 About Authors:
About Authors:
Gunjan Kalyani*, Vishal S. Deshmukh, Pranita Kashyap, Yogesh Vaishnav, Ajit Kumar Pandey
Shri Rawatpura Sarkar Institute of Pharmacy,
Kumhari, Durg, Chhattisgarh.
kalyani.gunjan@yahoo.in, rvg_54767@yahoo.co.in*
Abstract:
The IUPAC Name of Salmeterol is (RS)-2-(hydroxymethyl)-4-{1-hydroxy-2-[6-(4-phenylbutoxy) hexylamino]ethyl}phenol. Salmeterol is a prescription drug that is used for treating airway spasms in people with asthma or chronic obstructive pulmonary disease (COPD). By relaxing the muscles around the airways to allow more air into and out of the lungs, the medication can help prevent asthma attacks from occurring.Salmeterol comes in the form of an inhalation powder and is generally used twice a day. Present research work deals with UV spectrophotometric method for the estimation of salmeterol in pure form. For the estimation of salmeterol, solvent system employed was ethanol and wavelength of detection (λdet) was 252 nm. The linearity was obtained in the range 6 – 14 µg/ml, with a regression coefficient, R2= 0.999. The LOD & LOQ were found to be 4.99 µg/ml and 14.24 µg/ml respectively. Obtained results showed that there is minimum intra day and inter day variation. The developed method was validated and recovery studies were also carried out. Sample recovery using the above method was in good agreement with their respective labeled claims, thus suggesting the validity of the method and non-interference of formulation excipients in the estimation.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1478
1. Introduction:
The IUPAC name of Salmeterol is RS)-2-(hydroxymethyl)-4-{1-hydroxy-2-[6-(4-phenylbutoxy) hexylamino] ethyl} phenol.
1.1 Mechanism of action
Salmeterol's long, lipophilic side chain binds to exosites near beta(2)-receptors in the lungs and on bronchiolar smooth muscle, allowing the active portion of the molecule to remain at the receptor site, continually binding and releasing. Beta(2)-receptor stimulation in the lung causes relaxation of bronchial smooth muscle, bronchodilation, and increased bronchial airflow.
Salmeterol is a prescription drug that is used for treating airway spasms in people with asthma or chronic obstructive pulmonary disease (COPD). By relaxing the muscles around the airways to allow more air into and out of the lungs, the medication can help prevent asthma attacks from occurring. Salmeterol comes in the form of an inhalation powder and is generally used twice a day. However, it can also be used 30 minutes before exercise to help prevent exercise-induced asthma attacks.
Literature review suggested no UV Spectroscopic method. The objective of the work was to develop simple, accurate, precise and economic second order derivative Spectroscopic method to estimate the candesartan in bulk. The method should be simple, accurate, precise, reproducible and statistically valid.
UV spectrophotometry is generally preferred especially by small-scale industries as the cost of the equipment is less and the maintenance problems are minimal. The method of analysis is based on measuring the absorption of a monochromatic light by colorless compounds in the near ultraviolet path of spectrum (190-380nm). The fundamental principle of operation of spectrophotometer covering UV region consists in that light of definite interval of wavelength passes through a cell with solvent and falls on to the photoelectric cell that transforms the radiant energy into electrical energy.
Thus, the objectives of project:
I. To develop a simple, precise, accurate method, less time consuming & economical derivative spectroscopic method.
II. Under derivative spectroscopy, the development of Second Order derivative Method.
III. Validation of developed method using common parameters:
a) Linearity
b) Precision
c) Accuracy
d) Sensitivity
e) Limit of Detection (LOD)
f) Limit of Quantification (LOQ)
[adsense:468x15:2204050025]
2. Materials and Methods:
Year of experimentation: 2012
Site of experimentation: Shri Rawatpura Sarkar Institute of Pharmacy, Kumhari, Durg, Chhattisgarh.
2.1 Drug
The standard sample of Salmeterol was obtained as gift sample form Glenmark Pharmaceuticals, Solan, H.P., India.
2.2 Instrument specifications
UV Spectrophotometer, Shimadzu, model 1800.
2.3 Chemicals and reagents used
Ethanol obtained from local market, manufactured by Merck Pharmaceuticals.
2.4 Preparation of stock solution
The stock solution of salmeterol is prepared by dissolving 100 mg of drug in 100 ml methanol in volumetric flask with continuous shaking. 10 ml of sample was withdrawn and diluted to 100 ml methanol to get 10 μg/ml of solution. The solution was than scanned in UV range between 200-400 nm UV-VIS Spectrophotometer, Shimadzu, Japan to determine the absorption maxima of the drug against blank as ethanol.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
2.5 Wavelength scanning and determination of absorption maximum
From the stock solution of salmeterol, known concentration of 10μg/ml is prepared by suitable dilution with ethanol. Wavelength scanned for the maximum absorption of drug solution using UV-Visible spectrophotometer within the wavelength region of 200–400 nm against blank ethanol. The wavelength that shows the peak with a highest absorbance is considered as absorbance maximum of the drug. The result is presented in table 1.
2.6 Linearity studies for Candesartan analytical method
Stock solution was subsequently diluted with ethanol to get 2μg/ml, 4μg/ml, 6μg/ml, 80μg/ml, 10μg/ml, 12μg/ml 14μg/ml, 16μg/ml, 18μg/ml 20μg/ml. The results are tabulated and the linearity curve was constructed by plotting concentration vs. absorbance value. The result is presented in table 1 and fig. 3.
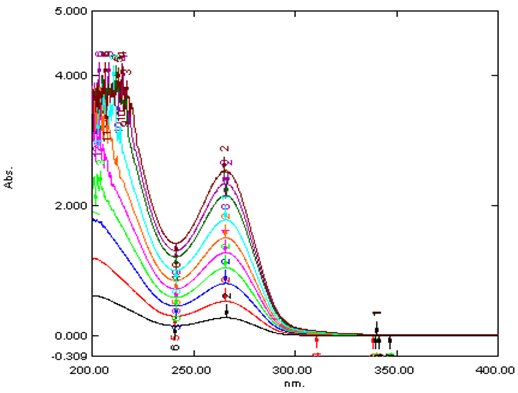
Fig 2 depicting linearity for concentration range.
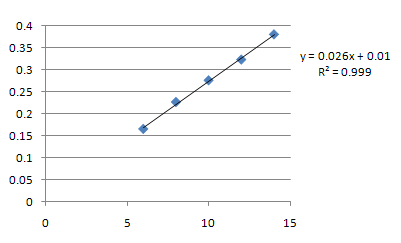
FIG. 3: Standard curve of salmeterol
Regression equation; y = 0.026x + 0.01
R2 = 0.999
X – Axis: Concentration
Y- Axis: Absorbance
2.7 Precision
The precision of method was ascertained; the percent relative standard deviation were calculated and presented.
2.7.1 Inter day and intraday studies for Candesartan analytical method
The prepared stock solution was subsequently diluted to get 10 μg/ml. The resulting solution absorbance was measured at detection wavelength of 252.4 nm using double beam UV spectrophotometer against blank of ethanol. The findings was made at different time intervals in day times in a day and performed continuously for six days. The results obtained were tabulated and studied for inter day and intraday variation. The results are tabulated in table 1.
2.8 Accuracy studies: The accuracy/recovery studies were carried out with the commercial preparation of salmeterol and percentage recoveries was calculated. The reproducibility of estimation was determined by performing the drug content of different samples. The results of accuracy studies were expressed in %. The result is presented in table 1.
2.9 Assay studies: The assay studies were carried out with the help of candesartan SMEDDS. The percentage purity was calculated. Convert the normal mode obtained spectra to first order derivative. The reproducibility of estimation was determined by performing the drug content of different samples. The results of assay studies were expressed in %. The result is presented in table 1.
|
S.NO. |
PARAMETERS |
OBTAINED RESULT |
|
1 |
Detection Wavelength |
252.4 nm |
|
2 |
Linearity and Range |
6 - 14 µg/ml |
|
3 |
Sensitivity |
0.18 µg/cm2/ 0.001 AU |
|
4 |
Limit Of Detection |
4.699 µg/ml |
|
5 |
Limit Of Quantification |
14.24 µg/ml |
|
6 |
Intraday precision |
% RSD less than 2%. |
|
7 |
Inter day precision |
% RSD less than 2%. |
|
8 |
Accuracy studies |
Within the limit 95 -105% |
|
9 |
Assay |
Within the limit 95 -105% |
Table 1 depicting the validation parameters
3. Results and Discussion
Literature review revealed that area under curve and first order derivative spectroscopy method is available for estimation of Salmeterol but the specific absorptivity and calibration curve method are not available so these methods are selected for the analytical method development of Salmeterol in bulk and pharmaceutical dosage forms. The developed method is validated for repeatability, reproducible and the accuracy and precision. In the inter day and intraday study of standard graph, the % RSD is less than 2% indicating the developed method is reproducible. The different levels of standard concentration solutions are measured forabsorbance value and actual concentration is calculated. The results showed that the amount recovered is 100% indicating the first order derivative spectroscopic method is accurate and precise.
4. Conclusion
Proposed study describes new UV spectrophotometric (Calibration curve, Specific absorptivity) method for the estimation of Salmeterol in all its formulation. The method was validated and found to be simple, sensitive, and accurate and precise. Percentage of recovery shows that the method is free from interference of the excipients used in the formulation. Therefore the proposed method can be used for routine analysis of estimation of Salmeterol in all its pharmaceutical dosage form.
5. Acknowledgements
I sincerely thank my Principal and management staff & lab technicians for their contribution in carrying forward the research work. I would also sincerely thank specially to my teacher, Mr. Yogesh Vaishnav, who has been my role model as well as my inspiration, for his active and Kind guidance and for providing the necessary support and basic facilities in lab.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
TABLE 1: Linearity of Candesartan
|
S.NO. |
CONCENTRATION (µg/ml) |
D2 VALUE AT DETECTION WAVELENGTH (284.3 nm) |
|
1 |
40 |
0.003 |
|
2 |
60 |
0.004 |
|
3 |
80 |
0.005 |
|
4 |
100 |
0.007 |
|
5 |
120 |
0.008 |
|
6 |
140 |
0.009 |
|
7 |
160 |
0.010 |
TABLE 2 (a) intraday precision
|
S.NO |
CONCENTRATION (µg/ml) |
D2 VALUE AT DETECTION WAVELENGTH (284.3 nm) |
||||
|
|
|
TIME (MINS) |
I |
II |
III |
MEAN |
|
1 |
140 |
1:30 PM |
0.008 |
0.008 |
0.008 |
0.008 |
|
2 |
140 |
1:45 PM |
0.008 |
0.008 |
0.009 |
0.00833 |
|
3 |
140 |
2:00 PM |
0.008 |
0.009 |
0.008 |
0.00833 |
|
4 |
140 |
2:30 PM |
0.009 |
0.008 |
0.008 |
0.00833 |
|
5 |
140 |
3:30 PM |
0.009 |
0.009 |
0.008 |
0.00866 |
|
6 |
140 |
4:30 PM |
0.008 |
0.009 |
0.009 |
0.00866 |
|
MEAN = |
0.008 |
|||||
|
SD = |
0.00014 |
|||||
|
% RSD = |
1.75 |
|||||
TABLE 2 (b) Inter day precision
|
S.NO |
CONCENTRATION (µg/ml) |
DAYS & DATE |
D2 at 268.8 nm |
|
1 |
140 |
8.2.2012 |
0.008 |
|
2 |
140 |
9.2.2012 |
0.008 |
|
3 |
140 |
10.2.2012 |
0.008 |
|
4 |
140 |
11.2.2012 |
0.008 |
|
5 |
140 |
12.2.2012 |
0.008 |
|
6 |
140 |
13.2.2012 |
0.009 |
|
MEAN = |
0.008 |
||
|
SD = |
0.000135 |
||
|
% RSD = |
1.68 |
||
TABLE 3 Accuracy studies
|
S.NO. |
TEST (µg/ml) |
STANDARD (µg/ml) |
D2 VALUE AT 284.3 nm |
CONC. (µg/ml) |
AMOUNT OF TEST RECOVERED (µg/ml) |
% RECOVERY |
|
1 |
5 |
60 |
0.004 |
65 |
5 |
100 |
|
2 |
10 |
60 |
0.005 |
70 |
10 |
100 |
|
3 |
15 |
60 |
0.006 |
75 |
15 |
100 |
References
1. Umarker et al jouneral of pharmacy research Simultaneous Estimation of Salmiterol and Diclofenac Potassium by UV Spectrophotometer Using Multicomponent Method vol 4(4) page no 978-979.
2. Rall, T.W: “Goodman and Gilman’s the pharmacological basics of Therapeutics”, Pergamon Press, New York, Eighth edition 1990.
3. Williams, A.D: Foye’s Principles of Medicinal Chemistry, Fifth edition 2002.
4. drugbank.ca/drugs/DB01222 accessed on 16/11/2011.
5. rxlist.com/rhinocort-aqua-drug.htm accessed on 16/11/2011.
6. Patel Jignesh et al. Q-Analysis spectrometric method for determination of candesartan cilexetil and hydrochlorothiazide in tablet dosage form. J chem. Pharm Res; 2010; 2(3): 10-14.
7. Chadburn, B. P. Proceedings Analytical Division Chemical Society, 1982, 19, 42.
8. European Brewery Convention, Analytica 4th ed Method 7.4.1., 1987, p. E123.
9. Fell, A. F. Proceedings Analytical Division Chemical Society, 1978, 15, 260.
10. Fell, A. F. U.V. Spectrum Croup Bulletin, 1979, 7, 5.
11. Fell, A. F. Proceedings Analytical Division Chemistry Society, 1980, 17, 512.
12. Budavari. S: The Merck Index, An Encyclopedia of Chemicals, Drugs and Biologicals, White House Station, N. J., Thirteenth edition. 2001.
13. Reynolds, J.E.F and Prasad B.A., IN; Martinadale, the Complete Drug Reference, the Pharmaceutical Press, London, 33rd Edn, 2002, 853.
14. Pfister, M et al: Pharmacokinetics and haemodynamics of Candesartan cilexetil in hypertensive patients on regular haemodynamics; British Journal of Clinical Pharmacology 1999, 47:645-651.
15. Sever, P., Menard, J., Eds: Angiotensin II antagonism refined: Candesartan cilexetil, Journal of Human Hypertens; 1997, 11 (Suppl 2): S 1- 95.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









