 About Authors:
About Authors:
P.Abisha1*, D.ANANTHA LAKSHMI2
1 II M.Sc Biochemistry,
2 HEAD OF THE DEPARTMENT
DEPARTMENT OF BIOCHEMISTRY
ST.PIOUS X DEGREE AND PG COLLEGE FOR WOMEN, NACHARAM, HYDERABAD
*godsbloved@yahoo.in
ABSTRACT
Sodium is an essential element for all animal life (including human) and for some plant species. In animals, sodium ions are necessary for regulation of blood and body fluids, transmission of nerve impulses, heart activity, and certain metabolic functions. Sodium is the chief cation in extracellular compartment of animal body.
Sodium plays a vital role not only in human body but also in various food types. Sodium chloride is the principal source of sodium in the diet, and is used as seasoning and preservative, such as for pickling and jerky; most of it comes from processed foods.
The sodium content in various foods can be analyzed by using Flame Photometry. Flame photometry also called flame atomic emission spectrometry is a branch of atomic spectroscopy in which the species examined in the spectrometer are in the form of atoms.
The samples used for analysis are junk foods which have become a common trend among all generations. A total of 15 junk foods were processed and analyzed for estimating the sodium content in them by Flame Photometry.
Based on the analysis the samples were categorized into low, moderate and high sodium containing foods. The analysis helps in creating awareness among all the generations to reduce the sodium intake in order to prevent problems like hypertension, hyponatremia, hypernatremia and associated medical complications in their future.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1412
INTRODUCTION
Sodiumis a chemical element with the symbol Na (from Latin: natrium) and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals such as feldspars, sodalite and rock salt. Many salts of sodium are highly soluble in water and are thus present in significant quantities in the Earth's bodies of water, most abundantly in the oceans as sodium chloride.
Sodium - silvery-white alkali metal

MELTING POINT: 97.8°C
BOILING POINT: 883°C
DENSITY: 0.971 g/cm 3
MOST COMMON IONS: Na +
Many sodium compounds are useful, such as sodium hydroxide (lye) for soap making, and sodium chloride for use as a deicing agent and a nutrient. Sodium is an essential element for all animals and some plants. In animals, sodium ions are used against potassium ions to build up charges on cell membranes, allowing transmission of nerve impulses when the charge is dissipated; it is therefore classified as a dietary inorganic macro-mineral. The free metal does not occur in nature, but instead must be prepared from its compounds; it was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide
Characteristics of Sodium:
Sodium at standard temperature and pressure is a soft metal that can be readily cut with a knife and is a good conductor of electricity. Freshly exposed, sodium has a bright, silvery luster that rapidly tarnishes, forming a white coating of sodium hydroxide and sodium carbonate. These properties change at elevated pressures: at 1.5 Mbar, the color changes to black, then to red transparent at 1.9 Mbar, and finally clear transparent at 3 Mbar. All of these allotropes are insulators and electrides.
When sodium or its compounds are introduced into a flame, they turn it yellow, because the excited 3s electrons of sodium emit a photon when they fall from 3p to 3s; the wavelength of this photon corresponds to the D line at 589.3 nm (Earnshaw, A, et al 1997)

A positive flame test for sodium has a bright yellow color
Sodium is generally less reactive than potassium and more reactive than lithium. Like all the alkali metals, it reacts exothermically with water, to the point that sufficiently large pieces melt to a sphere and may explode; this reaction produces caustic sodium hydroxide and flammable hydrogen gas (Emsley, John 2001) When burned in dry air, it mainly forms sodium peroxide as well as some sodium oxide, in moist air, sodium hydroxide results. Sodium metal is highly reducing, with the reduction of sodium ions requiring −2.71 volts but potassium and lithium have even more negative potentials. Hence, the extraction of sodium metal from its compounds (such as with sodium chloride) uses a significant amount of energy.
Sodium compounds are of immense commercial importance, being particularly central to industries producing glass, paper, soap, and textiles. The sodium compounds that are the most important are common salt (NaCl), soda ash (Na2CO3), baking soda (NaHCO3), caustic soda (NaOH), sodium nitrate (NaNO3), di- and tri-sodium phosphates, sodium thiosulfate (Na2S2O3·5H2O), and borax (Na2B4O7·10H2O). In its compounds, sodium is usually ionically bonded to water and anions, and is viewed as a hard Lewis acid.
Like the other alkali metals, sodium dissolves in ammonia and some amines to give deeply coloured solutions; evaporation of these solutions leaves a shiny film of metallic sodium. The solutions contain the coordination complex (Na(NH3)6)+, whose positive charge is counterbalanced by electrons as anions; cryptands permit the isolation of these complexes as crystalline solids. Cryptands, like crown ethers and other ionophores, have a high affinity for the sodium ion; derivatives of the alkalide Na- are obtainable by the addition of cryptands to solutions of sodium in ammonia via disproportionation.
History:
Salt has been an important commodity in human activities, as shown by the English word salary, which derives from salarium, the wafers of salt sometimes given to Roman soldiers along with their other wages. In medieval Europe, a compound of sodium with the Latin name of sodanum was used as a headache remedy. The name sodium is thought to originate from the Arabic suda, meaning headache, as the headache-alleviating properties of sodium carbonate or soda were well known in early times. The chemical abbreviation for sodium was first published by Jöns Jakob Berzelius in his system of atomic symbols, and is a contraction of the element's new Latin name natrium, which refers to the Egyptian natron, a natural mineral salt primarily made of hydrated sodium carbonate. Natron historically had several important industrial and household uses, later eclipsed by other sodium compounds. Although sodium, sometimes called soda, had long been recognised in compounds, the metal itself was not isolated until 1807 by Sir Humphry Davy through the electrolysis of sodium hydroxide.
Sodium imparts an intense yellow color to flames. As early as 1860, Kirchhoff and Bunsen noted the high sensitivity of a sodium flame test.
Commercial production:
Enjoying rather specialized applications, only about 100,000 tonnes of metallic sodium are produced annually. Metallic sodium was first produced commercially in 1855 by carbothermal reduction of sodium carbonate at 1100 °C, in what is known as the Deville process:
Na2CO3 + 2 C → 2 Na + 3 CO
A related process based on the reduction of sodium hydroxide was developed in 1886.
Sodium is now produced commercially through the electrolysis of molten sodium chloride, based on a process patented in 1924. This is done in a Downs Cell in which the NaCl is mixed with calcium chloride to lower the melting point below 700 °C. As calcium is less electropositive than sodium, no calcium will be formed at the anode. This method is less expensive than the previous Castner process of electrolyzing sodium hydroxide.
Reagent-grade sodium in tonne quantities sold for about US$3.30/kg in 2009; lower purity metal sells for considerably less. The market for sodium is volatile due to the difficulty in its storage and shipping; it must be stored under a dry inert gas atmosphere or anhydrous mineral oil to prevent the formation of a surface layer of sodium oxide or sodium superoxide. These oxides can react violently in the presence of organic materials. Sodium will also burn violently when heated in air. Smaller quantities of sodium cost far more, in the range of US$165/kg; the high cost is partially due to the expense of shipping hazardous material.
Sodium consumption:
Sodium chloride is the principal source of sodium in the diet, and is used as seasoning and preservative, such as for pickling and jerky; most of it comes from processed foods. The DRI for sodium is 2.3 grams per day, but on average people in the United States consume 3.4 grams per day, the minimum amount that promotes hypertension (Grobbee, D. E. 2004); this in turn causes 7.6 million premature deaths worldwide.
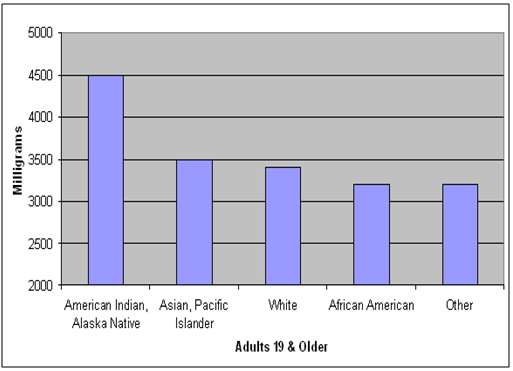
About 90% of Americans eat more sodium than is recommended for a healthy diet. Too much sodium increases a person's risk for high blood pressure. High blood pressure often leads to heart disease and stroke. More than 800,000 people die each year from heart disease, stroke and other vascular diseases, costing the nation $273 billion health care dollars in 2010. Most of the sodium we eat comes from processed foods and foods prepared in restaurants.
Sodium is already part of processed foods and cannot be removed. However, manufacturers and restaurants can produce foods with less sodium.
Sodium in biology:
Sodium is an essential element for all animal life (including human) and for some plant species. Sodium ions are necessary in small amounts for some types of plants, but sodium as a nutrient is more generally needed in larger amounts by animals, due to their use of it for generation of nervous impulses and finer regulation of fluid balance.
In animals, sodium ions (often referred to as just "sodium") are necessary for regulation of blood and body fluids, transmission of nerve impulses, heart activity, and certain metabolic functions. Sodium is the chief cation in extracellular compartment of animal body. A normal serum sodium level is 135 - 145 mEq/liter (mEq/L).
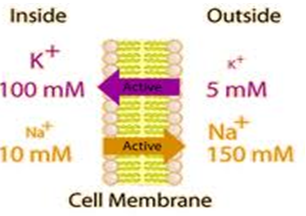
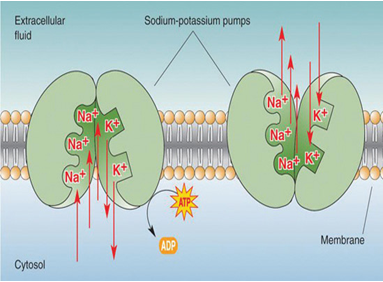
Sodium cations are important in neuron (brain and nerve) function, and in influencing osmotic balance between cells and the interstitial fluid, with their distribution mediated in all animals (but not in all plants) by the so-called Na+/K+-ATPase pump. In the nerve cells, this sodium-potassium flux generates the electrical potential that aids the conduction of nerve impulses. This electrical potential gradient, created by the "sodium-potassium pump," helps generate muscle contractions and regulates the heartbeat.
Sodium is an essential nutrient that regulates blood volume, blood pressure, osmotic equilibrium and pH; the minimum physiological requirement for sodium is 500 milligrams per day.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Sodium distribution in species:
In plants:
In C4 plants, sodium is a micronutrient that aids in metabolism, specifically in regeneration of phosphoenolpyruvate and synthesis of chlorophyll (Kering, M. K. 2008). In others, it substitutes for potassium in several roles, such as maintaining turgor pressure and aiding in the opening and closing of stomata. Excess sodium in the soil limits the uptake of water due to decreased water potential, which may result in wilting; similar concentrations in the cytoplasm can lead to enzyme inhibition, which in turn causes necrosis and chlorosis. (Zhu, J. K., 2001) To avoid these problems, plants developed mechanisms that limit sodium uptake by roots, store them in cell vacuoles, and control them over long distances; excess sodium may also be stored in old plant tissue, limiting the damage to new growth.
In animals:
Since only some plants need sodium and those in small quantities, a completely plant-based diet will generally be very low in sodium. This requires some herbivores to obtain their sodium from salt licks and other mineral sources. The animal need for sodium is probably the reason for the highly-conserved ability to taste the sodium ion as "salty." Receptors for the pure salty taste respond best to sodium, otherwise only to a few other small monovalent cations (Li+, NH4+, and somewhat to K+). Calcium ion (Ca2+) also tastes salty and sometimes bitter to some people but, like potassium, can trigger other tastes.
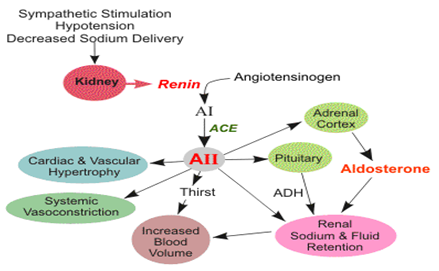
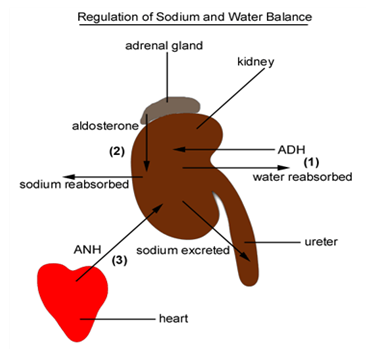
The renin-angiotensin system and the atrial natriuretic peptide regulate the amount of fluid in the body (Laragh, John H, 1985) Reduction of blood pressure and sodium concentration in the kidney result in the production of renin, which in turn produces aldosterone, retaining sodium in the urine. Because of this, the osmotic pressure changes and osmoregulation systems generate the antidiuretic hormone, causing the body to retain water and restore its total amount of fluid. Receptors in the heart and blood vessels sense the resulting distension and pressure, leading to production of the atrial natriuretic peptide, causing the body to lose sodium in the urine; the osmoregulation systems detect this and remove water, restoring the total fluid levels.
Excitable animal cells rely on the entry of Na+ to cause a depolarization. An example of this is signal transduction in the human central nervous system, which depends on sodium ion motion across the nerve cell membrane, in all nerves.
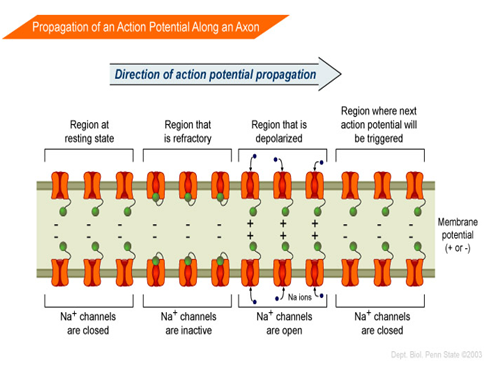
Some potent neurotoxins, such as batrachotoxin, increase the sodium ion permeability of the cell membranes in nerves and muscles, causing a massive and irreversible depolarization of the membranes, with potentially fatal consequences. However, drugs with smaller effects on sodium ion motion in nerves may have diverse pharmacological effects which range from anti-depressant to anti-seizure actions. Sodium is thus important in neuron function and osmoregulation between cells and the extracellular fluid; their distribution is mediated in all animals by Na+/K+-ATPase (Campbell, Neil 1987). Hence, sodium is the most prominent cation in extracellular fluid: the 15 liters of it in a 70 kg human have around 50 grams of sodium, 90% of the body's total sodium content.
Function of sodium ions:
Sodium is the primary cation (positive ion) in extracellular fluids in animals and humans. These fluids, such as blood plasma and extracellular fluids in other tissues bathe cells and carry out transport functions for nutrients and wastes. Sodium is also the principal cation in seawater, although the concentration there is about 3.8 times what it is normally in extracellular body fluids.
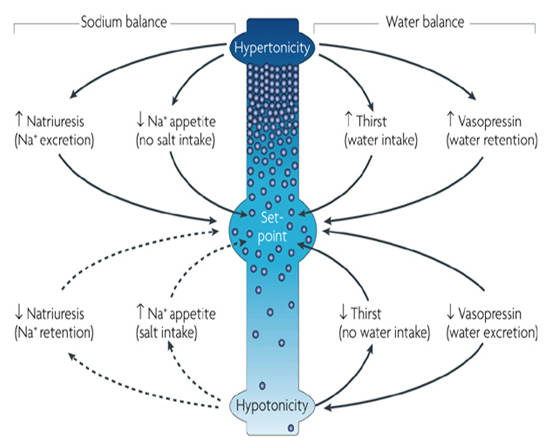
1. Human water and salt balance:
Although the system for maintaining optimal salt and water balance in the body is a complex one, one of the primary ways in which the human body keeps track of loss of body water is that osmoreceptors in the hypothalamus sense a balance of sodium and water concentration in extracellular fluids. Relative loss of body water will cause sodium concentration to rise higher than normal, a condition known as hypernatremia. This ordinarily results in thirst. Conversely, an excess of body water caused by drinking will result in too little sodium in the blood (hyponatremia), a condition which is again sensed by the hypothalamus, causing a decrease in vasopressin hormone secretion from the posterior pituitary, and a consequent loss of water in the urine, which acts to restore blood sodium concentrations to normal.
Severely dehydrated persons, such as people rescued from ocean or desert survival situations, usually have very high blood sodium concentrations. These must be very carefully and slowly returned to normal, since too-rapid correction of hypernatremia may result in brain damage from cellular swelling, as water moves suddenly into cells with high osmolar content.
In humans, a high-salt intake was demonstrated to attenuate nitric oxide production. Nitric oxide (NO) contributes to vessel homeostasis by inhibiting vascular smooth muscle contraction and growth, platelet aggregation, and leukocyte adhesion to the endothelium (Ken Okumura., et al 2002).
2. Urinary sodium
Because the hypothalamus/osmoreceptor system ordinarily works well to cause drinking or urination to restore the body's sodium concentrations to normal, this system can be used in medical treatment to regulate the body's total fluid content, by first controlling the body's sodium content. Thus, when a powerful diuretic drug is given which causes the kidneys to excrete sodium, the effect is accompanied by an excretion of body water (water loss accompanies sodium loss). This happens because the kidney is unable to efficiently retain water while excreting large amounts of sodium. In addition, after sodium excretion, the osmoreceptor system may sense lowered sodium concentration in the blood and then direct compensatory urinary water loss in order to correct the hyponatremic (low blood sodium) state.
Functions of Sodium In Specific Food Categories:-
Since sodium plays different roles in specific food types, it is helpful to discuss the functions of sodium in the context of food categories.
Grains
Whole grains are naturally low in sodium. However, a number of products made from grains have added sodium, and these products are major contributors to sodium intake.
Ready-to-Eat Cereals
Salt is frequently added to breakfast cereals to improve flavor and texture(Brady, 2002). A survey of children’s cereals from around the world found that, on average, these products are about 1 percent salt by weight.
Rice and Pasta
Rice and most pastas are very low in sodium (Brady, 2002; Van der Veer, 1985); however, salt is often added for flavor during preparation. Many flavored rice and pasta products contain salt in the seasoning, with salt sometimes being used as a bulk carrier to evenly distribute flavorings used in smaller quantities.
Baked Goods
Sodium plays multiple roles in breads and other baked goods. Salt, sodium bicarbonate, and sodium salts of leavening acids are the main sources of sodium in baked goods, accounting for 95 percent of the sodium in these products (Reichenbach and Singer, 2008). In most baked goods, salt is used to improve product taste and flavor. Without salt, many baked goods have an insipid taste (Van der Veer, 1985).
Salt is also responsible for fermentation control and texture in yeast-raised breads. In the mass production of bread, salt levels are used as a tool to control yeast activity. Salt reduces yeast activity by reducing water activity and damaging the membrane of the yeast cells. If too much salt is used, doughs may rise too slowly. However, if too little is added, fermentation may proceed too quickly or “wild” fermentations may occur, resulting in doughs that are gassy and sour with poor texture (Hutton, 2002; Vetter, 1981). Salt can also interact with gluten, one of the major proteins in flour responsible for the texture of baked goods, to ease the handling of dough during processing. The result of this interaction reduces the stickiness of the dough (Hutton, 2002; Vetter, 1981).
Quick breads, cakes, and cookies typically rely on chemical leavening agents rather than yeast to quickly create airy textures. Some of the most popular leavening agents contain sodium, including baking soda (sodium bicarbonate) and baking powder (a combination of sodium bicarbonate and one or a combination of the following: potassium hydrogen tartrate, sodium aluminum sulfate, sodium acid pyrophosphate, and calcium acid phosphate) (Bender, 2006).
Other additives used in bread may contribute minor amounts of sodium. One of these additives is sodium stearoyl lactylate, an emulsifier used to improve the volume of breads as well as to maintain the textural quality of frozen baked goods. Another sodium-containing additive is sodium metabisulfite. This acts as a dough-softening agent that can increase the extensibility of dough or be used to speed up dough development when high-speed mixing methods are not desirable (Cauvain, 2003).
Salt also helps to control the growth of molds and the Bacillus species of bacteria, thus extending the shelf life of baked goods (Betts et al., 2007).
Muscle Foods
Fresh Meats
Unprocessed cuts of meat have some naturally occurring sodium, but are generally considered low in sodium. Fresh meat products increasingly have been injected with salt- and phosphate-containing brines, increasing the sodium content of fresh products.
Processed Meats
Once meat is further processed into sausages or deli meats, the sodium content increases substantially. Sodium is used in meats not only for the flavor it imparts, but also for its role as a preservative and its impact on the textural qualities of the final product. Similar to fresh meats, salt addition to processed meats can help increase water binding in the muscle tissues, leading to increased yields (more products to sell) and greater tenderness. The mechanism by which salt increases water binding is not fully understood, but it is thought to be related to the ability of salt to create repulsion between myofibrillar proteins (Desmond, 2007). At times, phosphate salts containing sodium are also used to improve water binding of muscle foods and to lengthen the time before products turn rancid (Hedrick et al., 1994).
Salt is also used in the processing of products such as sausages and restructured meats. The presence of salt can solubilize myofibrillar proteins that are insoluble in water alone. Salting, in combination with processing steps such as blending and tumbling, helps to extract these salt-soluble proteins to the surface of the meat. Salt-soluble proteins extracted to the surface of the meat pieces are responsible for “gluing” the small pieces of meat together as they form a gel network during heating. In meat products made from batters (bologna, frankfurters, etc.), salt-soluble proteins coat fat particles, thereby keeping the fat and protein components from separating.
In cured meat products, such as hot dogs, smoked meats, bacon, and sausages, sodium can be introduced from three ingredients: salt, sodium nitrite, and the reductants sodium ascorbate and sodium erythorbate. Salt imparts flavor and plays a role in preservation by reducing water activity. The action of salt in reducing water activity is one hurdle against microbial growth in processed meats (Matthews and Strong, 2005). However, current levels of salt alone are too low to provide sufficient protection against spoilage and pathogen growth. Instead, sodium, in combination with other compounds such as sodium nitrite and with environmental conditions such as pH and storage temperature, works synergistically to create safe food products (Doyle et al., 2001; Matthews and Strong, 2005). Sodium nitrite is the ingredient responsible for the characteristic pink color of cured meats and for the preservation of meaty flavor. Sodium nitrite also has the function (in combination with salt) of inhibiting the growth of Clostridium botulinum (Doyle et al., 2001). If sodium nitrite and salt were not used in certain processed meat and seafood products, especially those that are vacuum or modified-atmosphere packaged, these products could no longer be produced or handled because they would pose a risk of botulism outbreaks (Betts et al., 2007; Hedrick et al., 1994; Matthews and Strong, 2005). The final sodium-containing cure ingredients are reductants. Sodium ascorbate and sodium erythorbate are commonly used reductants that play a role in increasing the rate of color formation in cured meats. The other essential role of sodium ascorbate or erythorbate is to retard the formation of N-nitrosamines, carcinogenic compounds that can form from residual nitrite especially during high-temperature cooking (Doyle et al., 2001).
Kosher Meats
Salting also plays a role in the kosher processing for meats. All blood must be removed from the tissues for a meat or poultry product to be considered kosher. To achieve this, meat is soaked and then salted. While the salt is used only on the surface of the meat, some is still able to penetrate, leading to increased salt content (Curtis, 2005).
Milk
Cow’s milk—as a source of essential nutrients for a growing mammal—naturally contains some sodium. Whole, low-fat, and skim milk all contain similar levels of sodium.
Cheese
Sodium in cheese is due to sodium naturally present in milk as well as added salt. While the characteristic salt taste of cheese is popular with consumers, salt also plays roles in the cheese making process that contribute to the texture, shelf life, and safety of the end product.
A function of salt in most cheese production is to draw water or whey out of cheese curds. Cheese curds are formed during the initial stages of cheese production when casein proteins in milk coagulate. The coagulation process also traps other milk components, such as fat, carbohydrates (lactose), minerals, and water. Often, more water is trapped in the curd than is desired in the final product. Commonly, cheese curds will be pressed prior to the ripening process to remove this excess water, but pressing alone is usually insufficient. Addition of salt by brine solution or dry rub is used to remove additional water by osmosis to reach desired moisture levels (Potter and Hotchkiss, 1995; Walstra et al., 1999).
Removal of water from cheese curds helps to reduce the water available for microbial growth, reducing the likelihood of microbial spoilage and pathogen growth. For some types of cheese, salting creates a hard rind that protects the cheese during ripening and transport. In addition, the presence of salt in the resulting moisture-reduced cheese decreases the water activity of the product. Lowering water activity controls the growth of cheese starter cultures, which can influence the pH, texture, and ripening of cheese(Guinee and Fox, 2004).
Texture is also altered by the removal of excess water and by the overall sodium content of the cheese. Cheeses with lower salt content are typically soft, pasty, and adhesive, while those with higher content are harder, drier, and crumblier (Guinee and Fox, 2004). Salt also impacts physical characteristics, such as melt ability, shredding, stretching, and flow (Reddy and Marth, 1991). Texture is also altered by the activity of proteolytic enzymes, and the activity of proteolytic enzymes is altered by salt (Guinee and Fox, 2004). Processed cheeses can have additional sodium in the form of sodium phosphates and sodium citrates, which are emulsifying agents important to the formation and final texture of these products (Guinee and O’Kennedy, 2007).
Non-salty tastes are also affected by the presence of salt. Undesirable bitterness in cheese is thought to be related to insufficient salt levels (Guinee and Fox, 2004). In addition, the activity of starter cultures is impacted by salt level and time of addition. Starter cultures are responsible for the production of a number of flavor compounds in addition to acid (Doyle et al., 2001).
Butter
Salt was initially added to butter as a preservative prior to widespread use of refrigeration. Salt still plays a preservation role today, but it is less important because access to refrigeration is possible throughout the supply chain. Instead, taste and flavor development are the main drivers for common levels of salt in butter and margarine (Brady, 2002; Hutton, 2002).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Other Dairy Products
Other dairy products, such as yogurt, ice cream, and puddings, contain sodium naturally, from low levels of sodium-containing additives, such as sodium alginate and carrageenan (both thickening agents) (Goff, 1995; Lal et al., 2006), or from added flavorings.
Sauces, Gravies, Stocks, Salad Dressings, and Condiments
Sauces, gravies, stocks, salad dressings, and condiments are often high in sodium. Reasons for sodium use include flavor, preservation, and improving the stability of emulsions (by improving the solubility of emulsifiers). Flavor is a main reason for adding salt to these products, and saltiness is often one of the major characteristics of these items (Hutton, 2002).
In most condiments, salt also plays a role in preservation (Brady, 2002), combined with other hurdles to microbial growth. Sodium-containing additives also may be added to salad dressings, sauces, and condiments to act as emulsifiers or preservatives. For soy sauce, which is very high in sodium, salt is needed to influence the fermentation process in its production (Doyle et al., 2001).
Fruits, Vegetables, Beans, and Legumes
Fresh fruits and vegetables are generally very low in sodium, although salt may be added to fresh produce during home or foodservice preparation. Fruits that are processed further typically remain low in sodium (Van der Veer, 1985). Frozen vegetables generally do not have additional sodium unless components such as breadings or sauces are added to the product (Van der Veer, 1985). Dried pulses (beans, lentils, peas) are naturally low in sodium but they are often salted during home and foodservice cooking.
Canned vegetables are typically much higher in sodium than their fresh counterparts. In canning, a liquid medium is important for heat transfer during processing, and salt brine is generally used because salt enhances the consistency and flavor of vegetables (Hutton, 2002; Van der Veer, 1985). Pickled vegetables such as sauerkraut and cucumbers are also high in sodium because of the salt added to drive the fermentation process and to maintain a crisp texture (Brady, 2002).
Mixed Dishes
Combination foods, such as pizza, soups, stews, casseroles, and ready-to-eat meals, are usually high in sodium. Sodium in these foods comes from many sources and has multiple functions; when combined into a single serving, the sodium from these varied sources can easily contribute significant levels to the total diet. Pepperoni pizza is a good example of this because each of the major ingredients contains sodium. The pepperoni has sodium for preservation, meat binding, and flavoring. Sodium in the cheese contributes to texture and preservation as well as taste and flavor. Tomato sauce is seasoned with salt in addition to other herbs and spices. Finally, the crust contains sodium to control the leavening process and dough stickiness. The combination of these ingredients leads to an average sodium content of 668 mg/100 g, according to FDA’s Total Diet Study market basket data (FDA, 2007).
Soups are classic examples of complex, high-sodium foods. Some soups have high-sodium ingredients, such as cheese or sausage. However, even foods made from low-sodium ingredients, such as vegetables, are high in sodium due to the use of salt for flavoring. In soups, salt contributes not only to salt taste, but also to overall flavor (Gillette, 1985; Rosett et al., 1997).
In chilled foods, sodium-containing compounds can play a role in preventing the growth of pathogens. Vacuum and modified-atmosphere packaging can create oxygen-free environments that favor the growth of Clostridium botulinum. Salt, in addition to other hurdles, can help prevent the growth of this organism. If oxygen is present, Listeria monocytogenes is often a concern because it can grow even at low temperatures. Salt addition can serve as one hurdle to the viability of this organism (Hutton, 2002).
Savory Snacks
Most savory snacks, including chips, nuts, pretzels, popcorn, French fries, and extruded snacks (cheese balls, shaped potato snacks, etc.), have added sodium in the form of salt. The function of salt in these foods is to contribute to salt taste and overall flavor. For many flavored snack products, salt is used to distribute minor ingredients, such as flavors and colors. Mixing minor ingredients with salt before application can help to ensure even distribution of these components over the surface of the snack (Matz, 1993).
Secondary functions of sodium in some extruded products are to modify texture and color. Extruded products have a puffy texture and the degree of expansion and airiness has been found to change with the salt concentration of the extrudate and is thought to be due to interactions between salt and starch (a main component of these snacks). Color has also been found to change with salt content, and this relationship has been proposed to be due to the ability of salt to change the water activity of the extrudate and thus change the rate of browning reactions (Ainsworth and Plunkett, 2007).
Confections
Hard candies are generally low in sodium, and other confections may have low levels of sodium-containing leavening or texture-modifying agents (Saulo, 2002). Dairy-based confections will contribute to sodium intake due to the sodium naturally present in milk. Chocolates may also contain small amounts of sodium to contribute to flavor and texture. Some confections are likely to contain added salt for flavoring purposes, particularly those with fillings, such as crèmes or jams (Van der Veer, 1985). Other confections that may include salt for flavoring purposes are caramels, taffy, and nut-containing candy.
Beverages
Water is relatively low in sodium, but sodium levels vary by water source and with the use of water-softening systems (Bradshaw and Powell, 2002; Pehrsson et al., 2008). Tea and coffee are also very low in sodium, although the level may increase slightly with the addition of milk and cream.
Sodium-containing preservatives are sometimes added to carbonated beverages and fruit drinks (Doyle et al., 2001). Even though these beverages contain sodium, the levels are generally low compared to those of many other solid food items.
The vegetable juice category of beverages is one in which sodium levels are traditionally quite high. Taste and flavor improvements are the reasons for addition of salt to tomato, carrot, and vegetable blend drinks.
Salt is often present in sports drinks for the stated purpose of rehydrating the body during or after physical activity, although the medical justification for the sodium contained in these drinks under the conditions consumed (e.g., high school sports activities) is not clearly demonstrated (Jeukendrup et al., 2009; Shirreffs et al., 2007).
Common Sodium-Containing Compounds and Their Functions in Food:
Emulsifying Agents:
· Sodium pyrophosphate
· Disodium hydrogen phosphate
· Sodium alginate
· Sodium phosphate
· Trisodium citrate
· Trisodium phosphate
· Sodium stearoyl lactylate
Buffering Agents:
· Aluminum sodium sulfate
· Disodium hydrogen phosphate
· Sodium adipate
· Sodium dihydrogen citrate
· Sodium dihydrogen phosphate
· Sodium DL-malate
· Sodium hydrogen carbonate
· Sodium phosphate
· Trisodium citrate
· Trisodium phosphate
Anticaking Agents:
· Sodium aluminosilicate
· Sodium ferrocyanide
Flavor-Enhancing Agents:
· Monosodium glutamate
· Disodium 5′-guanylate
· Disodium 5′-inosinate
· Disodium 5′-ribonucleotides
Leavening Agents:
· Sodium bicarbonate
· Disodium pyrophosphate
· Sodium acid pyrophosphate
· Sodium aluminum phosphate
· Sodium hydrogen carbonate
Dough-Conditioning Agents:
· Sodium stearoyl lactylate
· Sodium stearyl fumarate
Stabilizing Agents:
· Disodium ethylenediaminetetraacetic acid (EDTA)
· Disodium pyrophosphate
· Potassium sodium L-tartrate
· Sodium alginate
· Sodium carboxymethylcellulose
· Sodium caseinate
· Trisodium citrate
· Sodium stearoyl lactylate
Neutralizing Agents:
· Trisodium phosphate
· Sodium sesquicarbonate
· Sodium phosphate
· Sodium DL-malate
· Sodium dihydrogen phosphate
· Sodium dihydrogen citrate
· Sodium citrate
· Sodium adipate
· Aluminum sodium sulfate
· Sodium potassium tartrate
· Sodium acetate
Thickening Agents:
· Sodium alginate
· Sodium carboxymethylcellulose
Moisture-Retaining Agents:
· Sodium hydrogen DL-malate
· Sodium lactate
· Sodium lauryl sulfate
Texture-Modifying Agents:
· Sorbitol sodium
· Sodium tripolyphosphate
· Pentasodium triphosphate
· Disodium hydrogen phosphate
Bleaching Agent:
· Sodium metabisulfite
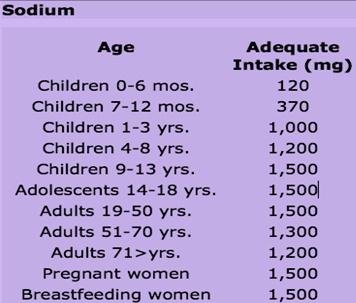
Recommended dietary allowance for Sodium:
Specific recommendations regarding sodium intake do not exist for infants, children, and adolescents. Eating habits and attitudes about food formed during childhood are likely to influence eating habits for life. For this reason, moderate intake of sodium is suggested.
FLAME PHOTOMETER
Principle: -Flame photometry (more accurately called flame atomic emission spectrometry) is a branch of atomic spectroscopy in which the species examined in the spectrometer are in the form of atoms. In all cases the atoms under investigation are excited by light. Absorption techniques measure the absorbance of light due to the electrons going to a higher energy level. Emission techniques measure the intensity of light that is emitted as electrons return to the lower energy levels. Flame photometry is suitable for qualitative and quantitative determination of several cations, especially for metals that are easily excited to higher energy levels at a relatively low flame temperature (mainly Na, K, Rb, Cs, Ca, Ba, Cu). This technique uses a flame that evaporates the solvent and also sublimates and atomizes the metal and then excites a valence electron to an upper energy state. Light is emitted at characteristic wavelengths for each metal as the electron returns to the ground state that makes qualitative determination possible. Flame photometers use optical filters to monitor for the selected emission wavelength produced by the analyte species. Comparison of emission intensities of unknowns to either that of standard solutions (plotting calibration curve), or to those of an internal standard (standard addition method), allows quantitative analysis of the analyte metal in the sample solution.

The following processes occur in the flame photometer.
i) Nebulization:A solution containing the relevant substance to be analyzed is aspirated into the burner and dispersed into the flame as a fine spray. This process is called nebulization.The most commonly employed flames in flame photometry can be grouped into two types:
1. Flames in which the fuel and oxidant as air or oxygen are well mixed before combustion, these are called pre-mix or laminar flames as they exhibit laminar flow.
2. Flames in which the fuel gas and the oxidant are first mixed in the flame itself. They are called unpremix or turbulent flames since they exhibit turbulence.
Flames are not uniform in composition, length or cross section. The structure of a premixed flame, supported as a laminar flow is shown below:
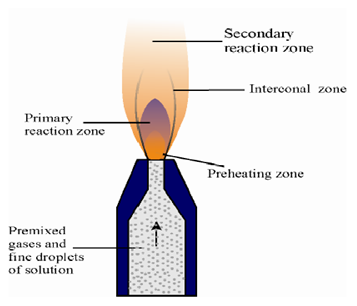
ii) Vaporization: The heat of the flame vaporizes the sample constituents. No chemical change takes place at this stage.
iii) Atomization: At this stage the metal ions that were in the solvent are reduced to metal atoms. For example, 2+ Mg (aq) + 2e _ Mg (g). By heat of the flame and action of the reducing gas (fuel), molecules and ions of the sample species are decomposed and reduced to give atoms.
iv) Excitation: The atoms at this stage are able to absorb energy from the heat of the flame. The amount of energy absorbed depends on the electrostatic forces of attraction between the negatively charged electrons and the positively charged nucleus. This in turn depends upon the number of protons in the nucleus. As electrons absorb energy they move to higher energy levels and are in the excited state.
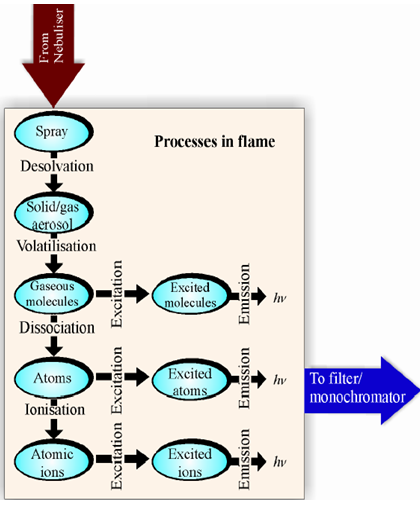
v) Emission of radiation: Electrons in the excited state are very unstable and move back down to the ground state or a lower energy state quite quickly. As they do so, they emit the energy in the form of radiation of characteristic wavelength, which is measured by a detector. For some metals this radiation corresponds to wavelengths of light in the visible region of the electromagnetic spectrum and is observed as a characteristic color of the flame. As electrons from different energy levels are able to emit light as they relax, the flame color observed will be a mixture of all the different wavelengths emitted by the different electrons in the metal atom under investigation.
Instrumentation:-
The sample is introduced into a flame wherein it undergoes a number of processes leading to the formation of excited atomic species which emit radiation. The radiation is then measured and suitably analyzed. The instrument used for the purpose is called flame photometerand it consists of the following basic components.
1. Flame atomiser: It converts the sample into excited atomic species and consists of the following.
o Nebuliser and mixing chamber: It is a means of transporting a homogeneous solution into the flame at a steady rate.Pneumatic nebuliseris the most commonly used nebuliser for introducing aqueous/liquid samples.
o Atomiser burner: Here the fuel and oxidant burn to give a flame that can be maintained in a constant form and at a constant temperature.Two types of atomisation burners have been used in flame photometry which are given below and explained in the following paragraphs.
a) Pre-mix or Lundegarh burner
b) Total consumption burner
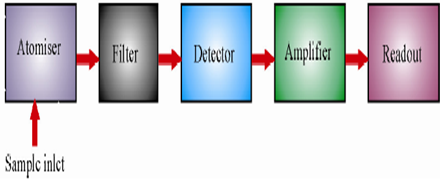
2. Monochromator (or filter): It isolates the light of the wavelength to be measured from that of extraneous emissions.Generally a grating or a prism monochromator is employed.
3. Detector: It helps in measuring the intensity of radiation emitted by the flame.Photoemissive cells or photomultiplier tubes are commonly employed for the purpose.
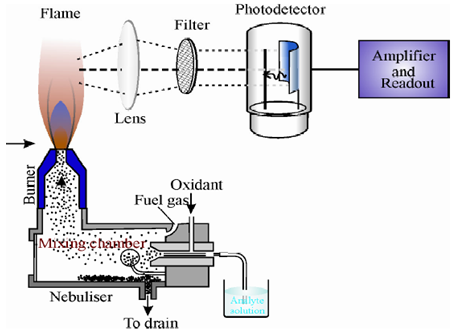
4. Amplifier and Readout Device: It is used to amplify the signal and provides a suitable output.Nowadays the instruments have microprocessor controlled electronics that provides outputs compatible with the printers and computers thereby minimising the possibility of operator error in transferring data.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Interferences in quantitative determinations:-
The success of the quantitative determination depends on how accurately the intensity of the emitted radiation represents the concentration of the analyte. It has been found that number of factors besides the analyte, affect the intensity of the emitted radiation. The analytical signals measured often include contributions from constituents other than the analyte. The constituents are called the matrix constituents. The contributions are known as interferences and are found to influence the outcome of the analytical procedure. These can be corrected by subtracting their contributions to the signal. The contribution of the interferent can be calculated from the magnitude of the interference and the concentration of the interferent. The interferences encountered can be classified as follows.
· Spectral interferences
· Ionised interferences
· Chemical interferences
1. Spectral Interferences
These refer to the interferences that affect the spectral intensity or resolution. There are several types of spectral interferences which are explained below.
The first type of interference arises when two elements exhibit spectra, which partially overlap, and both emit radiation at some particular wavelength. The detector cannot distinguish between the sources of radiation and records the total signal, thus resulting in incorrect answer. Such interferences are more common at high flame temperatures because numerous spectral lines are produced at high temperatures. For example, the Fe line at 324.73 nm overlaps with the Cu line at 324.75 nm. Such interference can be overcome either by taking measurements at an alternative wavelength which has no overlap, if available, or by removing the interfering element by extraction. Alternatively, one may make a calibration curve, which is prepared from a solution having similar quantities of the interfering element.
The second type of spectral interference deals with spectral lines of two or more elements which are close but their spectra do not overlap. This type of interference becomes a problem when a filter is used as the device to isolate spectral lines. A filter may allow spectral lines separated by 5.0-10.0 nm to pass through, thus resulting in an error in the analysis. Such interferences can be reduced by increasing the resolution of the spectral isolation system. However, the interference cannot be eliminated entirely due to the finite width of the spectral isolation system and the finite slit width in such systems.
A third type of spectral interference occurs due to the presence of continuous background which arises due to high concentration of salts in the sample, especially of alkali and alkaline earth metals. Some organic solvents also produce a continuous background. This type of interference can be corrected by using suitable scanning technique.
2. Ionisation Interferences
In some cases, high temperature flame may cause ionisation of some of the metal atoms, e.g., in case of sodium, it can be given as follows.
Na Na+ + e-
The Na+ ion possesses an emission spectrum of its own with frequencies, which are different from those of atomic spectrum of the Na atom. This reduces the radiant power of atomic emission. This interference can be eliminated by adding a large quantity of a potassium salt to the standards as well as sample solutions. The addition of potassium salt suppresses the ionization of sodium, as the potassium atom itself undergoes ionization due to low ionization energy. Thus, the sodium atom emission is enhanced. This type of interference is restricted to alkali metals.
3. Chemical Interferences
The chemical interferences arise out of the reaction between different interferents and the analyte. These are of different types. Some of these are given below.
Cation-anion interference
The presence of certain anions, such as oxalate, phosphate, sulphate and aluminate, in a solution may affect the intensity of radiation emitted by an element, resulting in serious analytical error. For example, calcium in the presence of phosphate ion forms a stable substance, as Ca3(PO4)2 which does not decompose easily, resulting in the production of lesser atoms. Thus, the calcium signal is depressed. Another similar example is that of determination of barium in presence of sulphate forming insoluble BaSO4. This type of interference can be removed either by extraction of the anion or by using calibration curves prepared from standard solutions containing same concentrations of the anion as found in the sample.
Cation-cation interference
In many cases, mutual interferences of cations have been observed, resulting in reduced signal intensity of the element being determined. These interferences are neither spectral nor ionic in nature and the mechanism of such interferences is not well understood. Thus, for example, aluminum interferes with calcium and magnesium. Also, sodium and potassium show cation-cation interference on one another.
Interference due to oxide formation
This type of interference arises due to the formation of stable metal oxide if oxygen is present in the flame, resulting in reduced signal intensity. The alkaline earth metals are subject to this type of interference. This type of interference can be eliminated by either using very high flame temperature to dissociate the oxides or by using oxygen-deficient environment to produce excited atoms.
4. Other Factors
A number of other factors affect the intensity of light emission from a given sample solution. For example, the organic solvents in a sample may also influence the intensity of the emission line by changing the viscosity and surface tension of the liquid, which in turn alter the rate at which the sample is aspirated into the flame. They also affect the flame temperature through their contribution to the heat of combustion. An increase in the line intensity is usually observed.
OBJECTIVES
· To determine the levels of sodium in junk food and understand the benefits of sodium in diet and risks of high sodium in diet.
· To analyze sodium levels in junk food, this can be used to create awareness among the younger generation to prevent problems like hypertension, hyponatremia, hypernatremia and associated medical complications in their future.
· To establish a procedure for analyzing sodium levels in various junk foods by Flame Photometry and have a comparative study with processed foods, vegetables and fruits from the literature.
Effects of Sodium:
Sodium has an important role in maintaining the water balance within cells and in the function of both nerve impulses and muscles. Any extra sodium is excreted by the kidneys. Consuming excess sodium may lead to edema or water retention. Women who consume excess sodium may be at higher risk for developing osteoporosis even if calcium intake is adequate. Some evidence suggests that for each teaspoon of salt (2,000 mg of sodium) consumed, considerable calcium is excreted in the urine.
Too much sodium may lead to high blood pressure in those who are sensitive to sodium. Patients with High Blood Pressure are recommended to reduce the sodium (salt) intake. Sodium may lead to a serious build-up of fluid in people with congestive heart failure, cirrhosis, or kidney disease. Such people should be on a strict sodium-restricted diet, as prescribed by their doctor.
Sodium is classified as a "dietary inorganic macro-mineral" for animals. High intake of sodium causes hypernatremia (elevated sodium level in the serum) whereas low intake of sodium leads to hyponatremia (lower sodium in serum than normal).
Clinical manifestations of hypernatremia can be subtle, consisting of lethargy, weakness, irritability, and edema. With more severe elevations of the sodium level, seizures and coma may occur. Symptoms of hyponatremia include nausea and vomiting, headache, confusion, lethargy, fatigue, appetite loss, restlessness and irritability, muscle weakness, spasms, or cramps, seizures, and decreased consciousness or coma.
Sodium occurrence:
Sodium occurs naturally in most foods. The most common form of sodium is sodium chloride, which is table salt. Milk, beets, and celery also naturally contain sodium, as does drinking water, although the amount varies depending on the source. Rock salt and sea salt are almost entirely sodium chloride, with only traces of other elements (minerals).
Sodium is also added to various food products. Some of these added forms are monosodium glutamate, sodium nitrite, sodium saccharin, baking soda (sodium bicarbonate), and sodium benzoate. These are ingredients in condiments and seasonings such as Worcestershire sauce, soy sauce, onion salt, garlic salt, and bouillon cubes. Processed meats, such as bacon, sausage, ham, canned soups and vegetables are all examples of foods that contain added sodium.
Fast foods are generally very high in sodium. Hence by analysis of such type of foods a general awareness about impact of it on health can be given. Accordingly the occurrence of serious health problems also can be prevented.
METHODOLOGY
Materials required:
a) Conical flasks
b) Beakers
c) Pipettes
d) Measuring cylinders
e) Standard flasks
f) Deionized water.
Equipment:
1. Flame photometer
2. Electrical balance.
Chemicals required:
1. Deionized water
2. Hydrochloric acid(HCl): 0.5% HCl (V/V) (to be used for standards)
3. Sodium chloride (NaCl): For 1000ppm weigh 635mg of NaCl and dissolve it in 250ml of deionized water in a 250ml standard flask.
4. Potassium chloride (KCl): For 1000ppm weigh 477mg of KCl and dissolve it in 250ml of deionized water in a 250ml standard flask.
Flame photometer measures the concentration of Sodium, Potassium ,Calcium and Lithium in terms of the quantity of the element itself in solution. Standard solutions prepared from the salts of these metals must be made up to contain the concentrations required in terms of the quantity of the element. Na, K, Ca or Li. The following values are typical for standards of 1mg. Na/100mL, 1mg.K/100mL, 10mg.Ca/100mL, and 10mg. Li/100mL.
Parts per million (ppm)
This is generally employed for those solutions in which a substance is present in a very small quantity. It represents gram of a solute per million ml of the solution.
ppm = mass of component
-----------------------------------------------
Total mass of the solution X 106
Or
ppm = gm or mL of the solute or substance
-----------------------------------------------
gm or mL of the solution X 106
Thus 1 ppm of a solution of Nacl /Kcl in water represents
1ppm= 1mg Kcl / L of solution.
= 1mg Kcl / 1000mL of solution.
= 1µg Kcl / mL of solution.
QUALITY OF SALTS REQUIRED
1000 ppm standard solution should have 1000mg of element in 1000mL salt solution of the element.
Thus 100mL standard solution should be made up of a quantity of salt of the element in mg., equivalent to (250 X Molecular Wt. of salt/Atomic Wt. of element), to contain 250mg. of element.
|
Element |
Atomic Wt of element |
Salt |
Molecular Wt. of salt |
Quantity of salt in mg. required for preparation of 100mL of 1000ppm standard solution |
|
Na |
22.99 |
NaCl |
58.44 |
254 |
|
K |
39.10 |
KCl |
74.56 |
190.8 |
|
Ca |
40.08 |
CaCO3 |
100.09 |
249.6 |
|
Li |
6.94 |
Li2CO3 |
73.89 |
532.4 |
Preperation of Standard Solutions
Stock Standard Solution
Weigh accurately 254mg of “Anal R” quality of Sodium Chloride (NaCl) into a 100mL volumetric flask through a funnel. Weigh accurately 190.8mg “Anal R” quality of Potassium Chloride (KCl) and transfer it into the same volumetric flask through the same funnel. Add double distilled water to the flask, dissolve the crystals and make up the solution to the mark with double distilled water. This stock standard solution contains 1000mEq/100mL of Sodium (Na) and 1000mEq/100mL Potassium (K)
Working Standard solutions
This Stock Standard solution is diluted 1:100 with double distilled water (10mL of Stock Standard solution make up to 1Litre). This diluted Stock Standard solution is further diluted as below to obtain a series of Working Standard solutions of Na & K.
|
S. No |
Diluted Stock Standard solution in mL |
Double distilled water in mL |
Concentration of mEq/L of Working Standard solution of Na & K (in ppm) |
|
1 |
4 |
96 |
40 |
|
2 |
6 |
94 |
60 |
|
3 |
8 |
92 |
80 |
|
4 |
10 |
90 |
100 |
|
5 |
12 |
88 |
120 |
Samples: The samples used are foods that are rich in sodium levels. They are:-
1. Amul Cheese
2. Nutrilite Butter
3. Pani puri masala
4. Pav bhaji masala
5. Maggie masala
6. Britannia Cashew Biscuits
7. Lays chips
8. Act II Popcorn
9. Kurkure
10. Kissan Tomato sauce
11. Weikfield Soya sauce
12. Cadbury chocolate
13. Appy fizz
14. Coca cola
15. Ajinomoto
A detailed description about each food item and the role of sodium in it is given below:
Cheese:
Sodium in cheese is due to sodium naturally present in milk as well as added salt. While the characteristic salt taste of cheese is popular with consumers, salt also plays roles in the cheese making process that contribute to the texture, shelf life, and safety of the end product. A function of salt in most cheese production is to draw water or whey out of cheese curds. Removal of water from cheese curds helps to reduce the water available for microbial growth, Undesirable bitterness in cheese is thought to be related to insufficient salt levels (Guinee and Fox, 2004). In addition, the activity of starter cultures is impacted by salt level and time of addition. Starter cultures are responsible for the production of a number of flavor compounds in addition to acid (Doyle et al., 2001).
Butter:
Salt was initially added to butter as a preservative prior to widespread use of refrigeration. Salt still plays a preservation role today, but it is less important because access to refrigeration is possible throughout the supply chain. Instead, taste and flavor development are the main drivers for common levels of salt in butter and margarine (Brady, 2002; Hutton, 2002).
Pani puri masala, Pav bhaji masala and Maggie masala:
Sodium in these foods comes from many sources and has multiple functions; when combined into a single serving; the sodium from these varied sources can easily contribute significant levels to the total diet. Vacuum and modified-atmosphere packaging can create oxygen-free environments that favor the growth of Clostridium botulinum. Salt, in addition to other hurdles, can help prevent the growth of this organism. If oxygen is present, Listeria monocytogenes is often a concern because it can grow even at low temperatures. Salt addition can serve as one hurdle to the viability of this organism (Hutton, 2002).
Biscuits:
Quick breads, cakes, and cookies typically rely on chemical leavening agents rather than yeast to quickly create airy textures. Some of the most popular leavening agents contain sodium, including baking soda (sodium bicarbonate) and baking powder (Bender, 2006) accounting for 95 percent of the sodium in these products (Reichenbach and Singer, 2008). Salt also helps to control the growth of molds and the Bacillus species of bacteria, thus extending the shelf life of baked goods (Betts et al., 2007). The Bacillus species is capable of forming rope-like structures, off-flavors, and discoloration, especially in baked goods high in sugar or fats (Doyle et al., 2001). Salt is also responsible for fermentation control and texture in yeast-raised breads. Salt can also interact with gluten, one of the major proteins in flour responsible for the texture of baked goods, to ease the handling of dough during processing. The result of this interaction reduces the stickiness of the dough (Hutton, 2002; Vetter, 1981). In most baked goods like biscuits, salt is used to improve product taste and flavor. Without salt, many baked goods have an insipid taste (Van der Veer, 1985).
Lays Potato chips, Popcorn and Kurkure:
Most savory snacks like chips, wafers and popcorn have added sodium in the form of salt. The function of salt in these foods is to contribute to salt taste and overall flavor. For many flavored snack products, salt is used to distribute minor ingredients, such as flavors and colors. Mixing minor ingredients with salt before application can help to ensure even distribution of these components over the surface of the snack (Matz, 1993). In fried products, antioxidants may also be incorporated in these mixtures to prevent the development of rancidity (Ainsworth and Plunkett, 2007).
Secondary functions of sodium in some extruded products are to modify texture and color. Extruded products have a puffy texture and the degree of expansion and airiness has been found to change with the salt concentration of the extrudate and is thought to be due to interactions between salt and starch (a main component of these snacks). Color has also been found to change with salt content, and this relationship has been proposed to be due to the ability of salt to change the water activity of the extrudate and thus change the rate of browning reactions (Ainsworth and Plunkett, 2007).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Tomato sauce and Soya sauce:
The reasons for sodium use in these sauces include flavor, preservation, and improving the stability of emulsions (by improving the solubility of emulsifiers). Flavor is a main reason for adding salt to these products, and saltiness is often one of the major characteristics of these items (Hutton, 2002). Salt also plays a role in preservation (Brady, 2002), combined with other hurdles to microbial growth. Sodium-containing additives also may be added to salad dressings, sauces, and condiments to act as emulsifiers or preservatives. For soy sauce, which is very high in sodium, salt is needed to influence the fermentation process in its production (Doyle et al., 2001).
Chocolate:
Dairy-based confections will contribute to sodium intake due to the sodium naturally present in milk. Chocolates may also contain small amounts of sodium to contribute to flavor and texture. Some confections are likely to contain added salt for flavoring purposes, particularly those with fillings, such as crèmes or jams (Van der Veer, 1985). Other confections that may include salt for flavoring purposes are caramels, taffy, and nut-containing candy.
Appy fizz and Coca cola:
Water is relatively low in sodium, but sodium levels vary by water source and with the use of water-softening systems (Bradshaw and Powell, 2002; Pehrsson et al., 2008). Sodium-containing preservatives are sometimes added to carbonated beverages and fruit drinks (Doyle et al., 2001).
Even though these beverages contain sodium, the levels are generally low compared to those of many other solid food items. Salt is often present in sports drinks for the stated purpose of rehydrating the body during or after physical activity, although the medical justification for the sodium contained in these drinks under the conditions consumed (e.g., high school sports activities) is not clearly demonstrated (Jeukendrup et al., 2009; Shirreffs et al., 2007). It is reported that the sodium in these products is not added for taste or preservative effects (Man, 2007).
Ajinomoto:
Monosodium glutamate, also known as sodium glutamate or ajinomoto , is the sodium salt of glutamic acid, one of the most abundant naturally occurring non-essential amino acids (Ninomiya K, 1998). It was classified by the U.S. Food and Drug Administration as generally recognized as safe (GRAS) and by the European Union as a food additive. Pure MSG does not have a pleasant taste until it is combined with a consonant savory smell.
There is also an interaction between MSG and salt (sodium chloride), and other umami substances such as nucleotides. With these properties, MSG can be used to reduce salt intake (sodium), which predisposes to hypertension, heart diseases and stroke (Yamaguchi S, Takahashi C, 1984). The taste of low-salt foods improves with MSG even with a 30% salt reduction. The sodium content (in mass percent) of MSG is roughly 3 times lower (12%) than in sodium chloride (39%).
Sample processing:
Prepare an HCl solution by diluting 500ml of concentrated HCl with 220ml of water. The sample is prepared by weighing 5g of each (diced or ground) into 500ml Erlenmeyer flasks. Add 50ml of the above HCl solution. Bring each to a boil on a hot plate, and then simmer for 5 min. Cool and filter each through Whatman #1 filter paper into flasks. Now quantitatively transfer the supernatant for each to separate 100ml volumetric flasks. Dilute to the mark with distilled water and shake. This extract is used to prepare the appropriate dilutions required for the experiment.
Sample Analysis:
The air pressure during the working of the instrument should be maintained between 10 to 15 mm hg. Then to check that the flame is appropriate it should be noted that the blue flame is of 8-10mm height. The protocol for the analysis of the sample containing sodium is as follows:
- Let the instrument warm up for 5-10 minutes.
- Set the pressure of the instrument using the black colored air knob.
- Using the gas knob ignite the flame with the help of the red colored ignition button.
- Select the appropriate options that are displayed on the screen depending on the number of elements to be assayed.
- Feed distilled water to the instrument when the instrument prompts. The distilled water is required for cleaning the nebulizer.
- Feed the blank. The blank used in the experiment is 0.5% HCl solution.
- Feed the standards of appropriate concentration i.e. 40ppm, 60ppm, 80ppm, 100ppm and 120ppm.
- Once the standards are over feed the unknown biological samples of appropriate dilutions.
- The potassium and the sodium values that are present in the unknown samples are viewed using the view option.
- Once the experiment is over the values can be saved in the instrument in the form of a file with the required file name and the date on which the experiment is performed.
Calculations
CALCULATION OF SODIUM ELEMENT
1000ppm of standard solution contain 254 mg of NaCl which contains 100mg of Na alone
Xppm of sample solution contain ?
X× 100mg
Amount of Sodium = ------------------------------
1000
Therefore, amount of Sodium = y mg/100mL
CALCULATION FOR SODIUM CHLORIDE
1000ppm of standard solution contain 254 mg of NaCl which contains 100mg of Na alone
Xppm of sample solution contain ?
X× 254mg
Amount of Sodium = ---------------------------------------
1000
Therefore, amount of Sodium Chloride = y mg/100mL
RESULT
The samples selected for the study were junk foods which are prone to create health problems like hypertension, obesity, cardiac diseases etc. A total of 15 samples were analyzed for the sodium content. The data obtained from the analysis is shown in table-1.
Table-1 Levels of Sodium in 1:10 diluted sample.
|
SAMPLE NAME |
ppm VALUES (1:10) |
MILLIGRAM SODIUM PER 5g SAMPLE |
MILLIGRAM SODIUM PER 100g SAMPLE |
|
CHEESE |
54.6 |
54.6 |
1092 |
|
NUTRILITE BUTTER |
35.6 |
35.6 |
712 |
|
PANI PURI MASALA |
243.5 |
243.5 |
4870 |
|
PAV BHAJI MASALA |
62.7 |
62.7 |
1254 |
|
MAGGIE MASALA |
184.4 |
184.4 |
3688 |
|
BISCUIT |
14 |
14 |
280 |
|
LAYS CHIPS |
25.7 |
25.7 |
514 |
|
ACT II POPCORN |
37.4 |
37.4 |
748 |
|
KURKURE |
36.7 |
36.7 |
734 |
|
KISSAN TOMATO SAUCE |
43.6 |
43.6 |
872 |
|
SOYA SAUCE |
195.2 |
195.2 |
3904 |
|
CADBURY CHOCOLATE |
6.8 |
6.8 |
136 |
|
APPY FIZZ |
0.1 |
0.1 |
2 |
|
COCA COLA |
1.8 |
1.8 |
36 |
|
AJINOMOTO |
263.9 |
263.9 |
5278 |
Based on the data obtained from the analysis a graph was plotted by taking the types of junk foods on x-axis and the concentration of sodium in mg on the y-axis.
Graph-1 Levels of Sodium in 1:10 diluted sample
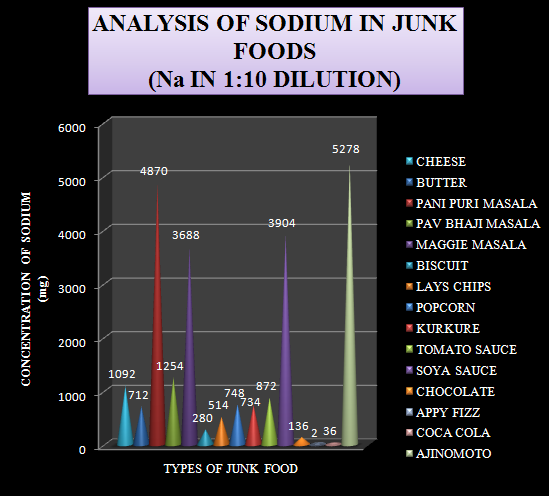
The same graph is plotted with the levels of sodium arranged in an increasing order. This graph is plotted in order to differentiate the junk foods into high, moderate and low levels of sodium containing foods.
Graph-2 Levels of Sodium in 1:10 diluted sample (Increasing order of values)
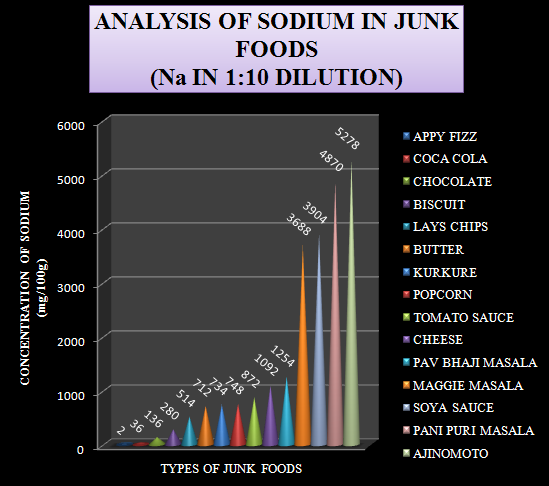
From the above graph the junk foods can be categorized as follows:
· Low sodium containing foods (0-200): E.g.: Appy fizz, coca cola and chocolate.
· Moderate sodium containing foods (300-1000): E.g.: biscuits, lays chips, butter, kurkure, popcorn and tomato sauce.
· High sodium containing foods (1000 & above): E.g.: cheese, pav bhaji masala, Maggie masala, soya sauce, Pani Puri masala and ajinomoto.
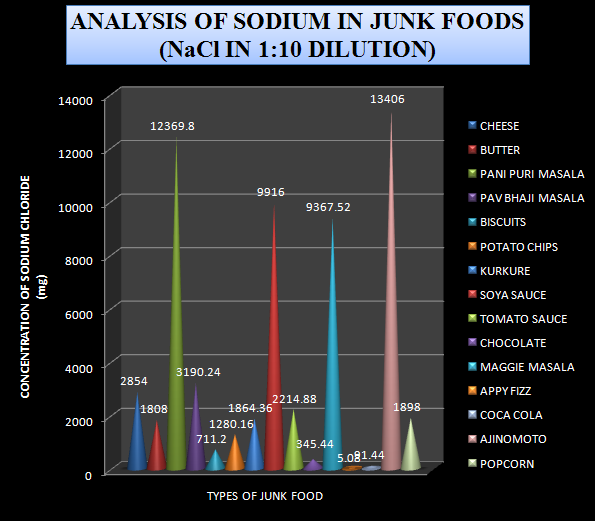
Using the Sodium data obtained from Flame Photometry the content of Sodium chloride was also calculated in the sample. The Sodium chloride content from various samples is shown in table-2
Table-2 Levels of Sodium chloride in 1:10 diluted sample
|
SAMPLE NAME |
ppm VALUES (1:10) |
MILLIGRAM NaCl PER 5g SAMPLE |
MILLIGRAM NaCl PER 100g SAMPLE |
|
CHEESE |
54.6 |
141.2 |
2824 |
|
NUTRILITE BUTTER |
35.6 |
90.4 |
1808 |
|
PANI PURI MASALA |
243.5 |
618.5 |
12369.8 |
|
PAV BHAJI MASALA |
62.7 |
159.5 |
3190.2 |
|
MAGGIE MASALA |
184.4 |
468.4 |
9367.5 |
|
BISCUIT |
14 |
35.6 |
711.2 |
|
LAYS CHIPS |
25.7 |
64 |
1280.2 |
|
ACT II POPCORN |
37.4 |
94.9 |
1898 |
|
KURKURE |
36.7 |
93.2 |
1864.4 |
|
KISSAN TOMATO SAUCE |
43.6 |
110.7 |
2214.9 |
|
SOYA SAUCE |
195.2 |
495.8 |
9916 |
|
CADBURY CHOCOLATE |
6.8 |
17.3 |
345.4 |
|
APPY FIZZ |
0.1 |
0.3 |
5.1 |
|
COCA COLA |
1.8 |
4.6 |
91.4 |
|
AJINOMOTO |
263.9 |
670.3 |
13406 |
Based on the data obtained from the analysis a graph was plotted by taking the types of junk foods on x-axis and the concentration of sodium chloride in mg on the y-axis.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Graph-3 Levels of Sodium chloride in 1:10 diluted sample
These values are used to plot a similar graph with the increasing order of sodium chloride content.
Graph-4 Levels of Sodium chloride in 1:10 diluted sample (Increasing order of values)
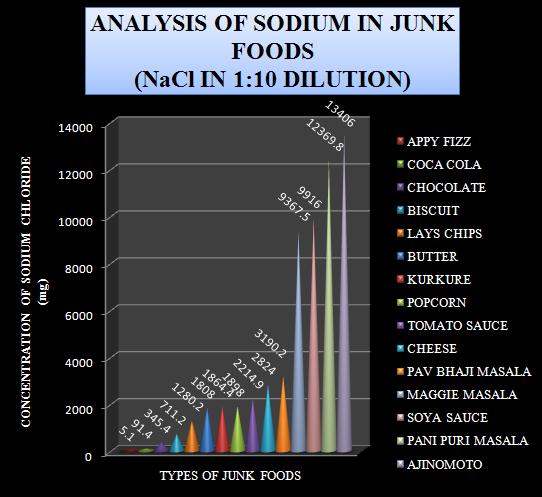
The above graph gave the data about the foods that are rich in sodium chloride content like ajinomoto, Pani Puri masala, soya sauce and Maggie masala.
CONCLUSION
Based on the analysis the various junk foods analyzed can be categorized into three:
1. Junk foods containing high levels of sodium
The junk foods that are rich in sodium levels are ajinomoto, soya sauce, Pani Puri masala, cheese, pav bhaji masala and Maggie masala. The sodium content in these foods is not only because of presence of sodium chloride but also because of other sodium containing compounds like sodium citrate, sodium glutamate, etc. The addition of salt to these foods is not only for the purpose of flavor but also it acts as a preservative. But consuming foods that are rich in sodium will lead to increased levels of sodium in body leading to hypernatremia. In order to avoid the problems associated with hypernatremia it is recommended that consumption of such foods should be limited in order to maintain the required sodium levels in the body.
2. Junk food containing moderate levels of sodium
The junk foods that contain moderate levels of sodium are biscuits, potato chips, butter, kurkure, popcorn and tomato sauce. In these foods sodium is not only present in the form of sodium chloride but they also contain other sodium containing additives. In butter, tomato sauce and cheese sodium is added as a preservative whereas in chips, popcorn, etc sodium is added for the purpose of flavor.
3. Junk foods containing low levels of sodium
Chocolate, Appy fizz and coca cola have very low levels of sodium. Sodium in these foods is added in the form of sodium carbonate, sodium bicarbonate etc. even though they have low sodium levels their consumption should be limited since more consumption of sodium carbonate and bicarbonate leads to gastro intestinal problems such as ulcers etc.
The results of this analysis showed a wide range in sodium levels within the packaged and take-away food categories included in this program. This may be due to brand-specific formulations in products such as biscuits and potato chips and discretionary salt usage by consumers and food preparation personnel for take-away foods such as pav bhaji, Pani Puri, cheese etc.
Healthy adults should limit sodium intake to 2,300 mg per day while individuals with high blood pressure should consume no more than 1,500 mg per day.
Those with congestive heart failure, liver cirrhosis, and kidney disease may need much lower amounts because Sodium may lead to a serious build-up of fluid in people with these diseases. Such people should be on a strict sodium-restricted diet, as prescribed by their doctor. In fact, a salt-restricted diet lowers blood pressure in people without hypertension as well. Because reducing salt intake causes no harm and diminishes the risk of hypertension and heart disease.
It is also recommended to use natural preservation processes in order to reduce the sodium intake. Thus it is concluded that moderate intake of sodium is suggested and people should consume foods containing moderate amounts of sodium like biscuits, potato chips, butter, kurkure, popcorn and tomato sauce.
BIBLIOGRAPHY
• Ainsworth P, Plunkett A. In: Reducing salt in snack products, Reducing salt in foods: Practical strategies. Kilcast D, Angus F, editors. Cambridge, UK: Woodhead P; 2007. pp. 296–315.
• Barbut S, Tanaka N, Maurer AJ. Effects of varying levels of chloride salts on Clostridium botulinum toxin production in turkey frankfurters. Journal of Food Science. 1986;51(5):1129–1131.
• Betts G, Everis L, Betts R. In: Microbial issues in reducing salt in food products, Reducing salt in foods: Practical strategies. Kilcast D, Angus F, editors. Cambridge, UK: Woodhead; 2007. pp. 174–200.
• Brownell, P.F. and Crossland, C.J. (1972). ‘The requirement for sodium as a micronutrient by species having the C4 dicarboxylic photosynthetic pathway’
• Brownell, P.F. (1979). ‘Sodium as an essential micronutrient element for plants and its possible role in metabolism’, Advances in Botanical Research, 7, 117–224.
• Brady M. Sodium: Survey of the usage and functionality of salt as an ingredient in UK manufactured food products. British Food Journal. 2002;104(2):84–125.
• Brashear G, Brewer MS, Meisinger D, McKeith FK. Raw material pH, pump level and pump composition on quality characteristics of pork. Journal of Muscle Foods. 2002;13(3):189–204.
• Cauvain S. Bread making: Improving quality. Boca Raton, FL: CRC Press; 2003.
• Church, Charles F., and Helen N.Church. Food Values of Portions Commonly Used : Bowes and Church. Philadelphia: J. B. Lippincott, 1970.
• Curtis PA. Guide to food laws and regulations. 1st ed. Ames, IA: Wiley-Blackwell; 2005.
• Cutler, Jeffrey A., Dean Follmann, and P. Scott Allender. "Randomized Trials of Sodium Reduction: An Overview." American Journal of Clinical Nutrition 65 (1997, Supp.): 643S–651S.
• Davidson PM. In: Chemical preservatives and natural antimicrobial compounds, Food microbiology: Fundamentals and frontiers. Doyle MP, Beauchat LR, Montville TJ, editors. Washington, DC: ASM Press; 2001.
• Desmond E. In: Reducing salt in meat and poultry products, Reducing salt in foods: Practical strategies. Kilcast D, Angus F, editors. Cambridge, UK: Woodhead; 2007. pp. 233–255.
• Doyle MP, Beuchat LR, Montville TJ, editors. Food microbiology: Fundamentals and frontiers. 2nd ed. Washington, DC: ASM Press; 2001.
• Emsley, John (2001). Nature's Building Blocks: An A-Z Guide to the
• Fennema OR. Food chemistry. 3rd ed. New York: Marcel Dekker; 1996.
• Gillette M. Flavor effects of sodium chloride. Food Technology. 1985;39(6):47–52.
• Glass K, Doyle ME. Safety of processed cheese FRI Briefings. Madison, WI: Food Research Institute; 2005. .
• Greenwood, N. N., and Earnshaw, A. (1997). Chemistry of the Elements, 2nd edition. Boston: Butterworth-Heinemann.
• G. L. Eskew, Salt, the Fifth Element (1948); D. W. Kaufmann, ed., Sodium Chloride (1968); G. Mamantov and R. Marassi, ed., Molten Salt Chemistry (1987).
• Guinee TP, O’Kennedy BT. In: Reducing salt in cheese and dairy spreads, Reducing salt in foods: Practical strategies. Kilcast D, Angus F, editors. Cambridge, UK: Woodhead; 2007. pp. 316–357.
• Hatch, M.D. and Slack, C.R. (1966). ‘Photosynthesis by sugar-cane leaves: a new carboxylation reaction and the pathway of sugar formation’, Biochemical Journal, 101, 103–111.
• He FJ, MacGregor GA. In: Dietary salt, high blood pressure and other harmful effects on health, Reducing salt in foods: Practical strategies. Kilcast D, Angus F, editors. Cambridge, UK: Woodhead; 2007. pp. 18–54.
• Hedrick HB, Aberle ED, Forrest JC, Judge MD, Merkel RA. Principles of meat science. 3rd ed. Dubuque, IA: Kendall/Hunt; 1994.
• Hutton T. Sodium: Technological functions of salt in the manufacturing of food and drink products. British Food Journal. 2002;104(2):126–152.
• Kono, Suminori, Masato Ikeda, and Michiharu Ogata. "Salt and Geographical Mortality of Gastric Cancer and Stroke in Japan." Journal of Epidemiology and Community Health 37 (1983): 43–46.
• Kilcast D, Angus F, editors. Reducing salt in foods: Practical strategies. Cambridge, UK: Woodhead; 2007.
• Kuntz LA. Fighting warmed-over flavor. Food Product Design. 2000. [accessed November 10, 2008].
• Laragh, John H. "Atrial Natriuretic Hormone, the Renin-Aldosterone Axis, and Blood Pressure—Electrolyte Homeostasis." New England Journal of Medicine 313 (1985): 1330–1340.
• Leistner L, Gorris LG. Food preservation by hurdle technology. Trends in Food Science and Technology. 1995;6(2):41–46.
• Leistner L, Gould JW. In: Update on hurdle technology approaches to food preservation, Antimicrobials in food. 3rd ed. Davidson PM, Sofos JN, Larry BA, editors. Boca Raton, FL: Taylor and Francis; 2005. pp. 621–631.
• Levens, Nigel R., Michael J. Peach, and Robert M. Carey. "Role of Intrarenal Renin-Angiotensin System in the Control of Renal Function." Circulation Research 48 (1981):157–167.
• Lewis RJ Sr. Food additives handbook. New York: Van Nostrand Reinhold; 1989.
• Lobstein T, Macmullan J, McGrath T, Witt J. Cereal offences: A wake-up call on the marketing of unhealthy food to children, Junk Food Generation. London: Consumers International; 2008.
• Man CM. In: Technological functions of salt in food products, Reducing salt in foods: Practical strategies. Kilcast D, Angus F, editors. Cambridge, UK: Woodhead; 2007. pp. 157–173.
• Matthews K, Strong M. Salt: Its role in meat products and the industry’s action plan to reduce it. Nutrition Bulletin. 2005;30(1):55–61.
• National Research Council. Recommended Dietary Allowances. 10th ed. Washington, D.C.: National Academy Press, 1989, pp. 247–261.
• Oliver, Walter J., Erik L. Cohen, and James V. Neel. "Blood Pressure, Sodium Intake and Sodium-Related Hormones in the Yanomamo Indians, a 'No-Salt' Culture." Circulation 52 (1975): 146–151.
• Reddy KA, Marth EH. Reducing the sodium content of foods: A review. Journal of Food Protection. 1991;54(2):138–150.
• Roberts TA, McClure PJ. Food preservatives and the microbiological consequences of their reduction or omission. Proceedings of the Nutrition Society. 1990;49:1–12.
• Rosett TR, Hamill T, Morris K, Klein BP. Taste qualities of reduced-sodium soups as affected by serving temperature. Journal of Food Science. 1997;62(2):421–424.
• Ruusunen M, Niemisto M, Puolanne E. Sodium reduction in cooked meat products by using commercial potassium phosphate mixtures. Agricultural and Food Science in Finland. 2002; 11(3):199–207.
• Rybka-Rodgers S. Improvement of food safety design of cook-chill foods. Food Research International. 2001; 34(5):449–455.
• Sanchez-Castillo, C. P., S. Warrender, T. P. Whitehead, and W. P. James. "An Assessment of the Sources of Dietary Salt in a British Population." Clinical Science 72 (1987): 95–102.
• Sheng, Hwai-Ping. "Sodium, Chloride, and Potassium." In Biochemical and Physiological Aspects of Human Nutrition, edited by Martha H. Stipanuk, pp. 686–710. Philadelphia: W. B. Saunders Co., 2000.
• Shelef LA, Seiter J. In: Indirect and miscellaneous antimicrobials, Antimocrobials in food. 3rd ed. Davidson PM, Sofos JN, Larry BA, editors. Boca Raton, FL: Taylor and Francis; 2005. pp. 573–598.
• Shirreffs SM, Casa DJ, Carter R III. Fluid needs for training and competition in athletics. Journal of Sports Sciences. 2007;25(Supplement 1):83–91.
• Sinar LJ, Mason M. Sodium in four canned vegetables. Journal of the American Dietetic Association. 1975;66(2):155–157.
• Stringer SC, Pin C. Microbial risks associated with salt reduction in certain foods and alternative options for preservation: Technical report. Norwich, UK: Institute of Food Research; 2005.
• Van der Veer O. The human intake of salt and the development of low-sodium and no-salt-added processed foods and salt substitutes; A review of literature. Wageningen, Netherlands: University of Agriculture, Department of Human Nutrition; 1985.
• Vermeulen RT, Sedor FA, Kimm SYS. Effect of water rinsing on sodium content of selected foods. Journal of the American Dietetic Association. 1983;82(4):394–396.
• Vetter JL. Technology of sodium in bakery products. Cereal Food World. 1981;6:64–66.
• Weinberger, Myron H. "Salt Sensitivity of Blood Pressure in Humans." Hypertension 27 (1996): 481–490.
• Zaika LL, Fanelli JS. Growth kinetics and cell morphology of Listeria monocytogenes Scott A as affected by temperature, NaCl, and EDTA. Journal of Food Protection. 2003;66(7):1208–1215.
• Zhu, J. K. (2001). "Plant salt tolerance"
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









