About Authors:
Bhuvanesh Sharma1*, Bhupendra Vyas1, Yuvraj Singh Sarangdevot1, Pankaj Sharma1, Abhishek Sharma1.
1Dept. of Quality Assurance, B.N. College of Pharmacy,
Udaipur (Raj) 313001
*bhuvan110@gmail.com
Abstract:-
A simple, specific, accurate stability indicating RP-HPLC method was developed for assay of Salmeterol xinafoate and Fluticasone propionate in Mdi using Inertsil C-8 (150 x 4.6 mm), 5 µm Column and a mobile phase composing of Buffer: Acetonitrile: Methanol (450:250:300) v/v. The flow rate was 2.0 ml/min and the effluent was monitored at 220 nm. The retention time was 3.2 ±0.1 min and 9.9±0.1 min. Drug was subjected to acidic, alkali, oxidation degradation. The degradation studies indicated the susceptibility of drugs to various degradations. The method was statistically validated for specificity, accuracy, precision, linearity, robustness and solution stability. Quantitative and recovery studies of the dosage form were also carried out and the % RSD was found to be less than 2. The developed method is simple, rapid and accurate and hence can be used for routine quality control analysis.
REFERENCE ID: PHARMATUTOR-ART-1817
INTRODUCTION
Pharmaceutical drug products formulated with individual or combination dosage forms. These dosage forms require various qualitative and quantitative analytical methods for the determination of each active ingredient. In this present study developed a single and high resolution RP-HPLC method for the determination of Salmeterol and fluticasone drug products.
The structural formula
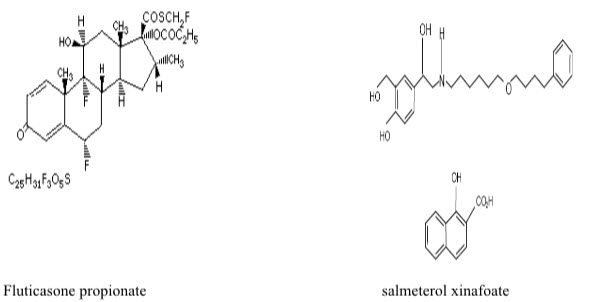
The chemical name of fluticasone propionate is S-Fluoromethyl 6, 9-difluoro-11ß-hydroxy- 16-methyl-3-oxo-17 -propionyloxy-androsta-1, 4-diene-17ß-carbothioate. It has a molecular formula of C25H31F3O5S and a molecular weight of 500.6.
The chemical name of salmetrol xinafoate is 4-Hydroxy'-[6-(4-phenylbutoxy)hexyl]amino]- methyl]-1,3-benzenedimethanol,1-hydroxyl-2-naphthoate. It has a molecular formula of C25H37NO4.C11H8O3 and a molecular weight of 603.8.
Fluticasone propionate is a white to off-white powder. It is freely soluble in dimethyl sulfoxide and dimethylformamide, sparingly soluble in acetone, dichloromethane, ethyl acetate and chloroform, slightly soluble in methanol and 95% ethanol, and practically insoluble in water.
Salmeterol xinafoate is a white to off-white crystalline powder. It is freely soluble in methanol, slightly soluble in ethanol, chloroform, and isopropanol, and sparingly soluble in water.
Literature surveys revealed that sensitive LC-MS methods are available for analysis of ant diabetic drugs and its metabolites in human plasma and urine. Several HPLC methods have been developed individually and combined dosage forms in human plasma. Even though stability indicating methods are developed for individual Anti asthametic drugs. Present study aimed for the easy specific precise and accurate earlier those than reported methods by reverse phase HPLC method. The method was validated according to the ICH (Q2A) guidelines.
EXPERIMENTAL WORK
Material and method
Table 1 : Instruments and Apparatus
|
NAME |
MAKE |
MODEL NO. |
|
U.V. Spectrophotometer |
Shimadzu |
1800 |
|
Analytical balance |
Mettler -Toledo |
AG 285 |
|
pH meter |
Mettler -Toledo |
780 |
|
HPLC |
Shimadzu Software: LC Solution Agilent Software: Chemstation |
CHT 2010 |
|
Milli-Q water dispenser |
Barnstead |
4326 |
|
Ultrasonic Bath |
Cadmach Ltd, India |
JAC 4020 |
* Chromatographic System
The HPLC system (Shimadzu Corporation, Japan), model Shimadzu CHT 2012, consisted of a system controller (CLASS-VP), on-line degasser (LC 2010C, Shimadzu), low pressure gradient valve (LC 2010C, Shimadzu), solvent delivery module (LC 2010C, Shimadzu), auto injector (LC 2010C, Shimadzu), column oven (LC 2010C, Shimadzu), and LC Solution software version = SPI, binary pump, auto injector (SIL-10AD VP, Shimadzu), column oven (CTO-10AS VP, Shimadzu) and PDA detector (PDA-SPD-M10A VP, Shimadzu Diode Array Detector) and Chem station (software).
* Materials and Reagents:
- A nylon 0.22 µm – 47 mm membrane filter (Gelman Laboratory, Mumbai, India)
- Salmeterol xinafoate and fluticasone propionate standard was used from Cadila Healthcare limited (Gujarat, India) having purity of 99.1 % and 98.0 % as such basis respectively.
- The commercial fixed dose product containing fluticasone propionate and Salmeterol xinafoate(250+20 µ) respectively and placebo pMDI were used from Cadila Healthcare limited (Gujarat, India).
- HPLC grade methanol (Merck Ltd, Mumbai, India), ammonium dihydrogen phosphate, O-Phosphoric Acid (Merck, India).
- The water for RP-HPLC was prepared by triple glass distillation and filtered through a nylon 0.22 µm – 47 mm membrane filter.
* Procedure
Buffer Preparation pH 3.7: Dissolve 1.3 gm of diammonium hydrogen phosphate in 1000 ml in Milli-Q water, stir well, and adjust the pH to 7.0 with diluted Orthophosphoric acid and Filtered.
Preparation of Mobile Phase: Prepare Sonicate and degassed mixture of Buffer: ACN: Methanol in the ratio of 450:250:300.
Diluent: Use mobile phase as a diluents
Preparation of Standard Stock Solution A of Salmeterol xinafoate: Transfer an accurately weight quantity of about 35 mg of working standard to a 200 ml volumetric flask, sonicate to dissolve and diluent to volume and mix well.
Preparation of Standard Stock Solution B of fluticasone propionate: Transfer an accurately weight quantity of about 25 mg of fluticasone propionate working standard to a 100 ml volumetric flask, add 25 ml of methanol sonicate to dissolve and add diluent to make volume and mix well.
Preparation of Final Standard Solution of Fluticasone propionate and Salmeterol xinafoate: Dilute 2 mL stock solution A and 10 mL stock solution of B were transfered into 100 mL volumetric flask and the volume was made upto 100 mL with diluents and mix. (It content 2.5 ppm and 25 ppm of salmeterol and fluticasone.
Sample Prepration for Assay:
Sample A: Take three fresh actuator, do the priming as mention below with another actuator thenTake the fresh actuator and actuate ten times with constant shaking for atleast five seconds in between each actuation. Repeat this procedure before each initial middle and end (sample B) collection.transfer these 3 actuator in 3 different 100 ml beaker and wash it with constant stirring by 20 ml diluents and sonocate it to dissolve it.fill the vial of these samples and mark it as actuator retention 1,2,3, respectively.
Sample B: Remove the pressurized container from the actuator and remove all labels and marking which may be present on the container with a suitable solvent. Dry the container, replace in its actuator, shake for about 30 sec and prime with 5 actuation the metering valve as follows. Discharge once to waste, wait for not less than 5 secand discharge again to waste. Remove the pressurized container fromits actuator, clean the valve stem and the wash with a suitable solvent. Dry it completely to ensure that all solvent is removed from the inside of the valve stem. Record the initial weight of canister (W1). Place a stainless steel base plate that has three legs and a central circular indentation with a hole about 1.5 mm in diameter in a small vessel in a clean and dry 100 ml beaker, add 35 ml of diluent. Hold the container in inverted position, shake for 5 seconds and deliver 1 actuation in beaker through the hole provided in the center of the stainless steel base plate. Similarly deliver further 9 actuation with constant shaking for at least 5 sec in between each delivery. Record the weight of canister after 10 actuation (W2). Transfer the beaker solution into a 100 ml volumetric flask. Wash the beaker and disc with diluents and transfer in the same volumetric flask and make up the volume with diluents. Separately inject mobile phase, Diluent, Standard preparation and sample preparartion into the chromatograph, record the chromatograms and measure the responses for the analyte peak and sequence was made according to test assay.
VALIDATION OF DEVELOPED STABILITY INDICATING RP- HPLC METHOD
Validation was done as per ICH guideline Q2 (R1). The developed HPLC method was validated with respect to parameters such as linearity, precision, accuracy, specificity, ruggedness, robustness and solution stability.
System Suitability
The system suitability test was performed as per method and checked before performing any parameter System suitability parameters of both Salmeterol xinafoate and Fluticasone propionate were determined and recorded in Table 4.
Linearity and Range
Linearity was determined at five levels over the range of 20% to 150%. A standard linearity solution was prepared to different concentration of 20%, 50%, 80%, 100%, 120%, and 150% of the test concentration.
Stock A for Salmeterol Xinafoate: Transfer an accurately weighed quantity of about 25.0 mg of Sameterol Xinafoate working standard to 100 mL volumetric flask, sonicate to dissolve and dilute to volume with diluents and mix. Take 10 ml of it and dilute to 100 ml with diluents and mix.
Stock B for Fluticasone Propionate:Transfer an accurately weighed quantity of about 25.0 mg of Fluticasone Propionate working standard to 100 mL volumetric flask, sonicate to dissolve and dilute to volume with diluents and mix. Now take 2,5,8,10,15 ml from stock A and B, and dilute to 100 ml with diluents and mix.
The area of each level was calculated and a graph of mean area versus concentration was plotted. The correlation co-efficient (r), Y-intercept, slope of regression line, residual sum of squares of Salmeterol xinafoate and Fluticasone propionate were calculated. The linearity results were recorded in Table 6.
Precision
Six different test samples were prepared from Salmeterol xinafoate and Fluticasone propionate. The sample should be prepared under the same condition and of same concentration. Then they were injected by one analyst and analysed on same day.Results related to method precision of Salmeterol xinafoate and Fluticasone propionate were recorded in Table 4-6.
Accuracy
The accuracy of the method was carried out at three levels in the range of 50-150% of the working concentration of sample. Calculated amount of Salmeterol xinafoate and Fluticasone propionate working standards were added in placebo containing volumetric flasks to prepare 50%, 100% and 150% level of the working concentration. Each level was prepared in triplicate manner. Results of accuracy study of Salmeterol xinafoate and Fluticasone propionate were recorded in Table 6.
Robustness
Following parameters were changed one by one and observed their effect on system suitability test and assay.
- Changed flow rate by 10 %. (i.e. 1.65 mL/min and 1.35 mL/min)
- Changed the minor components in the mobile phase by ±2 % absolute or ±30 % relatively whichever is lower.
- Changed the column temperature by ±5 °C. (i.e. 25 °C and 35 °C)
- Changed in mobile phase pH by ±0.2 unit (i.e. pH 3.0 and pH 3.4)
Results of robustness study of Salmeterol xinafoate and Fluticasone propionate were recorded in Table 6.
Forced Degradation Study
In order to prove the selectivity of analytical method, the API of Salmeterol xinafoate was studied under various stressed conditions to perform forced degradation study. Similarly, API of Fluticasone propionate was studied under various stressed conditions to perform forced degradation study. Stress study was carried out under the condition of acid/base hydrolysis, oxidation, thermal, humidity and photolytic, as mentioned in ICH Q1A (R2).
From the Forced degradation study, the optimized conditions for stress testing were selected.
Table 2 : Optimized Condition for Forced Degradation Study of Salmeterol xinafoate
|
Stress Type |
Stress Condition |
|
Acidic Alkali Humidity Thermal Peroxide |
5 mL of 2N HCL for 1 hour at 60 °C 5 mL of 1N NaOH for 15 min at 60 °C 40 °C, 75% RH for 5 day 100 °C for 24 hour 5 mL of 5 % H2O2 for 1 hour at 60 °C |
Table 3: Optimized Condition for Forced Degradation Study of Fluticasone propionate
|
Stress Type |
Stress Condition |
|
Acidic Alkali Humidity Thermal Peroxide |
5 mL of 2N HCL for 30 min at 100 °C 5 mL of 1N NaOH for 30 min at 100 °C 40 °C, 75% RH for 5 day 100 °C for 24 hour 3 mL of 5 % H2O2 for 30 min at room temp. |
RESULTS AND DISCUSSION
Figure no.1 : uv absorbance graph of mixed samples of salmeterol and fluticasone stock solution
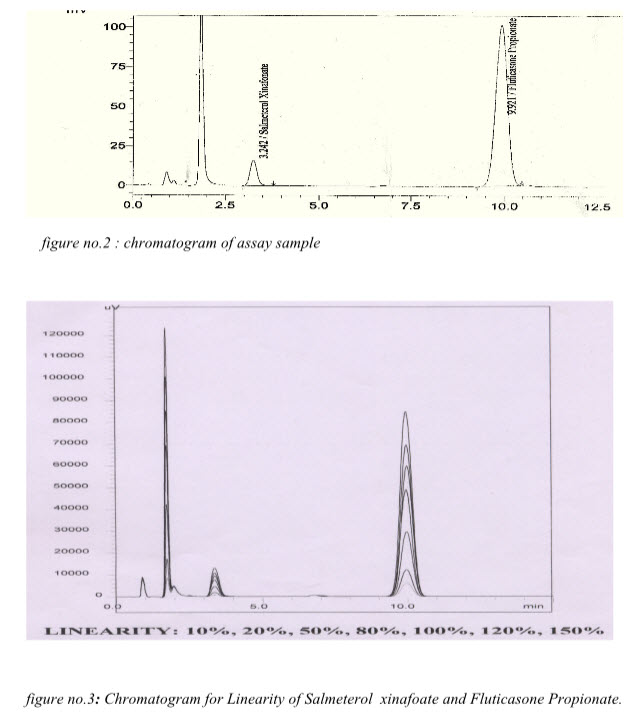
figure no.2 : chromatogram of assay sample
figure no.3: Chromatogram for Linearity of Salmeterol xinafoate and Fluticasone Propionate.
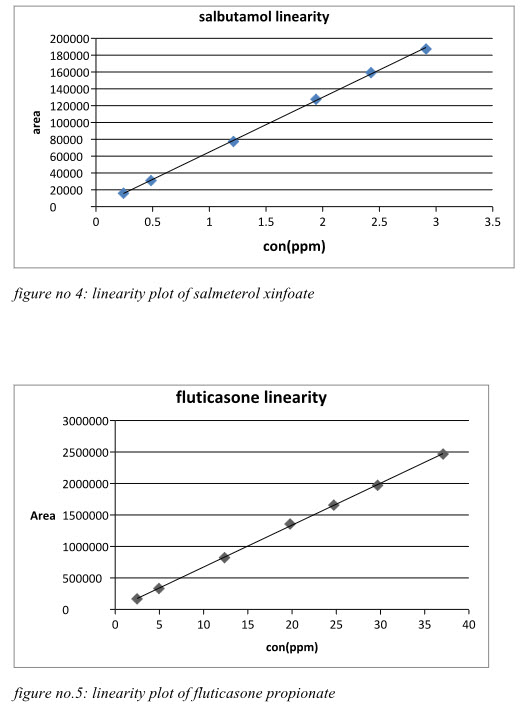
figure no 4: linearity plot of salmeterol xinfoate
figure no.5: linearity plot of fluticasone propionate
RESULTS OF METHOD VALIDATION PARAMETERS
Table 4: System Suitability and System Precision
|
Sr. No. |
Parameters (n= 5) |
Salmeterol xinafoate |
Fluticasone propionate |
|
1 |
Retention Time (min) |
3.2 |
9.9 |
|
2 |
Theoritical Plates |
1960 |
4391 |
|
3 |
% RSD |
0.1 |
0.3 |
|
4 |
Mean % assay |
104.8 |
107.6 |
Table 5 : Forced Degradation Data of Salmeterol xinafoate and Fluticasone propionate Sample Solution
|
For Salmeterol xinafoate |
For Fluticasone propionate |
|||||
|
Condition |
% Assay |
% Degradation |
Peak Purity Index |
% Assay |
% Degradation |
Peak Purity Index |
|
As such |
100 |
0.0 |
0.9999 |
100.0 |
0.0 |
0.9998 |
|
Acid |
88.7 |
11.3 |
0.9995 |
68.3 |
31.7 |
0.9998 |
|
Alkali |
94-4 |
5.6 |
0.9990 |
86.9 |
35.6 |
0.9998 |
|
Peroxide |
52.7 |
47.3 |
0.9997 |
65.0 |
35.0 |
0.9993 |
Summary of validation parameters:
Table. 6: Summary of validation Parameters.
|
Validation parameter |
Results |
|
|
Salmeterol xinafoate |
Fluticasone propionate |
|
|
Specificity:Interference from blank and placebo |
Complies |
Complies |
|
Linearity and Range |
Correlation coefficient: 0.999 Range: 0.25 – 3.5 µg/mL |
Correlation coefficient: 0.999 Range: 2.5 – 35 µg/mL |
|
Method precision (n = 6) (Repeatability) |
RSD: 0.03 % |
RSD: 0.07 % |
|
% Mean recovery |
100.20 % |
102. 7 % |
|
Robustness: Change in mobile phase ratio, column temperature, flow rate. |
Complies |
Complies |
|
System suitability |
Meets the system suitability criteria |
Meets the system suitability criteria |
Summary:
Analytical method development and simultaneous estimation of Salmeterol and fluticasone and its validation by RP-HPLC, various mobile phases like water, methanol, and different pH buffer solutions were tried in various proportions. Different temperature, different flow rate and different columns were tried and finally optimized chromatographic conditions are shown below,
- Column : Inertsil C-8 (150 x 4.6 mm), 5 µm
- Detector : 220 nm
- Injection Volume : 100 µL
- Flow Rate : 2.0 mL/min
- Temperature : 40 ºC
- Run Time : 15 minute
- Mobile Phase : Buffer: Acetonitrile:Methanol(450:250:300)
- Diluent : Mobile phase
- Buffer pH : 7.0
Above developed method was validated according to ICH guideline parameters like linearity, range, LOD, LOQ, system suitability, precision, accuracyand robustness and their results complies with official standards.
Conclusion
An reversed phase high performance liquid chromatographic method was developed and validated for the determination of Salmeterol and Fluticasone in pharmaceutical dosage form as a single component. This chromatographic assay fulfilled all the requirements needed for it to be identified as a reliable and feasible method, including accuracy, recovery and precision data. It is highly accurate, precise and selective. The analytical procedure and its chromatographic run time is less than 10 min. Therefore, the HPLC method can be used as a routine sample analysis for stability study purposes
References:
1.Munson J. W; Pharmaceutical Analysis Modern Methods, part B, Marcel Dekker Inc, New York, 2001, 54-63.
2.Sharma B. K., Instrumental Method of Chemical Analysis; 21th edition; Goel Publishing Housing, Krishna Prakashan Ltd, 2002; 3, 10.
3.Willard H. H., Merrit L. L. and John A. Instrumental methods of analysis, 7th edition, CBS Publishers, New Delhi, 1999; 38-51.
4.Meyer V.R. High Performance Liquid Chromatography (Practical), 2nd edition, John wiley and sons, London, 1993; 222-258
5.Torchilin V.P., “Recent advances in HPLC.” Int J. Pharm. 2005; 4, 145-160.
6.Singh SS and Bakshi M, “Development of Validated Stability Indicating Assay Methods: Critical Review.” J. Pharm. Biom. Anal, 2002; 28, 1011– 40.
7.ICH, Q2A, Text on Validation of Analytical Procedures, International Conference on Harmonization, Geneva, October 1994, 1-5.
8. Beckett A. H., Stenlake J. B., Principle Pharmaceutical Chemistry, CBS Publishers New Delhi, 2004, 275-314.
9. ICH topic Q-2B Validation of analytical procedure: Methodology CPMP/ICH/281/95) the European agency for the evaluation of medicinal product, Human medicines evaluation unit, 1-9.
10.Munson J. W; Pharmaceutical Analysis Modern Methods, part B, Marcel Dekker Inc, New York, 2001, 38-42.
11.Ravi Sankar S; Text Book of Pharmaceutical Analysis. 4th edition, Rx publications, Tirunelveli, 2005, 1-2.
12.Skoog D. A, Crouch S. R, Holler F. J; Analytical Methods, principles of instrumental analysis, 6th international edition. California, Cengage Learning Inc, 2006, 6-19.
13.Ahuja S, Scypinski S; Handbook of Modern Pharmaceutical Analysis, vol. III, academic press, USA, 2001, 349.
14.Connors K. A; Text Book of Pharmaceutical Analysis, 3rd edition, wiley interscience, New York, 2002, 353.
15.Mendham J, Denney R. C, Baraes J. D; Vogel’s Text Book of Quantitative Chemical Analysis, 5th edition, pears education, Singapore, 1991, 5-11.
16.Sethi P. D; Quantitative Analysis of Drugs in Pharmaceutical Formulations, 3rd edition, New Delhi, 2001, 1-15.
17.Basett J, Denney R. C, Jerrery G.H, Mendham J; Vogel’s Text Book of Quantitative Inorganic Analysis, 4th edition, longman group, England, 1986, 2.
18.Devala R. G; Text Book of Pharmaceutical Analysis, 2nd edition, vol-I, birla publications, New Delhi, 2005, 1-2.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









