual cycle yearly rhythm-bird migration |
TABLE 2.DISEASE INFLUENCED BY CHRONOTHERAPY:[13-19]
|
DISEASES |
CHRONOLOGICAL BEHAVIOUR |
DRUG USED |
|
Peptic ulcer |
Acid secretion is high in the afternoon and at night. |
H2blockers |
|
Cancer |
The blood flow to tumors is 3-fold greater during each daily activity phase of the circadian cycle than during the daily rest phase. |
Vinca alkaloids, Taxanes |
|
Duodenal ulcer |
Gastric acid secretion is highest at night while gastric and small bowel motility and gastric emptying are all slower at night. |
Proton pump inhibitors |
|
Neurological disorders |
The central pathophysiology of epilepsy and the behavioral classification of convulsive events. |
MAO-B inhibitor |
|
Hypercholesterolemia |
Cholesterol synthesis is generally higher during night than day time |
HMG CoA reductase Inhibitors |
|
Diabetes mellitus |
Increase in the blood sugar level after meal. |
Sulfonylurea, Insulin |
|
Arthritis |
Level of pain increases at night |
NSAIDs, Glucocorticoids |
|
Asthma |
Precipitation of attacks during night or at early morning. |
Β2 agonist, Antihistamines |
|
Attention deficit syndrome |
Increase in DOPA level in afternoon. |
Methylphenidate |
|
Cardiovascular diseases |
BP is at its lowest during the sleep cycle and rises steeply during the early morning. |
Nitroglycerin, calcium channel blocker, ACE inhibitors |
TABLE 3: FDA APPROVED ChrDDS IN US MARKET
|
Proprietory name; Dosage form |
Active |
Chronopharmaceutical technology |
Indication/rationale for |
|
Uniphyl® extended release tablets |
Theophyline |
CONTIN |
Asthma/increased bronchoconstriction in morning |
|
CoveraHS; extended release tablets |
Verapamil HCl |
OROS® |
Hypertension/increased |
|
Verelan® PM extended release capsules |
Verapamil HCl |
CODAS® |
Hypertension
|
|
Concerta® tablet |
Methylphenidate HCl |
OROS® |
Anti-psychotic
|
|
Cardizem®LA; |
Verapamil HCl
|
CEFORM® |
Hypertension
|
|
Innopran® XL; |
Propranolol HCl Verapamil HCl |
DIFFUCAPS® |
Hypertension
|
|
Invega™ |
Paliperidone |
OROS® |
Schizophrenia |
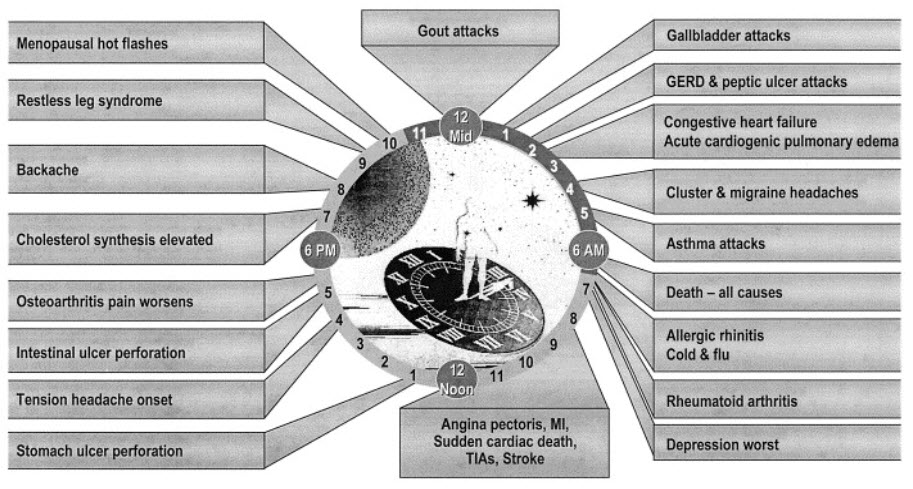
FIG 1. DISEASES INFLUENCED BY CHRONOTHERAPY
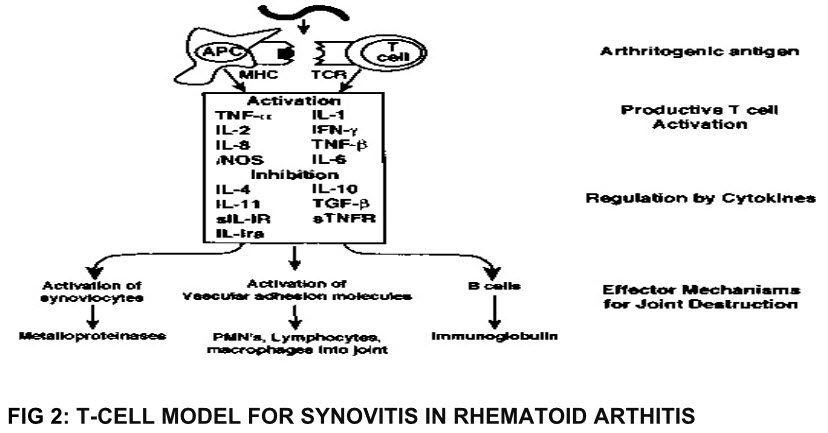
FIG 2: T-CELL MODEL FOR SYNOVITIS IN RHEMATOID ARTHITIS
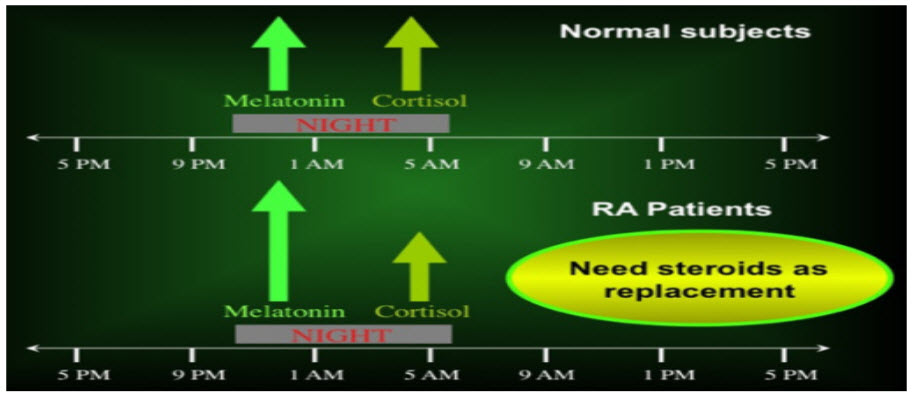
FIG 3: Altered balance between nocturnal hormones in rheumatoid arthritis. The altered balance between nocturnal hormone productions in chronic diseases such as rheumatoid arthritis (RA) is characterized by increased levels and steady-state duration of melatonin (enhancer of the immune/inflammatory reaction) and by decreased adrenal cortisol availability (downregulator of the immune/inflammatory reaction).
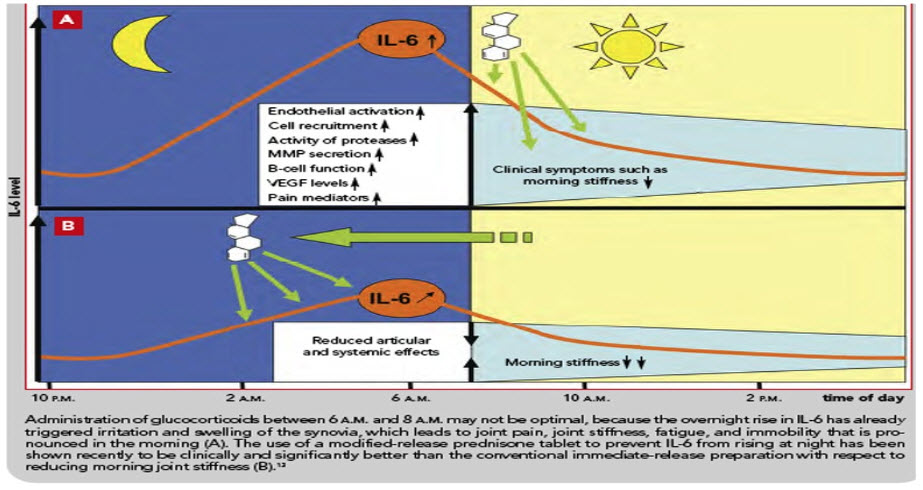
FIG 4. EFFECT OF IMMIDIATE & MODIFIED RELEASE PREDINISONE ON IL-6 LEVELS
FIG 5.DIFFUCAPS
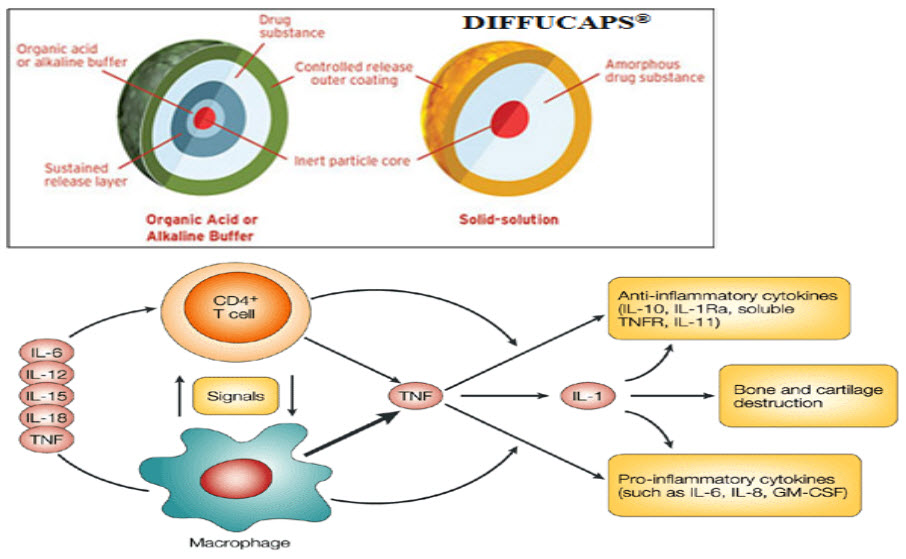
FIG 6. A schematic representation of some of the cytokine and cellular interactions in the tumour-necrosis factor (TNF)-dependent cytokine cascade to illustrate the crucial role of (TNF) in the cytokine network of rheumatoid arthritis.
References:
1. Dickmeis T, Glucocorticoids and the circadian clock, J Endocrinol, 2009;200:3–22.
2. Moore-Ede M, Fuller C, Sulzman F. The Clocks That Time Us. Boston: Commonwealth Fund Publications, Havard University Press; 1982: 448.
3. Warman VL, Dijk DJ, Warman GR, et al., Phase advancing human circadian rhythms with short wavelength light, Neuroscience Lett, 2003;342:37–40.
4. Botti B, Youan C. Chronopharmaceutics: Gimmick or clinically relevant approach to drug delivery, Journal of Control Release 2004: 337-353.
5. Jha N, Bapat S. Chronobiology and chronotherapeutics , Kathmandu University Medical Journal 2004; 2(8): 384-388.
6. Prisant LM. Chronobiology and Chronotherapeutics- Possible strategy for hypertension and ischemic heart disease. Business briefings: North Am Pharmacother. 2004; 2: 35- 36.
7. Maurizio Cutolo, Rainer H Straub, Buttgereit Frank. Circadian rhythms of nocturnal hormones in rheumatoid arthritis: translation from bench to bedside. Ann Rheum Dis. 2008; 67:905-908.
8. Straub RH, Cutolo M. Circadian rhythms in rheumatoid arthritis: implications for path physiology and therapeutic management. Arthritis Rheum. 2007; 56:399–408.
9. Youan CB. Chronopharmaceutics: gimmick or clinically relevant approach to drug deliv
10. Pranay, Ankita, Awani, Ashutosh. Chronopharmaceutics as a Novel Approach for Drug Delivery. J Pharm Sci Tech. 2009; 1:59-62.
11. Martin RJ, S Banks-Shlegel. Chronobiology of asthma. Am J Respir Crit Care. Med. 1998; 158:1002-1007, J Control Rel. 2004; 98:337-353.
12. Rupali Singh, Pramod Kumar Sharma and Rishabha M. Review on Chronotherapeutics - A New Remedy in the Treatment of Various Diseases. Eur J Bio Sci 2010; 2 (3): 67-76.
13. Moore JG, Englert Jr E. Circadian rhythm of gastric acid secretion in man. Nature 1970; 226:1261–1262.
14. Poirel C,Ennaji M. Chronobiological paradigms of mental life and clinical neuroscience. Encephhale 2000; 26: 57-66.
15. Smolensky M. H, Scott P. H, Harrist R. B. Administration time dependecy of the pharamocokinetic behavior and Therapeutic effect of a once-a-day theophylline in asthmatic children. Chronobiol. Int .1987; 4(3): 4
15. Lemmer B. Cardiovascular chronobiology and chronopharmacology. Biological Rhythms in Clinical and Laboratory Medicine 1992; 418– 427.
16. Swannell A. J. Biological rhythms and their effect in assessment of disease activity in rheumatoid arthritis. British Journal of Clinical Practice. 1983; 38 (Supplement 33): 16-19.
17. Waldhäusl W. Circadian rhythms of insulin needs and actions, Diabetes Research and Clinical Practice 1989; 6 (4).
18. Stein EA, Davidson MH, Dobs AS, Schrott H, Dujovne CA, Bays H, Weiss SR, Melino MR, Mitchel ME, Mitchel YB. Efficacy and safety of simvastatin 80 mg/day in hypercholesterolemic patients. Am J Cardiol 1998; 82: 311- 316.
19. Hori K, Zhang QH, Li HC, Saito S, Sato Y. Timing of cancer chemotherapy based on circadian variations in tumor tissue blood flow. Int J Cancer 1996; 65:360–364.
20. Fox DA. The role of T-cells in the immunopathogenesis of rheumatoid arthritis. Arthr Rheum. 1997; 40:598-609.
21. Arend WP, Dayer JM. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor-α in rheumatoid arthritis. Arthr Rheum. 1995; 2:151.
22. Struy KL, Hawes GE, Chatila MK. T-cell receptors in rheumatoid arthritis. Arthr Rheum. 1995; 5:577.
23. Bathon JM. Rheumatoid Arthritis Path physiology. John Hopkins Arthritis Centre. Available from: https://www.hopkins- arthritis.org/arthritis-info/rheumatoid- arthritis/rheum_clin_path.html.
24. Petrie K, Dawson AG, Thompson L. Brook R. A double-blind trial of melatonin as a treatment in international cabin crew. Biol Psychiatry. 1993; 33: 526-530.
25. Webb SM, Domingo MP. Role of melatonin in health and disease. Clin Endocrinol. 1995; 42: 221-234.
26. Folkard S, Arendt J. Can melatonin improve shift workers tolerance of the night shift? Some preliminary findings. Chronobiol Int. 1993; 1:315-320.
27. Cutolo1 M, Maestroni GJM. The melatonin- cytokine connection in rheumatoid arthritis. Ann Rheum Dis. 2005; 64:1109-1111.
28. Harkness JAL, Richter MB, Panayi GS, Geddawi M, K Van De Pette, Unger A, Pownall R. Circadian variation in disease activity in rheumatoid arthritis. Brit Med J. 1982; 284:2551-554.
29. Cutolo M, Maestroni GJ, Otsa K, Aakre O, Villaggio B, Capellino S. Circadian melatonin and cortisol levels in rheumatoid arthritis patients in winter time: a north and south Europe comparison. Ann Rheum Dis. 2005; 64:212–216.
30. Straub RH, Cutolo M, Circadian rhythms in rheumatoid arthritis: implications for pathophysiology and therapeutic management, Arthritis Rheum, 2007;56:399–408.
31. Buttgereit F, Doering G, Schaeffler A, et al., Efficacy of modified-release versus standard prednisone to reduce duration of morning stiffness of the joints in rheumatoid arthritis (CAPRA-1): a double-blind, randomised controlled trial, Lancet, 2008;371:205–14. 32Herold M, Günther R., Circadian rhythm of C-reactive protein in patients with rheumatoid arthritis. Prog Clin Biol Res., 1987: 227B: 271-9.
33.Crofford LJ, Kalogeras KT, Mastorakos G, Magiakou MA, Wells J, Kanik KS, Gold PW, Chrousos GP, Wilder RL., Circadian relationships between interleukin (IL)-6 and hypothalamic-pituitary-adrenal axis hormones: failure of IL-6 to cause sustained hypercortisolism in patients with early untreated rheumatoid arthritis. J Clin Endocrinol Metab., 1997; 82: 1279-83.
34.Prisant LM. Chronobiology and Chronotherapeutics- Possible strategy for hypertension and ischemic heart disease. Business briefings: North Am Pharmacother. 2004; 2: 35- 36.
35. Cutolo M, Seriolo B, Craviotto C, et al., Circadian rhythms in RA, Ann Rheum Dis, 2003;62:593–6.
36. Levi F, Le Louarn C, Reinberg A. Chronotherapy of osteoarthritis patients: optimization indomethacine sustained released (ISR). Annual Review of Chronopharmacology 1984; 1: 345-348.
37. Lvin M, Focan-Hensard D, Levi F. Chronobiological aspects of spondylarthritis. Annual Review of Chronopharmacology 1988; 5: 17-20.
38. Reinberg A, Manfredi R, Kahn MF. Tenoxicam chronotherapy of rheumatic diseases. Annual Review of Chronopharmacology 1990; 7: 293-296.
39. Levi F, Le Louarn C, Reinberg A., Timing optimizes sustained-release indomethacin treatment of osteoarthritis. Clin Pharmacol Ther., 1985; 37: 77-84.
40. Kowanko IC, Pownall R, Knapp MS. Circadian variations in the signs and symptoms of rheumatoid arthritis and in the therapeutic effectiveness of flurbiprofen at different times of the day. Brit J Clin Pharmacol. 1981; 11:477- 484.
41. Buttgereit F, Doering G, Schaeffler A, Witte S, Sierakowski S, Gromnica-Ihle E, Jeka S, Krueger K, Szechinski J, Alten R., Efficacy of modified-release versus standard prednisone to reduce duration of morning stiffness of the joints in rheumatoid arthritis (CAPRA-1): a double-blind, randomised controlled trial. Lancet., 2008; 371: 205-14.
42. Becker C, Barbulescu K, Hildner K, Meyer zum Büschenfelde KH, Neurath MF., Activation and methotrexate-mediated suppression of the TNFα promoter in T cells and macrophages. Ann. N. Y. Acad. Sci., 1998; 859: 219-22.
43. Choi HK, Hernán MA, Seeger JD, Robins JM, Wolfe F., Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002; 359: 1173-7.
44. Dolhain RJ, Tak PP, Dijkmans BA, De Kuiper P, Breedveld FC, Miltenburg AM., Methotrexate reduces inflammatory cell numbers, expression of monokines and of adhesion molecules in synovial tissue of patients with rheumatoid arthritis. Br J Rheumatol., 1998; 37: 502-8.
45. Percel P, Vishnupad K, Venkatesh G. Eurand Pharmaceuticals Ltd, United States, 2002; p 13.
46. Fuisz, R. Fusz Technologies Ltd, United States, 1996; p 34.
47. Panoz D, Geoghegan E. Elan Corporation, United States, 1989; p 49.
48. Prisant LM, Devane JG, Butler J. Am J Ther. 2000; 7: 345.
49. Leslie S. Euroceltique SA, United States, 1982; p 20.
50. Conte U, Maggi L. Biomaterials. 1996; 17: 889.
51. Crison JR, Vieira ML, Amidon GL. In: Rathbone MJ, Hadgraft J, Roberts MS, eds. Modified-Release Drug Delivery Technology, vol. 126. New York: Marcel Dekker; 2003: 249–256.
52. Katstra WE, et al. J Control Release. 2000; 66: 1.
53. Rowe CW, et al. J Control Release. 2000; 66: 11.
54. Monkhouse D, Yoo J, Sherwood J, Cima M, Bornancini E. Therics, Inc, United States, 2003; p 19.
55. Baichwal A, Staniforth J. Penwest Pharmaceuticals Co., United States, 2002; p 19.
56. Staniforth JN, Baichwal AR. Expert Opin Drug Deliv. 2005; 2: 587.
57. Altaf SA, Yu K, Parasrampuria J, Friend DR. Pharm Res. 1998; 15: 1196.
58. Narisawa S, et al. Pharm Res. 1994; 11: 111.
59. Narisawa S, Nagata M, Hirakawa Y, Kobayashi M, Yoshino H. J Pharm Sci. 1996; 85: 184.
60. BG De Geest, et al. Biomacromolecules. 2006; 7: 373.









