 About Authors:
About Authors:
Kapil Sharma*, Priyanka sharma
*Yaresun Pharmaceutical Pvt Ltd, Jaipur, India
*pharma_kapil@rediffmail.com
INTRODUCTION
Gas chromatography is a widely used technique for the separation of gaseous and volatile sub which are difficult to separate.The primary limit of s technique is that sample must be capable of being volatilized without undergoing decomposition because of this this technique is replaced by H.P.L.C.It is similar to column chromatography except that the gas is used as the mobile phase instead of the liquid.Here gas as a mobile phase or moving phase is passed through a column a column containing liquid adsorbent coated on inert solid support ,thus the adsorption or partition is possible.Gas solid chromatography(G.S.C) based upon the selective adsorption on solid,so the component of mixture distributes them selves between the gas phase and adsorbent and the separation is due to adsorptive properties.where as Gas liquid chromatography (G.L.C) is based upon partition between Gas and immobile liquid coated on solid,.so the component of mixture distributes them selves between the gas phase and liquid adsorbent coated on solid and the separation is due to partition properties1.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1490
Advantages1
(1)This technique has very high resolution power -complex mixture can be resolved into its components by this method .The separation identification ,and determination of compound with little diff in Boiling point is possible by this technique.Ex: separation of methyl ester of oleaic ,stearic ,and linoleic acid is possible.
(2) the sensitivity of this method is quite high.as the Detector used in this method with the detection limit as low 10-12 to10-14g.for example with the thermal conductive detector has 100 ppm while flame ,electron capture can detect ppm and parts per billions(picrograms)(10-12g)
(3) It involves the relative simple instrument operation of gas chromatography &related calculation do not required highly skilled personnel and thus this technique is suitable for routine analysis.
(4) The analysis is complete in short time and give good accuracy and precision.
(5) qualitative and quantitative analysis at a time is possible because the area produce for each peak is directly proportional to the concentration of the sample.
[adsense:468x15:2204050025]
TECHNIQUE 2
Gas chromatography is special form of the chromatography in which moving or mobile phase is a gas and the stationary phase is either solid(G.S.C) or liquid (G.L.C).The technique is suitable for separation of material which is volatile with out decomposition.In this method the sample is introduced in moving gas stream and is carried out through column. The column contain either adsorbent(G.S.C) or liquid on a solid support t(this act as e stationary phase)The component of mixture sample distribute b/w two phase .the adsorption or solubility properties may differ from components to components. and therefore the components are carried out along the column at different rate and finally they emerge at the outlet of the column in distinct zone(peaks) separated by carrier gas.On emerging, the vapours of the components are detected by suitable detector accompanied by automatic recording.When sample in vapour phase is introduced in to the column by carrier gas , some molecules of sample get rapidly dissolved in liqu d and dynamic equilibrium attain. At equilibrium the concentration of molecule of each type is constant ratio in two phases.Ex. molecule of compound X distribute equally between the two phase and molecule of compound Y on other hand may be highly soluble in liquid phase.(i.e stationary phase)with the result that very few molecule in the vapour phase when equilibrium is attained.In this position the carrier gas will drag molecule X leaving those of Y behind in the column.Molecule X will be carried to the region containing fresh liquid and some of them get dissolved until equilibrium is reached.Mean while a fresh mobile gas comes over the liquid containing Y some of molecule Y will again enter in gas to reach equilibrium.Thus the volatile molecule will dragged continuously to the head.while less volatile molecule will fall back. they too are picked up and forwarded by continuous gas stream. And if the condition are right than clean separation is attain.The resolution of the chromatography peak is depend upon wo factors.Column efficiency and solvent efficiency .The column efficiency is related to the peak broadening of initially compact band as it passes down a column.Here the broadening is result from the column design, and operating condition and can be quantitavely described by H.E.T.P i.e height equivalent to heretical plate .Solvent efficiency result from the solute –solvent interaction and determines the relative position of solute blend on a chromatogram.How ever improved separation can be obtained by controlling the variable that increase the band separation(increase solvent efficiency)an or decreases the band broadening(increases column efficiency)
COLUMN EFFICIENCY-
Column efficiency is measured by the number of theoretical plates.The original theory of chromatography i.e .plate theory Was able to describe the effect of variable that Influence the migration rates in quantitative term.How ever the plate theory is unable to describe the effect of factor which are responsible for band broadening.Hence plate theory is supplemented by rate theory.
(a)plate theory
The plate concept is adapted from the theory of distillation columns. According to the theory a chromatographic column is composed of discrete, but continuous narrow horizontal plate.It is assumed that during chromatographic process the equilibration of the solute between mobile and the stationary phase take place at each theoretical plate. with step wise transfer of solute and solvent from one plate to next.The separation efficiency of chromatographic column increase with increasing number of theoretical plate .thus, the number of theoretical plate is can be measured from the chromatogram.
N=16(T/W)2
Where t = distance from injection point to peak maxima (retention time)and w= peak width.in unit of time which can be determine by drawining the tangents about 2/3 rd of the height to the peak at the point of inflaction.
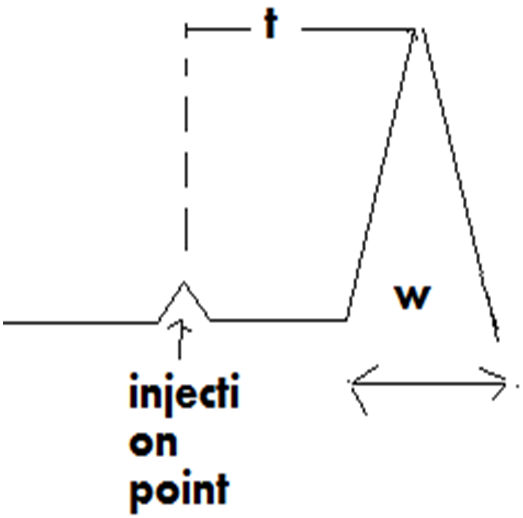
The second term affecting column efficiency is H.E.T.P which is the length of column necessary for attainment of solute equilibrium b/w mobile and stationary phase
H.E.T.P is related to the number of theoretical plate”N”
H.E.T.P=L/N= L/16(W/T)2
Where L=length of column usually in c.m
(b)Rate theory :The rate theory developed by van Deemter success fully describe the influence of variable that affect the band separation and band broadening.Van deemter equation is useful in optimizing the chromatographic performance and can be expressed as .
H.E.T.P=A+b/u+cu
Where A=eddy’s diffusion
B=longitudinal diffusion
C=mass transfer u =linear gas velocity (flow rate)through the chromatographic column.it is measured by
µ = length column(c.m)
retention time of air (second)
The influence of parameter ion separation efficiency has been discuss by keulemans .
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
(a) Eddys diffusion(Multiplepath)’A”:
In the packed column the solute and carrier gas travel along many path of different length thus solute molecule have different residence time. this result in the peak broadening .this broadening depend upon the size of the packing particles., the shape and manner of the packing and also on the column diameter. the term A i.e Eddys diffusion can be decresed by using smaller particle size but it is easier to obtain the packing with larger rather than smaller particle hence the particle size should be optimum .smaller particle also increase the pressure drop across the column leading to disturbance In linear gas velocity which ultimately decrease column efficiency . thus the Eddy diffusion can be minimized by using small particles of uniform size and smaller diameter columns . Generally the particle size up to 100-120 mesh range and the column with 1/8 inch inner diameter are used for good resolution.
(B) Molecular diffusion: Molecules from solution of high concentration tend to move to low concentration by diffusion. The phenomenon of broadening by diffusion occurs during the travel of band of the band .this is known as the loncitudinal diffusion.It is proportional to the solute diffusivity in the carrier gas .High solute diffusitivity leads to band broadening with consequent loss of efficiency. Solute diffusion in the liquid phase is extremely small than the gas ,hence can be neglected. Diffusivity is the property of both solute and carrier gas and may be reduced by increasing the pressure or molecular weight of the gas .thus the molecular diffusion can be decreased by using the optimum linear gas velocity and using high molecular weight of the carrier gas E.g nitrogen or argon than hydrogen or helium.
(c) Resistance to mass transfer “C” term.
This term describe the effect of the amount and viscosity of liquid on the solid support .A low viscosity low vapour pressure solvent with good absolute and differential solubility for the sample should be used also a low liquid loading (1-10%) have the advantage of fast analysis and lower temperature operation. Low liquid loadings, how ever reduce the sample capacity and may require highly in active solid supports .lowering the temperature improves the resolution and decrease the decomposition of the compounds but at the same time may increase the adsorption and time of analysis .Criteria for compound to be analysed by gas chromatography.
Two important criteria are:
1. Volatility: Unless a compound is volatile, it can not be mixed with mobile phase. Hence volatility is important.
2. Thermostability: All the compound are not in the form of vapour. there will be solid as well as liquid sample. Hence it is difficult to convert them in to vapour form so they have to be heated to a higher temperature.At that temperature the compound have to be thermo stable. If they are not thermo stable then they can not be analyzed by gas chromatography.
PRACTICAL REQUIRMENTS3
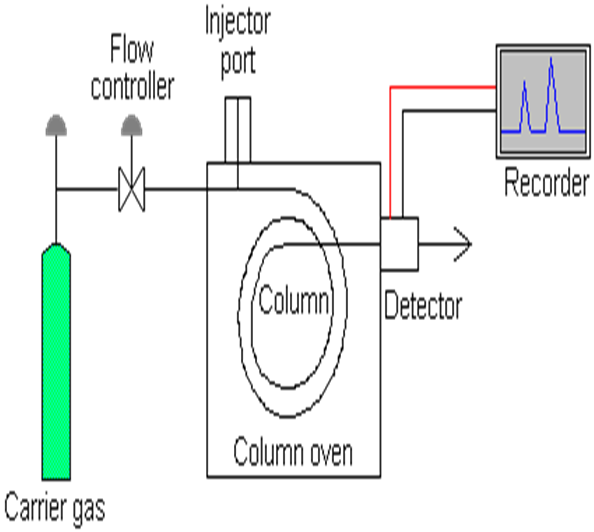
Fig: schematic diagram of gas chromatography.
1. carrier gas.
2. Flow regulator and flow meter.
3. Injection devices.
4. Columns.
5. Temperature control device.
6. Detector.
7. Recorders and integrators.
(1) CARRIER GAS: The main purpose of carrier gas is to transport the sample compoments through the column. For selection of the carrier gas, following factor must be conider.
(a) It should be chemically inert and should not interact with the sample or stationary phase.
(b) It should be suitable for the detector to be utilized and the type of the sample or stationary phase.
(c) It should give best column performance consistent with the desired speed of analysed.
(d) It should be readily available cheap and of high purity.
(e) It should not cause the risk of fire or explosion hazard.
Most widely used are Hydrogen, Helium, Nitrogen, and Argon.
Hydrogen: It has better thermal conductivity ,low density.it is useful in case of thermal conductivity detector and flame ionization detector. The disadvantages is that it reacts with unsaturated compounds and it is inflammable.
Helium:it also has excellent thermal conductivity, but it is expensive.it is good carrier gas when used with thermal conductivity detector.
Nitrogen: it is in expensive but has reduce sensitivity.
By considering the requirements ,and a compromising among the inertness efficiency and operating cost .Make the nitrogen and helium as the most common carrier gas. As carrier gas are compressible it must be stored under high pressure in cylinder and used when required.
(2)Flow regulator and flow meter:
As the carrier gas are stored at high pressure flow regulator are used to deliver the gas with uniform pressure or flow rate.Flow meter are used to measure the flow rate of carrier gas. They are Rotameter and soap bubble flow meter.
Rota meter: it is placed conveniently before the column inlet . it has an ordinary glass tube (like burette) with a float held on to a spring .the level of the float is determine by the flow rate of carrier gas and is precalibrated.

(a) soap bubble meter (b) Rotameter
Soap bubble meter: it is similar to rotameter and instead of float , soap bubble formed indicates the flow rate.it has a glass tube with a inlet tube at the bottom through which gas comes in .A rubber bulb is used to store the soap solution when the bulb is gently pressed , a drop of soap solution is converted in to a bubble by the pressure of carrier gas and travel up. The distance travelled upwards is measure of flow rate of carrier gas.
(3) Sample injection device: The sample injection system is very important because one of the feature of gas chromatography is the use of very small amount of the sample. this system must introduce the sample in a reproducible manner and must vaporized it instantaneously so that the sample will enter the column as a single slug.
Liquid samples are generally introduced by hypodermic syringe through a self sealing rubber septum in to a small inlet chamber, which may be heated to cause flash evaporation .
Solid sample must be dissolved in volatile liquids for introduction or may be introduced directly if they can be liquefied.
Gas samples require special gas sampling valves for introduction in to the carrier gas stream.
(4)Columns: The column can be constructed of glass or metal tubing and for analytical work it has 4.8 meter diameter . It can be of any length from few centimeter to over a hundred meter and can be of coiled ,bent or straight.
Three type of column are generally used.
(1)packed column.
(2) open tubular column.
(3) support coated open tubular column.(SCOT)
(a) Packed column.: packed column are prepared by packing metal or glass tubing with granular stationary phase. For G.S.C the column are packed with size graded adsorbent or porous polymer,where as for G.L.C the packing is prepared by coating the liquid phase over a size graded inert solid support. A wide variety of stationary phase like poly ethyleneglycols, high molecular weight ester, amides, hydrocarbons, polysiloxanes, microporous cross-linked poly aromatic beds.
The advantages of using porous material are
(i) There is no column –bleed.Most of the porous polymer are stable up to the 2500c and cause no base line drift. It there for ,allows the use of highly sensitive detector.
(ii) There is no adsorption of porous compound such as water alcohols, or acids and they are eluted rapidly as sharp symmetrical peaks.
(iii) Retention data are highly reproducible.
(iv) Some of the separation provided are unique.
(v) Porous chemical band are mechanically strong and can be packed on column.
(b) Open tubular column:These column are also called as capillary column OR golay column. They are made up of long capillary tubing (30-90meters) having uniform and narrow inernal diameter of(0.025-0.075c.m).they are made up of stainless steel ,copper, nylon ,or glass etc. the stainless steel being the most popular. The inside wall of the capillary tubing is coated with liquid phase in the form of a thin (0.5-1micron)and uniform film .
These column offers the least restance to the flow of carrier gas and hence they are most efficient than the packed column. But here disadvantages is that more sample can not be loaded.
(c) Support coated open tubular column (S.C.O.T): This is an improved version of golay column or capillary columns.As golay or capillary column have small sample capacity ,they can be modified in to S.C.O.T column.
These columns are made by depositing a micron size porous layer of support material on the inner wall of of the capillary column and then coated with a thin film of liquid phase. These column also have reistance to the flow of carrier gas but offer the advantages of more sample load.
(5) Temperature control devices.
Preheater: pre heater are used in Gas chromatography to convert the sample in to its vapour form and mix them with the mobile phase or carrier gas. The preheater are present along with the injection devices.As soon as liquid sample are injected ,they are converted in to vapour form.
Thermostatically controlled oven: The principle of separation in gas chromatography is partition. Partition co-efficient is the ratio of concentration of of a solute distributed between two immiscible liquids. Since partition co-efficient as well as solubility of a solute is depend upon temperature, so the temperature maintenances in column in highly essential for efficient temperature. Hence column as well as injection device should be maintained at a particular temperature.
For this two type of operation are available:
(i)Isothermal programming : (iso means same)
In which the same temperature is maintained throught the process.
(ii)Linear programming: in which the oven is heated lineary over a period of time Eg 1500C initially to 2000Cat the end of separation.with increase the temperature rate of 50c/minutes. this required when the sample contain mixture of low nd high boiling point temperature.
(6)Detectors:- detectors are the most important part of the gas chromatographic instruments.they are consider as a heart of the apparatus. A detector uses some property by which it can detect the difference between a pure carrier gas and elutd components.
The requirements of an ideal detector are:
i. Applicability to wide range of samples.
ii.High sensitivity to even small concentration.
iii.Rapidity of responces.
iv. Linerity i.e less response to low concentration and proportional response to high concentration.
v. Responance should be un affected by the temperature, flow rate, and character of carrier gas
vi. simple and easy to maintain.
vii. in expensive.
(1) Thermal conductivity detect OR Katharometer:
The Principle is based upon thermal conductivity difference between carrier gas and that of the components. Kathrometer has two platinum wire of uniform dimension which form a part of a wheatstone bridge.Through one of them pure carrier gas always flows through and through the other ,the effulents of the column passes. The two platinum wires are heated electrically and hence assume equilibrium condition of temperature and electrical resistance. When pure gas passes through both of them , there is no difference in temperature or resistance of the wire.Hence this produce a difference in resistanceand so conductivity between two wires,which ic amplified and recorded as a signal.
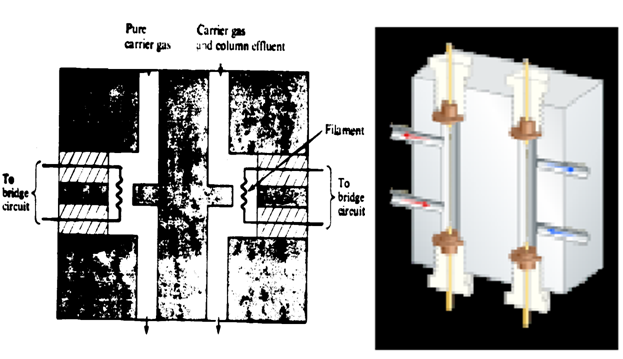
Fig: thermal conductivity detector.
The thermal conductivities of some carrier gases are given as follows.
|
H2
|
He |
N2
|
Methane |
Hexane |
|
32.7 |
33.9 |
6.5 |
5.2 |
3 |
From the above colum it can be state that the Hydrogen and Helium have higher thermal conductivity and they are the best carrier gas for Kathrometer. Hydrogen is inflammable and helium is expensive but both of them have good responces.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Advantages:
• Responds to all compounds
• Adequate sensitivity for many compounds
• Good linear range of signal
• Simple construction
• Signal quite stable provided carrier gas glow rate, block temperature, and filament power are controlled
• Nondestructive detection
Disadvantages:
· Low sensitivity.
· Affected by fluctuations in temperature and flow rate.
· The response in only relative and absolute.
· Biological sample can not be analysed.
(2)Flame ionisation Detector.
A tiny film of hydrogen is maintained at the capillary jet made of quartz or platinum, air or oxygen is introduced through a side by inlet for supporting the combustion. column effluent are led in to the flame where in ionization of components may take place. An electrode system located close by picks up the ionization current which is then amplified and fed to recorder when only
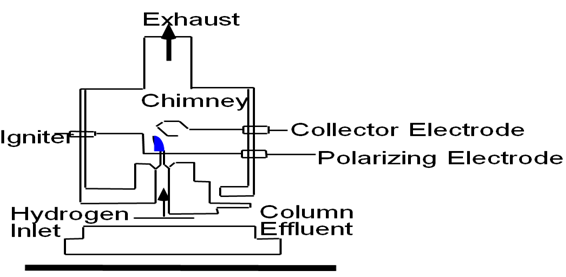
Fig: scematic diagram of Flame ionization detector.
carrier gas passing through flame,there is or very small and constant ionization current recorded as study base line.when the sample compoments elutes and passs through the flame,its molecule are ionized and the resulting ionization current after amplification is fed in to suitable recorder.A FID is sensitive to all organic compound but not insensitive to the noble gases, oxygen, nitrogen, co,co2, water, nitrous oxide,H2s,so2,cs2.
Advantages:
This detector is extermly sensitive and back ground noise is low hene µg quantities of sample can be determine.Stable and in sensitive to small changes in the flow rate to carrier gas.
(3) Electron capture device:The electron capture detector has two electrode ,with column effluentpassing between them. One of the electrode is treated with radioactive isotope which emits the electron as it decays.the emitted electron produce secondary electrons, which are collected by the anode,when the potential of 20V is applied between them.
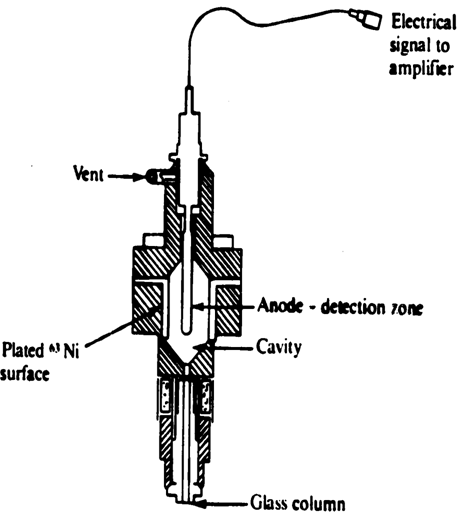
Fig: schematic diagram of electone capture detector
when carrier gas alone pass through all secondary electron are collected by the positively polarized electrode. Hence a steady base line is recorded.Effluent molecule which have affinity for electron ,capture these electrons when they pass through the electrodes.Hence the amount of steady state current is reduced. This diff is amplified and recorded as out put signal.
The carrier gas used in this type of detector depends upon the electron affinity of the compound analyzed .for compound having more electron affinity argon is used as a carrier gas, compound having less affinity to electron nitrogen , co2 is used as carrier gas.
Advantages Highly sensitive. Even Nano gram quantities can be determine but it has dis advantages that this can be used only for which has affinity to electron.
RECORDER:
Recorders are used to record the response obtained from the detectors after amplification in gas chromatography generally potentiometric detector is used.In this type of recorder the input response is continuously balanced by feed back response. a pan connected through this system moves proportionally along the width of the chart paper, thus recording the signal .At the same time the chart paper moves at a fixed speed along its length. Before the operating a recorder ,its zero should be recorded .
Integrators.
An integrator is employed for simultaneous measurement of areas under chromatographic peaks by the electric /mechanical means .Manually measurement of this techniques is tedious ,time consuming, and less precise. Electronic integrators print out of the peak area digital and give highest peak area
Application 4,5
1 Quality analysis : it is nothing but the identification of a compound .this is done by comparising the retention time of sample as well as standard. Under identical condition .the retention time of the standard and sample are same . if there is deviation than they are not same compound.Retention time meansit the difference between the point of injection and the peak maxima . it is the time required for 50% of component to be eluted from the column . it measured in time in second or minutes.
2 checking the purity of the compound: By comparing the chromatogram of the standard and that of the sample, the purity of the compound can be reported. If the additional peak are present and hence the compound is not purified from the percentage area of the peaks obtained. The percentage purity can also be reported.
3 presence of impurity: This can be seen by the presence of additional peaks when compared to the reference or standard material. The percentage impurities may also be calculated from peak areas.
4 Quantitative analysis: The quantity of the compound can be determine by the following method.
ADirect comparing method.By injecting a sample and standard separately and comparing their peak areas , the quantity of the sample can be determine.
A1/A2=W1/W2
Where A1and A2are peak of the sample and standard W1 and W2 are the concentration or weight of sample and standard.
B calibration curve method.
In this method the standard of various concentration are used to determine their peak areas. A graph of peak area v/s concentration is plotted .from the peak area of the unknown sample the concentration of the unknown is calculated by interpolation method.
C.Internal standard method.
In this method , a compound with similar retention characteristics is used. A known concentration of the internal standard is added separately to the standard solution and sample solution whose concentration is not known. The chromatogram is recorded and the peak area ratio of the sample and internal standard is determine. By using the peak area ratio of standard and internal standard , the concentration of the unknown solution is determined. By using the peak ratio of sample and internal standard , the concentration of the unknown solution is determined .this method is useful when more extraction step are involved in sample preparation and the sample preparation and the sample matrix is complex.
5 Multi components analysis or Determination of mixture of drugs: Similar to the quantification of a single drug , multi component, analysis is determined by using any one of the above methods. Marketed formulation are available which contain several drugs and each component can be determined quantitatively .
6 Isolation and identification of drug or metabolites in urine , plasma, serum etc can be carried out.
7 Isolation and identification of mixture of components like amino acids ,plant extracts, volatile oil etc.
REFERENCES
1. Sharma B.K. Instrumental Methods of Chemical Analysis, 18th Edition, 1999. Goel Publishing House, Meerut.
2. Jeffrey, et. al., Introduction: Vogel’s Textbook of Quantitative Chemical Analysis, 5th Edition, 1997, ELBS, Longman.
3. Skoog D.A. Holler F.J., Nieman D.A.; Introduction to UV Spectroscopy in, Principle of Instrumental Analysis, 5th ed., Thomson book/cole, 2004.
4. Willard, Merrit, Dean and Settle; H.P.L.C. Methods and Applications in; Instrumental methods of Analysis; 7th ed., New Delhi: C.B.S. Publishers and Distributors, 2003.
5. British Pharmacopoeia, Vol-1, 4th ed., Controller of Her Majesty,s Stationary Office, London, 2004.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









