About Author:
KIRANKUMAR K. VAGHASIYA
M.Sc. Biotechnology
Bhagwan Mahavir College Of Biotechnology, Surat
* vaghasiyakiran51@yahoo.co.in
Abstract:
Biosensors can be excellent analytical tools for monitoring programs working to implement legislation.Biosensor are analytical devices which are capable of providing either qualitative or quantitative results. Biosensor technology to be in a very early stage of development. There are many market opportunities. In this article, biosensors for environmental analysis and monitoring are extensively reviewed. Examples of biosensors for the most important families of environmental pollutants, including some commercial devices, are presented. Finally, future trends in biosensor development are discussed. In this context, bioelectronics, nanotechnology, environmental pollution, and especially biotechnology seem to be growing areas that will have marked influence on the development of new biosensing strategies in the next future.At present There are various types of biosensor available for the number of industrial and diagnostic Applications.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1464
Introduction:
Defination:
“Biosensor” – Any device that uses specific biochemical reactions to detect chemical compounds in biological samples.[1]
Current Definition A sensor that integrates a biological element with a physiochemical transducer to produce an electronic signal proportional to a single analyte which is then conveyed to a detector.[2]
A biosensor is a device incorporating a biological sensing element like (e.g. tissue, microorganisms, organelles, cell receptors, enzymes, antibodies, nucleic acids, natural products etc.), a biologically derived material (e.g. recombinant antibodies, engineered proteins, aptamers etc) or a biomimic (e.g. synthetic receptors, biomimetic catalysts, combinatorial ligands, imprinted polymers etc) either intimately connected to or integrated within a transducer. Specific molecular recognition is a fundamental prerequisite, based on affinity between complementary structures such as enzyme-substrate, antibody-antigen and receptorhormone, and this property in biosensor is used for the production of concentration–proportional signals. Biosensor’s selectivity and specificity highly depend on biological recognition systems connected to a suitable transducer[3,4,5]. Biosensors can be excellent analytical tools for monitoring programs working to implement legislation. Public concern and legislation are nowadays demanding better environmental control. In addition, the increase in the number of analytes to monitor requires more suitable analytical methods. Conventional methods are actually reaching the highest accuracy with low detection limits, but are expensive, time-consuming, and require the use of highly trained personnel.[6] The current tendency to carry out field monitoring has driven the development of biosensors as new analytical tools able to provide fast, reliable, and sensitive measurements with lower cost; many of them aimed at on-site analysis. In this sense, biosensors would not compete with official analytical methods, but they can be used both by regulatory authorities and by industry to provide enough information for routine testing and screening of samples.[7] A biosensor is a device that detects, transmits and records information regarding a physiological or biochemical change. Technically, it is a probe that integrates a biological component with an electronic transducer thereby converting a biochemical signal into a quantifiable electrical response.[8,9,10] Biosensors are defined as analytical devices incorporating a biological material, a biologically derived material, or biomimic, intimately associated with or integrated within a physicochemical transducer or transducing microsystem.[11]Biosensors usually yield a digital electronic signal which is proportional to the concentration of a specific analyte or group of analytes. While the signal may in principle be continuous, devices can be configured to yield single measurements to meet specific market requirements. Examples of Biosensors include immunosensors, enzyme-based biosensors, organism- and whole cell-based biosensors. They have been applied to a wide variety of analytical problems including uses in medicine, biomedical research, drug discovery, the environment, food, process industries, security and defence.
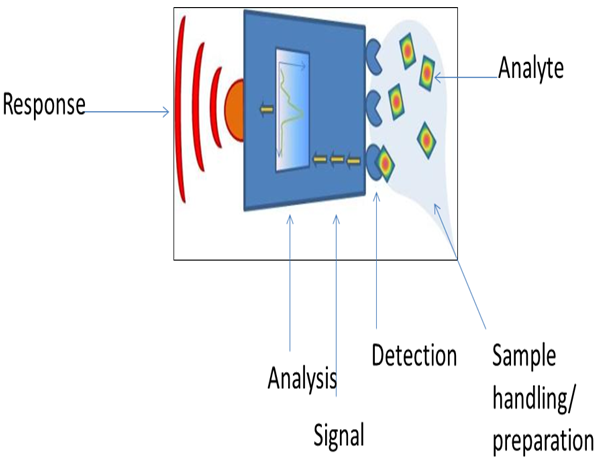
Biosensors should be distinguished from a bioassay or a bioanalytical system, which require additional processing steps, such as reagent addition.[12]The increasing number of potentially harmful pollutants in the environment calls for fast and cost-effective analytical techniques to be used in extensive monitoring programs. Additionally, over the last few years, a growing number of initiatives and legislative actions for environmental pollution control have been adopted in parallel with increasing scientific and social concern in this area.[13,14,15,16]
[adsense:468x15:2204050025]
The requirements for application of most traditional analytical methods to environmental pollutants analysis, often constitute an important impediment for their application on a regular basis. The need for disposable systems or tools for environmental applications, in particular for environmental monitoring, has encouraged the development of new technologies and more suitable methodologies. In this context, biosensors appear as a suitable alternative or as a complementary analytical tool. Biosensors can be considered as a subgroup of chemical sensors in which a biological mechanism is used for analyte detection [13,15,16] A biosensor is defined by the International Union of Pure and Applied Chemistry (IUPAC) as a self-contained integrated device that is capable of providing specific quantitative or semi-quantitative analytical information using a biological recognition element (biochemical receptor), which is retained in contact direct spatial with a transduction element.[17] permanently fixed in the construction of the device. Biosensors are being developed for different applications, including environmental and bioprocess control, quality control of food, agriculture, military, and, particularly, medical applications. In fact, most of the commercially available biosensor systems are applied in the clinical and pharmaceutical markets. Accordingly, most research and development has been devoted to this area. In the food industry, the detection of contaminants, verification of product content, monitoring of raw materials conversion, and product freshness [18] The beer industry has already identified ways for improving and controlling their products through the use of biosensors [19] Biosensing systems and methods are being developed as suitable tools for different applications, including bioprocess control, food quality control, agriculture, military and in particular, for medical applications. The function of a biosensor depends on the biochemical specificity of the biologically active material. The choice of the biological material will depend on a number of factors viz the specificity, storage, operational and environmental stability. Selection also depends on the analyte to be detected such as chemical compounds, antigens, microbes, hormones, nucleic acids or any subjective parameters like smell and taste. Enzymes, antibodies, DNA, receptors, organelles and microorganisms as well as animal and plant cells or tissues have been used as biological sensing elements. Some of the major attributes of a good biosensing system are its specificity, sensitivity, reliability, portability, (in most cases) ability to function even in optically opaque solutions, real-time analysis and simplicity of operation. [8,9,20,21,22,23,24] so, Biosensors are powerful tools aimed at providing selective identification of toxic chemical compounds at ultratrace levels in industrial products, chemical substances, environmental samples (e.g., air, soil, and water) or biological systems (e.g., bacteria, virus, or tissue components) for biomedical diagnosis. Combining the exquisite specificity of biological recognition probes and the excellent sensitivity of laser-based optical detection, biosensors are capable of detecting and differentiating big/chemical constituents of complex systems in order to provide unambiguous identification and accurate quantification.A new generation of biosensors discussed in this presentation uses antibody and DNA probes.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
History:

Professor Leland C Clark Jnr1918–2005
Father of the Biosensor
- 1916 First report on immobilization of proteins : adsorption of invertase on activated charcoal
- 1922 First glass pH electrode
- 1956 Clark published his definitive paper on the oxygen electrode.
- 1962 First description of a biosensor: an amperometric enzyme electrodre for glucose (Clark)
- 1969 Guilbault and Montalvo – First potentiometric biosensor:urease immobilized on an ammonia electrode to detect urea
- 1970 Bergveld – ion selective Field Effect Transistor (ISFET)
- 1975 Lubbers and Opitz described a fibre-optic sensor with immobilised indicator to measure carbon dioxide or oxygen.
-
1975 First commercial biosensor ( Yellow springs Instruments glucose biosensor)
- 1975 First microbe based biosensor, First immunosensor
- 1976 First bedside artificial pancreas (Miles)
- 1980 First fibre optic pH sensor for in vivo blood gases(Peterson)
- 1982 First fibre optic-based biosensor for glucose
- 1983 First surface plasmon resonance (SPR) immunosensor
- 1984 First mediated amperometric biosensor: ferrocene used with glucose oxidase for glucose detection
- 1987 Blood-glucose biosensor launched by MediSense ExacTech
- 1990 SPR based biosensor by Pharmacia BIACore
- 1992 Hand held blood biosensor by i-STAT
- 1996 Launching of Glucocard
- 1998 Blood glucose biosensor launch by LifeScan FastTake
- 1998 Roche Diagnostics by Merger of Roche and Boehringer mannheim
- Current Quantom dots, nanoparicles, nanowire, nanotube, etc [25]
Main components of a biosensor:
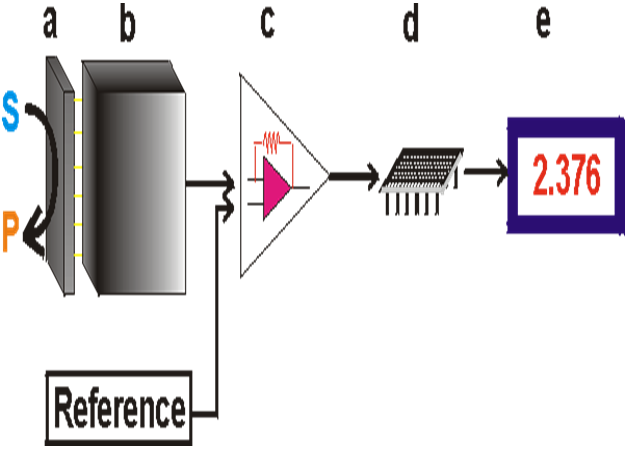
(a) the biocatalyst converts the substrate to product.
(b) the transducer which converts the reaction to an electrical signal
(c) the amplifier,
(d) the processor and
(e) the display[26]
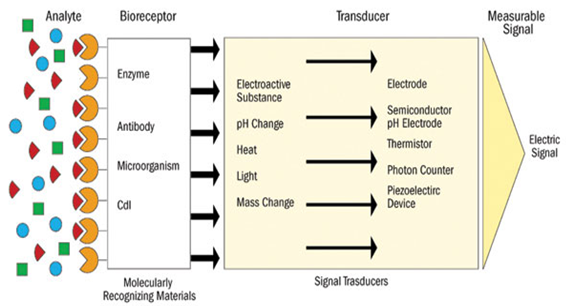
Biosensor has three major components:
(i) a bio-recognition element such as DNA, enzyme, antibody etc. for recognition of an anlyte,
(ii) a matrix such as a self-assembled monolayer (SAM), Langmuir-Blodgett film, thick polymer film, hydrogel and nanomaterials etc. for the immobilization of a biomolecule and
(iii) a transducer unit for conversion and amplification of the biochemical reaction product into a recognizable.[27]
Main principle of a biosensor:[28]
Types of biosensor
Whole cell biosensor / advanced bioreporter technologies :
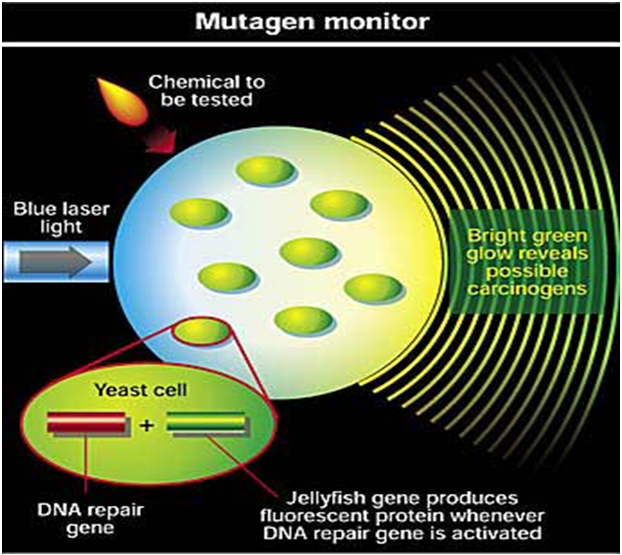
Bacteria can be used as biosensors to demonstrate the toxicity of a variety of environmental media including soil, sediment, and water by coupling bacteria to transducers that convert a cellular response into detectable signals [29]. These bacterial biosensors are engineered by pairing a reporter gene that generates a signal with a contaminant-sensing component that responds to chemical or physical change, such as exposure to a specific analyte. When the biosensor is exposed to such a change, the sensing component stimulates the reporter gene through a biochemical pathway in the cell. The reporter gene then produces a measurable response, such as emitting visible light, which is indicative of the degree of chemical or physical change [29, 30,31,32] Several biosensors have been developed that indicate toxicity of any chemical or physical change; new biosensors are being developed to respond to particular analytes. Such biosensors have been developed for heavy metals and metalloids including arsenic, cadmium, mercury, and lead [33] Biosensors measure the bioavailable concentration for the contaminant they are designed to detect [30] test the measurements made by biosensors, a chelating agent known to decrease bioavailability of lead was added to a lead solution. Measurements of the lead solution containing chelating agents were taken and compared to measurements of the lead-only solution. A decrease in the biosensor.s luminescence matched a decrease in bioavailable concentration of lead in the solution. This demonstrates that biosensors are sensitive to the bioavailable fraction of the contaminant and their luminescence reflects the bioavailable concentration [30]. Biosensors have been further tested by comparing their results with the results from chemical analysis of arsenic contaminated samples [31].Chemical analyses such assequential extraction procedure, can be used to determine the total arsenic, water-soluble arsenic,and acid-soluble arsenic content of the sample. The acid and water soluble contents are used todetermine the mobility of arsenic, in an attempt to clarify true exposure potential. The resultsfrom a biosensor correlated moderately with the water-soluble arsenic determined by chemicalanalysis, but not with the acid-soluble or total arsenic content. Therefore, the acid-soluble orwater-soluble arsenic content does not completely represent the bioavailable content of arsenic [31]. Bioreporters refer to intact, living microbial cells that have been genetically engineered to produce a measurable signal in response to a specific chemical or physical agent in their environment.
Bioreporters contain two essential genetic elements, a promoter gene and a reporter gene. The promoter gene is turned on (transcribed) when the target agent is present in the cell’s environment. The promoter gene in a normal bacterial cell is linked to other genes that are then likewise transcribed and then translated into proteins that help the cell in either combating or adapting to the agent to which it has been exposed. In the case of a bioreporter, these genes, or portions thereof, have been removed and replaced with a reporter gene. Consequently, turning on the promoter gene now causes the reporter gene to be turned on. Activation of the reporter gene leads to production of reporter proteins that ultimately generate some type of a detectable signal. Therefore, the presence of a signal indicates that the bioreporter has sensed a particular target agent in its environment [32].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
General Mechanisms of Biosensor
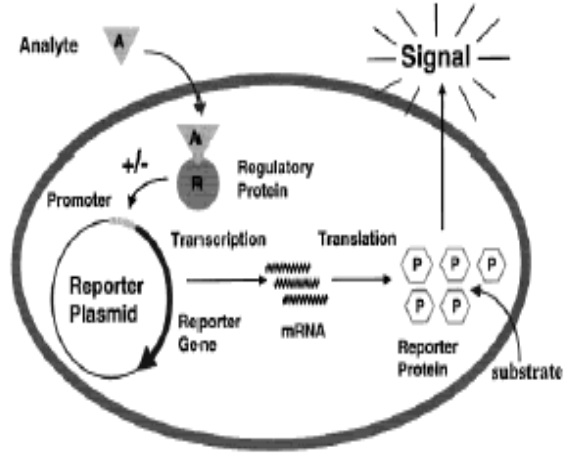
Reporter Gene Systems
Several types of reporter genes are available for use in the construction of bioreporter organisms, and the signals they generate can usually be categorized as either colorimetric, fluorescent, luminescent, chemiluminescent or electrochemical. Although each functions differently, their end product always remains the same – a measurable signal that is proportional to the concentration of the unique chemical or physical agent to which they have been exposed. In some instances, the signal only occurs when a secondary substrate is added to the bioassay (luxAB, Luc, and aequorin). For other bioreporters, the signal must be activated by an external light source (GFP and UMT), and for a select few bioreporters, the signal is completely self-induced, with no exogenous substrate or external activation being required (luxCDABE).
Bacterial luciferase (Lux):Luciferase is a generic name for an enzyme that catalyzes a light-emitting reaction. Luciferases can be found in bacteria, algae, fungi, jellyfish, insects, shrimp, and squid, and the resulting light that these organisms produce is termed bioluminescence. In bacteria, the genes responsible for the light-emitting reaction (the lux genes) have been isolated and used extensively in the construction of bioreporters that emit a blue-green light with a maximum intensity at 490 nm[33]. Three variants of lux are available, one that functions at < 30ºC, another at < 37ºC, and a third at < 45ºC. The lux genetic system consists of five genes, luxA, luxB, luxC, luxD, and luxE. Depending on the combination of these genes used, several different types of bioluminescent bioreporters can be constructed.[33]

Bioluminescence emitted from individual colonies of microbial cells containing the genes for bacterial luciferase.

luxAB bioreporters: luxAB bioreporters contain only the luxA and luxB genes, which together are responsible for generating the light signal. However, to fully complete the light-emitting reaction, a substrate must be supplied to the cell. Typically, this occurs through the addition of the chemical decanal at some point during the bioassay procedure. Numerous luxAB bioreporters have been constructed within bacterial, yeast, insect, nematode, plant, and mammalian cell systems.
|
Chemical and biological agents detectable by luxAB-based bioreporters |
|
|
Antibiotic effectiveness |
Gene expression/regulation |
|
Antimicrobial agents |
Growth phase regulation |
|
Bacterial biofilms |
Immunoassays |
|
Bacterial biomass |
In vivo expression technology (IVET) |
|
Bacterial stress response |
Industrial waste runoff |
|
Bacterial transport mechanisms |
Metabolic regulation |
|
Bioremediation process monitoring |
Mutagenicity tests |
|
Cell viable counts |
Plant pathogens |
|
Circadian rhythms |
Toxicity assays |
|
DNA damaging agents |
Tumor burden |
|
Environmental contaminants |
Viral infection |
|
Foodborne pathogens |
Xenobiotic detection |
luxCDABE bioreporters: Instead of containing only the luxA and luxB genes, bioreporters can contain all five genes of the lux cassette, thereby allowing for a completely independent light generating system that requires no extraneous additions of substrate nor any excitation by an external light source. So in this bioassay, the bioreporter is simply exposed to a target analyte and a quantitative increase in bioluminescence results, often within less than one hour. Due to their rapidity and ease of use, along with the ability to perform the bioassay repetitively in real-time and on-line, makes luxCDABE bioreporters extremely attractive. Consequently, they have been incorporated into a diverse array of detection methodologies ranging from the sensing of environmental contaminants to the real-time monitoring of pathogen infections in living mice.
|
Selected examples of luxCDABE-based bioreporters |
|||
|
Analyte |
Time for induction |
Concentration |
Reference |
|
2,3 Dichlorophenol |
2 h |
50 mg/ L |
35 |
|
2,4,6 Trichlorophenol |
2 h |
10 mg/ L |
35 |
|
2,4-D |
20 – 60 min |
2 μM – 5 mM |
36 |
|
3-Xylene |
Hours |
3 μM |
37 |
|
4-Chlorobenzoate |
1 h |
380 μM – 6.5 mM |
38 |
|
4-Nitrophenol |
2 h |
0.25 mg/ L |
35 |
|
Aflatoxin B1 |
45 min |
1.2 ppm |
39 |
|
Alginate production |
1 h |
50 – 150 mM NaCl |
40 |
|
Ammonia |
30 min |
20 μM |
41 |
|
Antibiotic effectiveness against Staphylococcus aureus infections in mice |
4 h |
100 CFU |
42 |
|
BTEX (benzene, toluene, ethylbenzene, xylene) |
1 – 4 h |
0.03 – 50 mg/L |
43 |
|
Cadmium |
4 h |
19 mg/kg |
35 |
|
Chlorodibromomethane |
2 h |
20 mg/ L |
35 |
|
Chloroform |
2 h |
300 mg/ L |
35 |
|
Chromate |
1 h |
10 μM |
45 |
|
Cobalt |
Not specified |
2.0 mM |
46 |
|
Copper |
1 h |
1 μM – 1 mM |
47 |
|
DNA damage (cumene hydroperoxide) |
50 min |
6.25 mg/ml |
48 |
|
DNA damage (mitomycin) |
1 h |
0.032 μg/ml |
49 |
|
Gamma-irradiation |
1.5 h |
1.5 – 200 Gy |
50 |
|
Heat shock |
20 min |
Various, depending on chemical inducer used |
51, 52 |
|
Hemolysin production |
Not specified |
5 mM cAMP |
53 |
|
Hydrogen peroxide |
20 min |
0.1 mg/L |
54 |
|
in vivo monitoring of Salmonella typhimurium infections in living mice |
4 h |
100 CFU |
55 |
|
Iron |
Hours |
10 nM – 1 μM |
56 |
|
Isopropyl benzene |
1 – 4 h |
1 – 100 μM |
57 |
|
Lead |
4 h |
4036 mg/kg |
44 |
|
Mercury |
70 min |
0.025 nM |
58 |
|
N-acyl homoserine lactones |
4 h |
Not specified |
59 |
|
Naphthalene |
8 – 24 min |
12 – 120 μM |
60 |
|
Nickel |
Not specified |
0.3 mM |
46 |
|
Nitrate |
4 h |
0.05 – 50 μM |
61 |
|
Organic peroxides |
20 min |
Not specified |
48 |
|
PCBs |
1 – 3 h |
0.8 μM |
62 |
|
p-chlorobenzoic acid |
40 min |
0.06 g/l |
38 |
|
p-cymene |
< 30 min |
60 ppb |
63 |
|
Pentachlorophenol |
2 h |
0.008 mg/L |
58 |
|
Phenol |
2 h |
16 mg/L |
58 |
|
Salicylate |
15 min |
36 μM |
60 |
|
Tetracycline |
40 min |
5 ng/ml |
64 |
|
Trichloroethylene |
1 – 1.5 h |
5 – 80 μM |
65 |
|
Trinitrotoluene |
Not specified |
Not specified |
66 |
|
Ultrasound |
1 h |
500 W/cm2 |
67 |
|
Ultraviolet light |
1 h |
2.5 – 20 J/m2 |
68 |
|
Zinc |
4 h |
0.5 – 4 μM |
69 |
Nonspecific lux bioreporters: Nonspecific lux bioreporters are typically used for the detection of chemical toxins. They are usually designed to continuously bioluminesce. Upon exposure to a chemical toxin, either the cell dies or its metabolic activity is retarded, leading to a decrease in bioluminescent light levels. Their most familiar application is in the Microtox assay where, following a short exposure to several concentrations of the sample, the decreased bioluminescence can be correlated to relative levels of toxicity [34].
Firefly luciferase (Luc): Firefly luciferase catalyzes a reaction that produces visible light in the 550 – 575 nm range. A click-beetle luciferase is also available that produces light at a peak closer to 595 nm. Both luciferases require the addition of an exogenous substrate (luciferin) for the light reaction to occur.
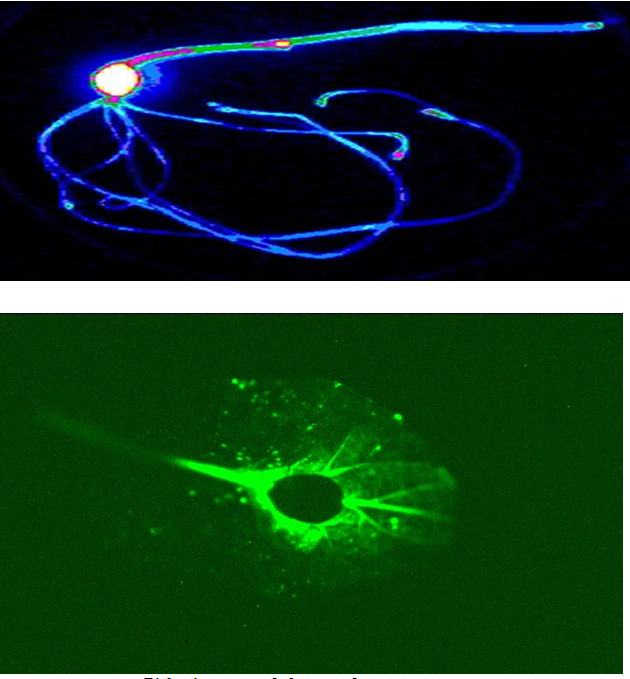
Bioluminescence of the genes for firefly luciferase
Numerous luc-based bioreporters have been constructed for the detection of a wide array of inorganic and organic compounds of environmental concern. Their most promising application, however, probably lies within the field of medical diagnostics. Insertion of the luc genes into a human cervical carcinoma cell line (HeLa) illustrated that tumor-cell clearance could be visualized within a living mouse by simply scanning with a charge-coupled device camera, allowing for chemotherapy treatment to rapidly be monitored on-line and in real-time[70]. In another example, the luc genes were inserted into human breast cancer cell lines to develop a bioassay for the detection and measurement of substances with potential estrogenic and antiestrogenic activity.[71]
Aequorin: Aequorin is a photoprotein isolated from the bioluminescent jellyfish Aequorea victoria. Upon addition of calcium ions (Ca2+) and coelenterazine, a reaction occurs whose end result is the generation of blue light in the 460 - 470 nm range. Aequorin has been incorporated into human B cell lines for the detection of pathogenic bacteria and viruses in what is referred to as the CANARY assay (Cellular Analysis and Notification of Antigen Risks and Yields) [72].
Green fluo









