About Authors:
Dolita Shah*, Mayur Mistry1
*M.Pharm (Q.A, Gold Medalist),
Asst. Professor, Smt. R. B. Patel Mahila Pharmacy College
1Production officer, Intas Pharmaceutical ltd,
Ahmedabad, India
Introduction
A new molecule can cost several millions of rupees or dollars to progress and any blunder causes greater impact on company’s status. As medicines play a vital role in human’s life there must be regulations for medicines ensuring Quality, Safety and Efficacy of drugs. The regulatory affairs professional is the only one who is completely responsible for holding products in compliance and maintaining all the records. One of the vital activities of the regulatory specialist is to ensure that the all the information regarding medicines has been correctly established to the patient covering labelling also. Even a small mistake in any of the activities related to regulatory can make the product to be recall in addition to loss of several millions of the money.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1239
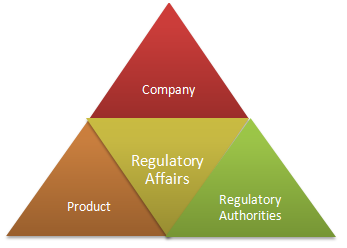 Drug development to commercialization is highly regulated. Every drug before getting market approval must undergo rigorous scrutiny and clinical trials to ensure its safety, efficacy and quality. These standards are set by regulatory authorities of their respective countries such as FDA in US and DCA in India etc. Regulation affects all aspects of the pharmaceutical world, from independent innovators and pharmaceutical companies to regulatory and administrative bodies and patients also. Regulatory department is crucial link between company, products and regulatory authorities whose positive or negative standpoint foster the insight of the regulatory authority into the industry, for good or for bad. So, the better the scientific precision, the greater will be the chances for a product to come to the market within the expected time.
Drug development to commercialization is highly regulated. Every drug before getting market approval must undergo rigorous scrutiny and clinical trials to ensure its safety, efficacy and quality. These standards are set by regulatory authorities of their respective countries such as FDA in US and DCA in India etc. Regulation affects all aspects of the pharmaceutical world, from independent innovators and pharmaceutical companies to regulatory and administrative bodies and patients also. Regulatory department is crucial link between company, products and regulatory authorities whose positive or negative standpoint foster the insight of the regulatory authority into the industry, for good or for bad. So, the better the scientific precision, the greater will be the chances for a product to come to the market within the expected time.
Regulation and regulatory Affairs1, 2
Regulation involves extensive evaluation of a particular drug product to ensure protection of public health, promotion of the product, Drug registration, marketing authorization, import and distribution, pharmacovigilance.
Regulatory Affairs is a comparatively new profession which has developed from the desire of governments to protect public health, by controlling the safety and efficacy of products in areas including pharmaceuticals, veterinary medicines, medical devices, pesticides, agrochemicals, cosmetics and complementary medicines.
Regulatory Affairs is a unique mixture of science and management to achieve a commercially important goal within a drug-development organization. Regulatory Affairs takes care of Development plan, supervising-writing / reviewing and assembling and submission management. They give strategic and technical advice at the highest level in their companies, right from the beginning of the development of a product, making an important contribution both commercially and scientifically to the success of a development programme and the company as a whole.
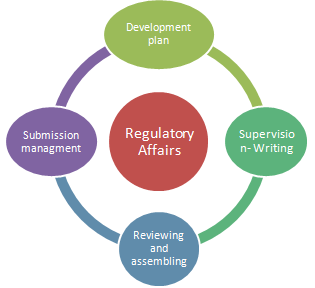
Generation of various Regulations2
Major incidences made us to understand that rules and regulations are required to prove safety along with efficacy of drug. Such incidences are as follows:
-Diphtheria Epidemic led to 1902 Biologics Control Act
-Publication of The Jungle by Upton Sinclair led to 1906 Pure Food and Drugs Act
-Elixir of Sulfanilamide led to the 1938 Food Drug and Cosmetic Act
-Thalidomide led to the 1962 Kefauver-Harris Amendments
-Dalkon Shield led to the 1976 Medical Device Amendments
-Bjork-Shiley Heart Valves led to the 1990 Safe Medical Devices Act
Passing of the 1938 Food Drug and Cosmetic Act, 4
Elixir sulfanilamide was an improperly prepared sulfanilamide medicine that caused mass poisoning in the United States in 1937. It caused the deaths of more than 100 people. The public outcry caused by these incident and other similar disasters led to the passing of the 1938 Federal Food, Drug, and Cosmetic Act.
In 1937, S. E. Massengill Company, a pharmaceutical manufacturer, created a preparation of sulfanilamide using diethylene glycol (DEG) as a solvent, and called the preparation "Elixir Sulfanilamide". DEG is poisonous to humans, but Harold Watkins, the company's chief pharmacist and chemist, was not aware of this (although it was known at the time). Watkins simply added raspberry flavoring to the sulfa drug which he had dissolved in DEG and the company then marketed the product. Although animal testing should have been routine in most drug company operations, Massengill performed none and there were no regulations requiring premarket safety testing of new drugs.
The company started selling and distributing the medication in September 1937. By October 11, the American Medical Association received a report of several deaths caused by the medication. The Food and Drug Administration was notified, and an extensive search was conducted to recover the distributed medicine. Frances Oldham Kelsey assisted on a research project that verified that the excipient DEG was responsible for the fatal adverse effects. At least 100 deaths were blamed on the medication.Congress responded to public outrage by passing the 1938 Food, Drug, and Cosmetic Act, which required companies to perform animal safety tests on their proposed new drugs and submit the data to the FDA before being allowed to market their products.
Qualities of a good RA professional
Ø Authoritative
Ø Team Player
Ø Decisive
Ø resourceful
Ø Good Communication Skill
Ø Analytical Skill- Ability to evaluate the strengths and weakness of the technical and legal options open to a company.
Ø Good Informational Technology skills
Ø Negotiating Skills
Ø Able to reapply scientific and regulatory principles
Ø Ability to work with other disciplines
Ø Flexible- Always willing to learn.
Responsibilities of Regulatory Affairs Department
Ø Keep in touch with international legislation, guidelines and customer practices
Ø Keep up to the date with a company’s product range
Ø Ensure that a company’s products comply with the current regulations.
Ø The Regulatory Affairs professional’s job is to keep track of the ever-changing legislation in all the regions in which the company wishes to distribute its products. They also advise on the legal and scientific restraints and requirements, and collect, collate, and evaluate the scientific data that their research and development colleagues are generating.
Ø Formulate regulatory strategy for all appropriate regulatory submissions for domestic, international and/or contract projects.
Ø Coordinate, prepare and review all appropriate documents for example dossier and submit them to regulatory authorities within a specified time frame in conjugation with the organization.
Ø Prepare and review of SOPs related to RA. Review of BMR, MFR, change control and other relevant documents.
Ø Monitor the progress of all registration submission.
Ø Maintain approved applications and the record of registration fees paid against submission of DMF’s and other documents.
Ø Respond to queries as they arise, and ensure that registration/ approvalare granted without delay.
Ø Impart training to R&D, Pilot plant, ADl and RA. Team members on current regulatory requirements.
Ø Advising their companies on the regulatory aspects and climate that would affect proposed activities. i.e. describing the "regulatory climate" around issues such as the promotion of prescription drugs and Sarbanes-Oxley compliance.
Ø Manage review audit reports and compliance, regulatory and customer inspections.
Ø Regulatory Affairs professionals help the company avoid problems caused by badly kept records, inappropriate scientific thinking or poor presentation of data. In most product areas where regulatory requirements are imposed, restrictions are also placed upon the claims which can be made for the product on labelling or in advertising.
Ø Have a duty to provide physicians and other healthcare professionals with accurate and complete information about the quality, safety and effectiveness of the product.
Why is Regulatory Affairs important? 1
In today’s competitive environment the reduction of the time taken to reach the market is vital to a product’s and hence the company’s success. The proper conduct of its Regulatory Affairs activities is therefore of considerable economic significance for the company.
Inadequate reporting of data may prevent a timely positive evaluation of a marketing application. A new drug may have cost many millions of pounds, Euros or dollars to develop and even a three-month delay in bringing it to the market has considerable financial considerations. Even worse, failures to fully report all the available data or the release of product bearing incorrect labelling, may easily result in the need for a product recall.
A good Regulatory Affairs professional will have a ‘right first time’ approach and will play a very important part in coordinating scientific endeavour with regulatory demands throughout the life of the product, helping to maximise the cost-effective use of the company’s resources. The Regulatory Affairs department is very often the first point of contact between the government authorities and the company. Officials respond much better to a company whose representatives are scientifically accurate and knowledgeable than to one in which these qualities are absent.
Regulatory Affairs Profession
The (Healthcare) Regulatory Affairs Profession is still an emergent profession but has two major international professional membership societies:
The Regulatory Affairs Professionals Society, RAPS,
The Organisation for Professionals in Regulatory Affairs, TOPRA,
In Canada, the major professional membership society is: The Canadian Association of Professional Regulatory Affairs, CAPRA,
Major Regulatory Authorities
|
Country |
Regulatory Authority |
|
India |
Central Drugs Standard Control Organization Drug controller general of India (DCGI) |
|
US |
Food and Drug Administration (US FDA) |
|
UK |
Medicines and Health care products regulatory Agency (MHRA) |
|
Australia |
Therapeutic Goods Administration (TGA) |
|
Japan |
Japanese Ministry of health, Labour and Welfare (MHLW) |
|
Canada |
Health Canada |
|
Brazil |
Agency Nacional degradation Vigilancia Sanitaria (ANVISA) |
|
South Africa |
Medicines Contol Council (MCC) |
|
Europe |
European Directorate for Quality of Medicines (EDQM) |
|
|
European Medicines Evaluation agencies (EMEA) |
References:
1. topra.org/careers/what-regulatory-affairs (Jan 2012)
2. en.wikipedia.org/wiki/Regulatory_affairs (Dec 2011)
3. Quality in the Manufacture of Medicine and other healthcare products, Pharmaceutical press, chapter.3, 29-41.
4. reg-info.com/
5. www.raps.org
6. topra.org
7. capra.ca
8. farmavitar.net
9. cdsco.nic.in
10. fda.gov
11. mhra.gov.uk
12. tga.gov.au
13. mhlw.go.jp
14. hc-sc.gc.ca
15. anvisa.gv.hr
16. mccza.com
17. edqm.eu
18. ema.europe.eu
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









