About Authors:
S.Kameshwaran1*, V.Suresh1, M.Mohanraj2
1Department of pharmacology,
JKK munirajah medical research foundation and College of Pharmacy,
B.Komarapalayam, Namakkal, Tamilnadu - 638183
2Marineboitoxinology labs, CAS in marine biology, Annamalai university,
Chidambaram, Tamilnadu-608502
*kamesh.pharm@gmail.com
ABSTRACT
Tecoma stans flowers have been traditionally used for many ailments including cancer. In the present study, anti cancer activity of methanolic flower extract of T.stans (METS) was evaluated using both in vitro and in vivo methods. METS was subjected to preliminary qualitative phytochemical investigations by using standard procedures. In vitro antitumor activity of METS was evaluated by the MTT assay methodusing Vero and HEP-2 cell lines. Then the extract subjected to in vivo anti cancer activity using Ehrlich ascites carcinoma (EAC) tumor model. The activity was assessed Increase in life span, average increase in body weight, changes in food intake, tumor volume, tumor weight, viable cell count, non viable cell count, PCV, Total cell count and hematological studies. The potency of the extract was compared with standard 5-flurouracil (20 mg/kg i.p.).In in vitro anti cancer activity METS exhibited significant cytotoxic activity against both cell lines even at different concentrations. Oral administration of METS at the dose of 200 and 400 mg/Kg, significantly (p < 0.001) increased the survival time, non viable cell count and decreased the average body weight and food intake, viable cell count of the tumor bearing mice. After 14 days of inoculation, METS was able to reverse the changes in the hematological parameters, protein and PCV consequent to tumor inoculation.The results indicate that METS possess significant antitumor activity on dose dependent manner.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1275
INTRODUCTION
Cancer, the second leading cause of death worldwide next to cardiovascular diseases, is a group of more than 100 different diseases, characterized by uncontrolled cellular growth, local tissue invasion and distant metastases1. Cancer is the cause of more than six million deaths each year in the world. In 2001, about 1,268,000 new cancer cases and 553,400 deaths were reported in the United States2. It can be treated with surgery, radiation, chemotherapy, hormone therapy and biological therapy. Chemotherapy is still a major challenge to the cancer patients because such highly potent drug can be toxic and less than 1% of injected drug molecules can reach their target cells whereas the rest may damage healthy cells and tissue especially bone marrow, epithelial tissues, reticulo-endothelial system and gonads3 Since medieval times, plants have been the source of medicines for the treatment of diseases. Regardless of the availability of a wealth of synthetic drugs, plants remain– even in the 21st century – an integral part of the health care in different countries, especially the developing ones. In the late 90’s, the WHO stated that a big percentage of the world’s population depends on plant based therapies to cover the needs of the primary health care (WHO 1999)4. The areas of cancer and infectious diseases have a leading position in utilization of medicinal plants as a source of drug discovery. Among FDA approved anticancer and anti-infectious preparations drugs of natural origin have a share of 60% and 75% respectively5. It is worthy to mention the vivid current interest in discovery of natural drugs for cancer treatment and chemoprevention6,7. Huge number of plant species is screened and bioassayed for this purpose worldwide8.
Tecoma stans(Common name: Yellow bell) also known as Yellow Trumpet bush belonging to the family Bignoniaceae. Itis an ornamental plant. It is an erect, branched, sparingly hairy or nearly smooth shrub 2 to 4 meters in height. The leaves are opposite, odd-pinnate, and up to 20 centimeters in length with 5 to 7 leaflets. The leaflets are lanceolate to oblong-lanceolate, 6 to 13 centimeters long, pointed at both ends, and toothed on the margins. Trumpet-shaped flowersare yellow, faintly scented, and borne in short, dense, terminal clusters .The calyx is green, 5 to 7 millimeters long, and 5-toothed. Flowering can begin as early as April and continue into fall. The flowers are followed by 6-inch-long, tanpods that are filled with small, papery winged seeds9.
Leaves of the T.stans contain the alkaloids tecomine and tecostamine are potent hypoglycaemic agent when given intravenously. Anthranilic acid is responsible for the antidiabetic activity; roots are powerful diuretic and vermifuge10.Tecoma is not a toxic because this plant is used in Latin America as a remedy for diabetes and moreover for feeding cattle and goats in Mexico11. The preliminary phytochemical screening of methanolic extract of flower extract of Tecoma stansshowed the presence of flavonoids, phenol, alkaloids, Tannin, steroids, triterpenes, anthraquinones and saponins etc.
Flavonoids are a group of more than 4000 polyphenolic compounds that occur naturally in foods of plant origin. These compounds possess a common phenylbenzopyrone structure (C6-C3-C6), and they are categorized according to the saturation level and opening of the central pyran ring, mainly into flavones, flavanols, isoflavones, flavonols, flavanones, and flavanonols12,13. The weight of the epidemiological evidence for a protective effect of flavonoids against cancer is impressive. A growing number of epidemiological studies suggest that high flavonoid intake may be correlated with a decreased risk of cancer14. flavonoids may inhibit various stages in the carcinogenesis process, namely tumor initiation, promotion, and progression. Also in vivo and in vitro studies, many mechanisms of action may be involved. These include carcinogen inactivation, antiproliferation, cell cycle arrest, induction of apoptosis and differentiation, inhibition of angiogenesis, antioxidation and reversal of multidrug resistance or a combination of these mechanisms15. Based on this information about flavanoids as anti cancer agents, the present study focus on the anti cancer activity (in invitro and in vivo) of the methanolic extract of Tecoma stans flowers.
MATERIALS AND METHODS
Collection and extraction
The flowers of Tecoma stans were collected in the month of May from Rasipuram (Namakkal District) Tamil Nadu. The plant was identified by Dr.G.V.S.Murthy, Joint Director of Botanical Survey of India, Southern circle, TNAU Campus, Coimbatore who authenticated the plant from available literature. The flower petals were dried in shade and powdered and 100 g of the dried powder was extracted with methanol by hot soxhlet apparatous. The solvent was removed under reduced pressure and controlled temperature by using rotary flash evaporator. Phytochemical screening of the extract revealed the presence of tannin, flavonoids, phenol, alkaloids, steroids, triterpenes and saponins etc.
Tumor cell lines
Vero and Hep-2 (Human larynx carcinoma cell line), The cell lines were purchased from Amla Research Institute, Trichur . EAC cells were obtained under the courtesy of Amala Cancer Research Centre, Trissur, Kerala, India. They were maintained by weekly intraperitoneal inoculation of 106 cells/ mouse.
Animals
Male swiss albino rats weighing 150-180g were used in this experiment. They were housed in standard environmental condition like, ambient temperature (25oC ± 1oC), relative humidity (55±5%), and 12/12h light dark cycle. Animals had free access to standard pellet diet and water ad libitum. All animal experiments were carried out in accordance with the guidelines of CPCSEA. The institute animal ethical committee has given the approval for conducting animal experiments (approval No. 1158/ac/07/CPCSEA).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
ANTI CANCER ACTIVITY
In vitro anticancer activity
MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5- diphenyl teterazolium bromide) assay16. Cells were grown in RPMI-1640 medium at 37ºC under incubated for 6-7hrs. 5% CO2 in a humified incubator. Cells were harvested, counted (3×104cells/ml), and transferred into a 24 well plate, and incubated for 24hrs. Prior to the addition of test compound. Serial dilutions of test samples were prepared by dissolving compounds in DMSO followed by dilution with RPMI-1640 medium to give final concentration at 250, 500, 1000μg /ml. Stock solutions of samples were prepared. Sample at 10μl and cell lines at 90μl were incubated for 72hrs. MTT solution at 5mg/ml was dissolved in 1ml of Phosphate Buffer Solution (PBS), and 10μl of it was added to each of the 24wells. The wells were wrapped with aluminium foil and incubated at 37ºC for 4hrs. The solution in each well containing media, unband MTT and dead cells were removed by suction and 150μl of DMSO was added to each well. Then the plants were shaken and optical density was recorded using a microplate reader (spectrophotometer) at595nm. DMSO as a blank. Controls and samples were assayed and replicated for each concentration and replicated three times for each cell line. After 24h incubation of the mononuclear cells with plant extracts, the cytotoxicity on the cancer cell lines was evaluated using MTT assay. The cytotoxicity was obtained by comparing the absorbance between the samples and control. The values were then used to iteratively calculate the concentration of plant extracts required to cause a 50% reduction (IC50) a growth (cell number) for each cell lines.
Cell viability (%) = Mean OD/Control OD x 100
IN VIVO ANTICANCER ACTIVITY
Determination of anti-tumour activity
The animals were acclimatized to our laboratory conditions. They were divided into five groups viz. Normal control (G1), Cancer induced animal (G2), cancer induced animal treated with 20mg/kg of 5-fluorouracil treated group (G3) 200mg/kg of METS flowers (G4), and 400mg/kg of METS flowers (G5) of ten each and used for the study17. The EAC cells were procured from Amala Cancer Institute, Thrissur, Kerala and injected intraperitonially (2×106 cells/mice) to all groups of animals. On the second day the animals of G4 and G5 were treated with 200 and 400mg/kg/day p.o. of METS flowers while G3 with 5- fluorouracil (20 mg/kg. i.p.) and the treatment was continued for next 14 days. G2 was not allocated any treatment after inoculation with EAC cells. The mice were observed for next 14 days for the development of ascitic tumour. On day 15, Twenty-four hours of last dose and 18 h of fasting, 6 animals of each group were sacrificed by cervical dislocation to measure antitumor and hematological parameters and the rest were kept with food and water ad libitum to check percentage increase in life span of the tumor host.The antitumor activity of the extract was measured in EAC animals with respect to the following parameters. Tumor volume, tumor weight, Packed cell volume (PCV), Viable cell count, non viable cell count, percentage of viable cells, percentage of non viable cells, Increase in life span (ILS), average increase in body weight, changes in food intake17-18.
Determination of Haematological Parameters
In order to detect the influence of METS flowers on hematological status of EAC bearing mice, a comparison was made among five groups (n = 6) of mice on the 15th day after inoculation. The groups were comprised of (I) Normal Control mice, (II) Tumor bearing mice, (III) Tumor bearing mice treated with 5- fluorouracil (20 mg/kg. i.p.. for 14 days).(IV) METS flowers (200 mg/Kg/ day, p.o. for 14 days), and (V) Tumor bearing mice treated with METS flowers (400 mg/Kg/day, p.o. for 14 days). Blood was drawn from each mouse by the retroorbital plexus method and the white blood cells (WBC), red blood cells (RBC), hemoglobin and protein were determined19-21. Serum preparation of the same blood use to evaluate the biochemical parameters.
STATISTICAL ANALYSIS
All values were expressed as mean ± SEM. Statistical analysis was performed with one way analysis of variance (ANOVA) followed by by Tukey Kramer multiple comparison test. P values < 0.001 were considered as highly significant and <0.05 were considered significant when compared to control.
RESULTS
In vitro anti cancer activity
From MTT assay, after treatment with various concentrations of METS Flowers parameters like percentage cell viability, percentage cytotoxicity were compared with untreated (control) cells. Decrease in cell viability & increase in cytotoxicity by METS Flowers was observed on both cell lines in dose dependent manner, but a significant decrease in cell viability (P < 0.001) was observed for 1000 μg/ml 500 μg/ml and 250 μg/ml doses of METS Flowers produced significant growth inhibition. The growth inhibitory activity of Tecoma stans flowers was more significant as the concentration of METS Flowers increases in case of both Vero and Hep-2 cell lines. [Table 1]
TABLE – 1
In vitro cytotoxic activity of METS on VERO and HEP2 cell lines
|
S. NO |
CONC (μg /ml) |
VERO CELL LINE |
HEP 2 CELL LINE |
||
|
% OF CELL VIABILITY |
% OF CYTOTOTOXICITY |
% OF CELL VIABILITY |
% OF CYTOTOTOXICITY |
||
|
1. |
Negative control |
100 |
0 |
100 |
0 |
|
2. |
Positive control |
14.21537 |
85.78463 |
18.46954 |
81.53046 |
|
3. |
METS (250) |
42.86211 |
57.13789 |
53.56631 |
46.43369 |
|
4. |
METS (500) |
28.95541 |
71.04459 |
32.24892 |
67.75108 |
|
5. |
METS (1000) |
18.17544 |
81.82456 |
23.51262 |
76.48738 |
In vivo anti cancer activity
Tumor growth and survival parameters
Antitumor activity of METS Flowers against EAC tumor bearing mice was assessed by the parameters such as tumor volume, tumor weight, viable and non viable cell count, mean survival time and % increase of life span, average increase in body weight, changes in the food intake. The tumor volume, tumor weight and viable cell count were found to be significantly (p<0.001) increased and non-viable cell count, increase in body weight and food intake was significantly (p<0.001) low in EAC control animals when compared with normal control animals. Furthermore, the mean survival time was increase to 31.66±6.02 (% ILS = 75.39) and 26±1.0 (% ILS = 61.9) on administration of METS Flowers at the dose of 400mg/kg and 200 mg/kg respectively. Finally, the change in body weight of the animals suggests the tumor growth inhibiting property of T.stans flowers. Food intake also reduced in the tumor control animals when compared to the normal control animals. All these results clearly indicate that the extract has a remarkable capacity to inhibit the growth of solid tumor induced by EAC cells [Table-2].
TABLE-2
Effect of the METS on mean survival time (MST), percentage increase life span (% ILS), average increase in body weight, changes in food intake, tumor volume, tumor weight, PCV, viable and non-viable tumor cell count in EAC bearing mice
|
PARAMETER |
EAC CONTROL |
5 - FU (20mg/kg) |
METS 200 mg/kg |
METS 400 mg/kg |
|
MST (Days) |
17.66±1.52 |
35.33±5.85*** |
26±1.0* |
31.66±6.02*** |
|
% ILS |
42.05 |
84.12** |
61.9** |
75.39** |
|
AVG INCREASE IN BODY WT (gms) |
13.47±0.32 |
4.0±0.50* |
7.7±0.53 |
6.86±8.88 |
|
CHANGES IN FOOD INTAKE (gms) |
25.66±2.93 |
48.83±2.32*** |
34.32±0.41*** |
41.71±0.78*** |
|
TUMOR VOLUME (ml) |
12.23±0.85 |
3.73±0.40*** |
6.2±0.20*** |
5.53±0.40*** |
|
TUMOR WEIGHT (gms) |
3.26±0.26 |
0.63±0.07*** |
2.32±0.11*** |
1.20±0.17*** |
|
PCV |
8.83±0.28 |
3.16±0.28*** |
6.33±0.28*** |
5.5±0.50*** |
|
VIABLE CELLS (×106 cells/ ml) |
9.24±0.28 |
2.21±0.20*** |
3.98±0.10*** |
3.28±0.21*** |
|
NON VIABLE CELLS (×106 cells/ ml) |
0.52±0.06 |
3.89±0.12*** |
1.91±0.07*** |
2.74±0.09*** |
|
VIABLE % |
96.55 |
23.12** |
41.65** |
34.33** |
|
NON VIABLE % |
12.96 |
97.25** |
47.83** |
68.58** |
|
TOTAL CELLS (×106 cells/ ml) |
9.76±0.34 |
6.00±0.32*** |
5.89±0.17*** |
6.02±0.30*** |
n=6 animals in each group, Values are represented as mean ± SEM of six animals.*P<0.05, **P<0.01 and ***P<0.001 between disease control and treated groups. (Analysed by ANOVA Tukey-Kramer multiple comparison test)
Hematological parameters
Hematological parameters of tumor bearing mice on 15 day were found to be significantly altered compared to the normal group. The total WBC count was found to be increased with a reduction of Hb content of RBC. The total number of RBC showed a modest change. At the same time interval on extract at a dose of 400 mg/kgand 200mg/kg restored all the altered hematological parameters to almost near normal [Table-3].
TABLE-3 Effect of METS on hematological parameters of EAC-bearing mice
|
DESIGN OF TREATMENT |
NORMAL |
TUMOR CONTROL |
5-FU (20 mg/kg) |
METS (200 mg/kg) |
METS (400 mg/kg) |
|
|
Hb (gm %) |
14.43±1.25 |
5.1±0.9 |
12.53±1.0*** |
10.06±1.0* |
11.7±0.40*** |
|
|
RBC (106 cells/mm3) |
5.63±0.75 |
2.6±0.1 |
5.46±0.25*** |
3.46±0.11 |
4.6±0.43* |
|
|
WBC (103 cells/mm3) |
7±0.62 |
14.26±0.70 |
9.66±0.55*** |
12.3±0.43 |
11.5±0.62* |
|
|
Differential Count (%) |
Lymphocytes |
70.4±0.40 |
32.7±0.70 |
66.56±0.50*** |
49.26±0.65*** |
58.23±0.65*** |
|
Neutrophils |
31.63±0.92 |
36.23±0.25 |
30±0.20*** |
33.73±0.47* |
32.26±0.30*** |
|
|
Monocytes |
2.33±0.15 |
4.7±0.20 |
29±0.20*** |
4.13±0.15 |
3.2±0.26*** |
|
n=6 animals in each group, Values are represented as mean ± SEM of six animals.*P<0.05, **P<0.01 and ***P<0.001 between disease control and treated groups. (Analysed by ANOVA Tukey-Kramer multiple comparison test).
Biochemical parameters
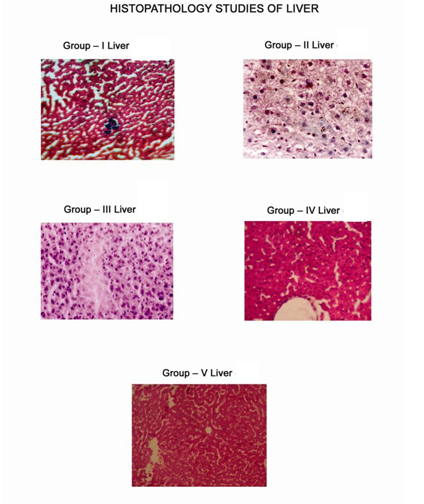
Various biochemical parameters like Tot.Protein , SGPT, SGOT, ALP , LPO, GSH, SOD, CAT values of METS At the dose of 400mg/kg were comparable to the standard drug 5- fluorouracil (20 mg/kg. i.p.) (Table-4). Histopathology study of liver of each group of animal given in Fig -2.
TABLE-4: Effect of METS on biochemical parametersof EAC-bearing mice.
|
DESIGN OF TREATMENT |
NORMAL CONTROL |
TUMOR CONTROL |
5-FU (20 mg/kg) |
METS (200 mg/kg) |
METS (400 mg/kg) |
|
TOT.PROTEIN. mg/dl |
8.46±0.47 |
11.78±0.17 |
7.88±0.30 |
10.16±0.05*** |
8.26±0.12* |
|
SGPT (U/ l) |
27.48±2.09 |
58.032±3.69*** |
33.25±1.10 |
43.84±1.87*** |
36.85±0.811* |
|
SGOT (U/ l) |
33.78±1.18 |
63.18±3.09*** |
40.46±1.36 |
46.07±2.29** |
38.03±2.13 |
|
ALP (IU/L) |
79.75±2.71 |
120.02±4.33*** |
81.61±1.48 |
99.72±2.59** |
86.75±4.26 |
|
LPO (nmol MDA/mg protein) |
0.96 ± 0.031 |
3.26± 0.039*** |
1.27± 0.041*** |
2.42 ± 0.075** |
1.33 ± 0.015*** |
|
GSH (mg/ g wet tissue) |
2.35 ± 1.532 |
0.91± 1.333 |
2.09± 0.977 |
1.77 ± 1.872 |
2.11 ± 0.931 |
|
SOD (U/mg protein) |
4.49 ± 3.74 |
1.56 ± 1.01 |
3.62 ± 3.17 |
2.47 ± 4.00 |
3.78 ± 8.32 |
|
CAT (U/mg protein) |
26.4 ± 0.023 |
9.67± 0.114 |
21.9± 0.011 |
19.4 ± 0.055 |
21.3 ± 0.063 |
n=6 animals in each group, Values are represented as mean ± SEM of six animals.*P<0.05, **P<0.01 and ***P<0.001 between disease control and treated groups. (Analysed by ANOVA Tukey-Kramer multiple comparison test)
DISCUSSION
The ehrlich tumor was initially described as a spontaneous murine mammary adenocarcinoma. It is a rapidly growing carcinoma with very aggressive behavior and is able to grow in almost all strains of mice. In ascetic form it has been used as a transplantable tumor model to investigate the antitumor effects of several substances22. The reliable criteria for judging the value of any anticancer drug are the prolongation of life span inhibition of gain in average body weight and the decrease in WBC23,24. The results of the present study showed an anticancer effect of METS Flowers against EAC in Swiss albino mice. A significant (p < 0.001 and 0.05) enhancement of MST and decrement of gain in average body weight was observed [Table – 1]. There was a regular and rapid increase in ascetic fluid volume of EAC bearing mice. Ascitic fluid is the direct nutritional source of tumor growth; it meets the nutritional requirements of tumor cells25. METS Flowers treatment decreased the tumor volume, tumor weight, PCV of solid tumor.
Reduction in viable cell count and increased non viable cell count towards normal in tumor host suggest antitumor effect against EAC cell in mice. In this study, METS Flowers increase the non viable cell count upto 68.58% and 47.83% at a dose of 400mg/kg , 200 mg/kg respectively [Table-2], which suggested that crude extract have direct relationship with tumor cells as these tumor cells are absorbed the anticancer drug by direct absorption and this anticancer agent lysis the cells by direct cytotoxic mechanism
Anemia and myelosuppression have been frequently observed in ascites carcinoma (26). Anemia encountered in ascites carcinoma mainly due to iron deficiency, either by haemolytic or myelopathic conditions which finally lead to reduced RBC number27. The analysis of the hematological parameters showed minimum toxic effect in the mice treated with METS Flowers after 15 days of transplantation, METS Flowers was able to reverse the changes in the hematological parameters consequent to tumor inoculation. This indicates that protective effect of METS Flowerson the hemopoietic system without inducing myelotoxicity, the most common side effects of cancer chemotherapy [Table-3].
The antitumor activity of EPG was comparable to that of 5-fluorouracil which is commonly used as an active antitumor agent in vast series of clinical and preclinical studies28.
Phytochemical studies indicates that the presence of tannin, flavonoids, phenol, alkaloids, steroids, triterpenes and saponins etc. Polyphenolic compounds might inhibit cancer cells by xenobiotic metabolizing enzymes that alter metabolic activation of potential carcinogens, while some flavonoids could also alter hormone production and inhibit aromatase to prevent the development of cancer cells29. The mechanism of action of anticancer activity of phenolics could be by disturbing the cellular division during mitotis at the telophase stage. It was also reported that phenolics reduced the amount of cellular protein and mitotic index, and the colony formation during cell proliferation of cancer cells30,31.
It was finally suggested that The in vitro and in vivo anticancer activities of methanolic extract of Tecoma stans flowers are probably due to the presence of alkaloid, phenolic compounds, flavonoids as well as terpenoids. Further studies are in progress to characterize the active principles and to elucidate the mechanism of action.
REFEREENCES
1. Dashora, N., V. Sodde, J. Bhagat, K.S. Prabhu, R. Lobo. Antitumor activity of dendrophthoe falcate against ehrlich ascites carcinoma in swiss albino mice. Pharm. Crops 2010; 2: 1-7.
2. Izevbigie EB. Discovery of Water-Soluble Anticancer Agents (Edotides) from a Vegetable Found in Benin City, Nigeria, Exp. Biol. and Med 2003; 228:293-98.
3. Kathiriya, A., K. Das, E.P. Kumar and K.B. Mathai, Evaluation of antitumor and antioxidant activity of Oxalis corniculata Linn. against ehrlich ascites carcinoma on mice. Iran. J. Cancer Prev 2010; 4: 157-65.
4. Dikshit A, Shahi SK, Pandey KP, Patra M, Shukla AC, Aromatic plants a source of natural chemotherapeutants. Nat. Acad. Sci. Letters 2004; 27(5&6): 145-64.
5. Newman DJ, Cragg GM, Snader KM, Natural products as sources of new drugs over the period 1981-2002. J Nat Prod 2003; 66(7): 1022-37
6. Kucuk O, New opportunities in chemoprevention research. Cancer Invest 2002; 20: 237-45.
7. Balunas MJ, Kinghorn AD, Drug discovery from medicinal plants Life Sci 2005; 78: 431-41.
8. Richardson M.A, Biopharmacologic and herbal therapies for cancer: research update from NCCAM. J. Nutr 2001; 131(11): 3037S-40S.
9. Parrotta J A,. Healing plants of Peninsular India. CABI publishing 2001; 701-02.
10. K.N.V. Rao et al, establishment of two varieties in tecoma stans ofindian origin pharmacognostically and pharmacologically. J Phytology 2010; 2: 92-102
11. Khare, CP.Indian medicinal plants and illustrated Dictionary. Springer Science publishers, New Delhi, 2007.
12. Middleton E, Jr., Kandaswami C, The impact of plant flavonoids on mammalian biology: Implications for immunity, inflammation and cancer. In: Harborne JB, editor. The flavonoids advances in research since 1986. 1st ed. London: Chapman and Hall 1994; 619–52.
13. Harborne JB, Williams CA, Advances in flavonoid research since 1992. Phytochemistry 2000; 55:481–504.
14. Le Marchand L, Cancer preventive effects of flavonoids—A review. Biomed Pharmacother 2002; 56:296–301
15. Wenying Ren, Zhenhua Qiao, Hongwei Wang, Lei Zhu, Li Zhang. Flavonoids: promising anticancer agents. Med Research Reviews 2003; 23(4): 519-534.
16. Mossman T, Rapid colorimatirc assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J of Immuno Mtds 1983; 65: 5-63.
17. Lakshmi K.S., Shrinivas.S., Sharma, rajesh.T., Chithra.V, Anti tumor activity of ethanolic extract of leaves of Holoptelea integrifolia on Dalton’s ascetic lymphoma in swiss albino mice. int.j of Green pharm 2010; 4 ( 1 ): 44-7.
18. Ronok Zahan, M. Badrul Alam, M. Saiful Islam, Gopal C, Anticancer Activity of Alangium salvifolium Flower in Ehrlich Ascites Carcinoma Bearing Mice. Int J of Cancer Res 2011; 7: 254-62.
19. D’Amour FF, Blood FR and Belden DA, The Manual for Laboratory Work in Mammalian Physiology. The University of Chicago Press, Chicago1965; 148-50.
20. Lowry OH, Rosebrough NJ, Farr AL and Randall RJ,. Protein measurement with Folin - phenol reagent. J. Biol. Chem 1951; 193: 265-75.
21. Docie JV, Practical Haemotology. J & A Churchill Ltd, London, 1958. 38-42.
22. Segura, J.A., L.G. Barero and J. Marquez, Ehrlich ascites tumor unbalances splenic cell populations and reduced responsiveness of T-cells to Staphylococcus aureus enterotoxin B stimulation. Immunomol. Lett 2000; 74: 111-15.
23. Clarkson BD and Burchenal JH. Preliminary screening of antineoplastic drugs. Prog. Clin. Cancer 1965; 1: 625- 29
24. Oberling C and Guerin M, The role of viruses in the production of cancer. Adv. Cancer Res 1954; 2: 353- 423.
25. Feng Q, Kumangai T, Torii Y, Nakamura Y, Osawa T and Uchida K, Anticarcinogenic antioxidants as inhibitors against intracellular oxidative stress. Free Radic. Res 2001; 35: 779-88.
26. Hogland, H.C, Hematological complications of cancer chemotherapy. Semi. Oncol 1982; 9: 95-102.
27. Gupta, M., U.K. Mazumder, P.K. Halder, C.C. Kandar, L. Monikandan and G.P. Senthil, Anticancer activity of Indigofera aspalathoides and Wedelia calendulaceae in swiss albino mice. Iran. J. Pharm. Res 2007; 6: 141-45.
28. Tomlinson SK, Melin SA, Higgs V, White DR, Savage P, Case D and Blockstock AW, 2002.
29. Schedule selective biochemical modulation of 5-fluorouracil in advanced colorectal cancer a phase II study. BMC Cancer 2004; 2: 9.
30. M. Zhao, B. Yang, J. Wang, Y. Liu, L. Yu, and Y. Jiang, Immunomodulatory and anticancer activities of flavonoids extracted from litchi (Litchi chinensis Sonn.) pericarp. Int Immunopharmacology 2007; 7:162–66.
31. A. Gawron and G. Kruk, Cytotoxic effect of xanthotoxol (8-hydroxypsoralen) on TCTC cells in vitro. Polish Jl of Pharmacolog & Pharmacy 1992; 44 (1): 51–57.
Fig1
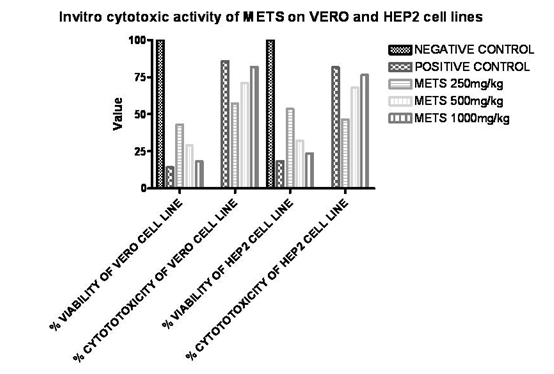
Fig-2 Histopathology study of livers of all five groups of animals.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









