ABOUT AUTHOR
R.VENKATESAN1*, S.GOKULRAJ1
KAMALAKSHI PANDURANGAN COLLEGE OF PHARMACY,
TIRUVANNAMALAI-606601, TAMILNADU, INDIA.
venkatesan20200@gmail.com
ABSTRACT
Drug discovery is a process which aims at identifying a compound therapeutically useful in curing and treating disease. This process involves the identification of candidates, synthesis, characterization, validation, optimization, screening and assays for therapeutic efficacy. Once a compound has shown its significance in these investigations, it will initiate the process of drug development earlier to clinical trials. New drug development process must continue through several stages in order to make a medicine that is safe, effective, and has approved all regulatory requirements. One overall theme of our article is that the process is sufficiently long, complex, and expensive so that many biological targets must be considered for every new medicine ultimately approved for clinical use and new research tools may be needed to investigate each new target. From initial discovery to a marketable medicine is a long, challenging task. It takes about 12 - 15 years from discovery to the approved medicine and requires an investment of about USD 1 billion. On an average, a million molecules screened but only a single is explored in late stage clinical trials and is finally made obtainable for patients. This article provides outline of the processes of new drug discovery and development.
INTRODUCTION:
Drug discovery is a multifaceted process, which involves identification of a drug chemical therapeutically useful in treating and management of a disease condition. Typically, researchers find out new drugs through new visions into a disease process that permit investigator to design a medicine to stopover or contrary the effects of the disease.[1] The process of drug discovery includes the identification of drug candidates, synthesis, characterization, screening, and assays for therapeutic efficacy. When a molecule avails its satisfactory results in these investigations, it will commence the process of drug development subsequent to clinical trials. Drug discovery and development is an expensive process due to the high budgets of R&D and clinical trials. It takes almost 12-15 years to develop a single new drug molecule from the time it is discovered when it is available in market for treating patients.[2] The average cost for research and development for each efficacious drug is likely to be USD 900 million to USD 2 billion. This figure includes the cost of the thousands of failures: For every 5,000-10,000 compounds that enter the investigation and development pipeline, ultimately only one attains approval. These statistics challenge imagination, but a brief understanding of the R&D process can explain why so many compounds don‘t make it and why it takes such a large, lengthy effort to get one medicine to patients.[3] The Success requires immense resources the best scientific and logical minds, highly sophisticated laboratory and technology; and multifaceted project management. It also takes persistence and good fortune.[4] Eventually, the process of drug discovery brings hope, faith and relief to billions of patients.[5]
STEPS INVOLVE IN DRUG DICOVERY AND DEVELOPMENT PROCESS INCLUDES:
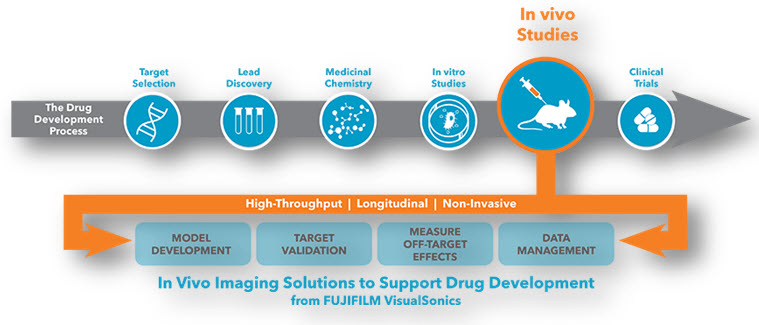
1. Target selection
2. Lead discovery
3. Medicinal chemistry
4. In vitro studies
5. In vivo studies
6. Clinical trials and therapeutics
1. TARGET SELECTION:
• Target selection in drug discovery is defined as the decision to focus on finding an agent with a particular biological action that is anticipated to have therapeutic utility is influenced by a complex balance of scientific, medical and strategic considerations.
• Target identification: to identify molecular targets that are involve in disease progressions.
• Target validation: to prove that manipulating the molecular target can provide therapeutic benefit for patients.
• Biochemical classes of drug target
• G-Protein coupled receptors -45%
• Enzymes -28%
• Hormones and factors -11%
• Ion channels -5%
• Nuclear receptors -2%
Techniques for target identification:
1. Cellular and genetic target:
Involves the identification of the function of a potential therapeutic drug target and its role in the disease process. For small molecular drugs, this step in the process involves identification of the target receptors or enzyme whereas for some biologic approaches the focus is at the gene or transcription level. Drugs usually act on either cellular or genetic chemicals in the body, known as targets, which are believed to be associated with disease.
Scientific use a variety of techniques to identify and isolate individual targets to learn more about their functions and how they influence disease. Compounds are then identified that have various interactions with the drug targets that might be helpful in treatment of a specific disease.
2. Genomics:
The study of genes and their function. Genomics aims to understand the structure of the genome, including the mapping genes and sequencing the DNA. Seeks to exploit the findings from the sequencing of the human and other genomes to find new drug targets. Human genome consists of a sequence of around 3 billion nucleotide (the A C G T bases) which in turn probably encodes 35,000-50,000 genes.
Drew’s estimates the number of genes implicated in disease, both those due to defects in single genes and those arising from combinations of genes, is about 1,000. Based on 5 or 10 linked proteins per gene, he proposes that the number of potential drug targets may lie between 5,000 and 10,000.single nucleotide polymorphism (SNP) libraries: are used to compare the genomes from both healthy and sick people and to identify where their genomes vary.
3. Proteomics:
It is the study of the proteome, the complete set of proteins produced by a species, using the technologies of large scale protein separation and identification. it is becoming increasingly evident that the complexity lf biological systems lies at the level of the proteins, and that genomics alone will not suffice to understand these systems. it also at the protein level that disease processes become manifest, and at which most(91%) drugs act.therefore,the analysis of proteins (including protein-protein, protein-nucleic acid, and protein ligand interactions )will be utmost importance to target discovery.
Proteomics is the systematic high throughput separation and characterization of proteins within biological systems. Target identification with proteomics is performed by comparing the protein expression levels in normal and diseased tissues. 2D PAGE is used to separate the protein, which are subsequently identified and fully characterized with LC-MS/MS.
4. Bioinformatics:
Bioinformatics is a branch of molecular biology that involves extensive analysis of biological data using computers, for the purpose of enhancing biological research.
It plays a key role in various stages of the drug discovery process including
• Target identification
• Computer screening of chemical compounds and
• Pharmacogenomics [7].
2. LEAD DISCOVERY:
A. Lead identification:
Between 5 and 50000 compounds are examined in the laboratory, of which only 100 to 200 are perfected in order to be tested on systems in vitro and in vivo.
Once the therapeutic target has been identified, scientists must then find one or more leads (e.g., chemical compounds or molecules) that interact with the therapeutic target so as to induce the desired therapeutic effects, e.g., through antiviral or antibacterial activity.
In order to discover the compounds whose pharmacological properties are likely to have the required therapeutic effects, researchers must test a large variety of them on one or more targets.
First of all, biologists ensure that the chosen compounds have the desired therapeutic or antiviral effects on the target. Then, they test the compounds relative toxicity or in the case of vaccine, their viral activity using in vitro cellular and/or tissue systems. Finally, they check their bioavailability in vivo on animals. in vitro cellular and/or tissue systems. Finally, they check their bioavailability in vivo on animals.
B.Lead Optimization:
Duration: From 4 to 6 months
The 100 to 200 chosen compounds are examined in the laboratory in order to perfect their physiochemical properties, their pharmacokinetic behavior and the therapeutic effectiveness. Around twenty (20) will be selected to be tested on.
The purpose of this stage is to optimize the molecules or compounds that demonstrate the potential to be transformed into drugs, retaining only a small number of them for the next stages.
To optimize these molecules, scientists use very advanced technique. For example, using X-ray crystallography and in silico (computer) modeling, they study how the selected molecules link themselves to the therapeutic target, for example, a protein or an enzyme. These data allow the medical chemists to modify the structure of the selected molecules or compounds, if necessary, by screening, thereby creating structural analogues.[8]
3. MEDICINAL CHEMISTRY:
Medicinal chemists prepare and/or select appropriate compounds for biological evaluation that, if found to be active, could serve as lead compounds. They then evaluate the structure–activity relationships (SARs) of analogous compounds with regard to their in vitro and in vivo efficacy and safety. Today, medicinal chemists who are engaged in drug discovery are part of interdisciplinary teams, and must therefore understand not only the field of organic chemistry, but also a range of other disciplines to anticipate problems and interpret developments to help move the project forward. As highlighted in this article, the role of the medicinal chemist has changed significantly in the past 25 years. In the early era (‘then’) of drug discovery (1950 to about 1980), medicinal chemists relied primarily on data from in vivo testing. In the more recent (‘now’) period (about 1980 to the present), the development of new technologies, such as high-throughput in vitro screening, large compound libraries, combinatorial technology, defined molecular targets and structure based drug design, has changed that earlier and relatively simple landscape. Although these new technologies present many opportunities to the medicinal chemist, the multitude of new safety requirements that have arisen has also brought unanticipated hurdles for the task of translating in vitro activity to in vivo activity. Simultaneously, the knowledge base that supports drug research has expanded considerably, increasing the challenge for chemists to understand their fields of expertise. The demonstration of adequate clinical safety and efficacy in humans has also become more complex, and ever-increasing amounts of data are now required by regulatory agencies. In fact, despite the use of many new technologies, and the growing resources and funding for drug research, the number of launches of new medicines in the form of new molecular entities (names) has been generally decreasing for more than a decade. Clearly, the difficulty and complexity of drug research has increased in the past two decades. It is our aim with this article to discuss how these changes have influenced the role of medicinal chemists and to suggest ways to help them to contribute more effectively to the drug discovery process [9].
4. INVITRO STUDIES:
In vitro studies are an essential part of research geared toward the discovery of drug candidates. Driven by the need for predictive information, in vitro techniques have been developed to study many aspects of drug disposition. These include: absorption, metabolic stability, elucidation of elimination pathways, potential for inhibition of CYP enzymes, potential for induction of CYP450 enzymes and metabolite profiling in various model species and humans. These studies are not necessarily conducted following regulatory statute. On the other hand, many of these in vitro experiments become essential support for submission of IND and post IND filing from both the preclinical and clinical arena [10].
“This part prescribes good laboratory practices for conducting nonclinical laboratory studies that support or are intended to support applications for research or marketing permits for products regulated by the Food and Drug administration…”
““Nonclinical Laboratory Study” means in vivo or in vitro experiments in which test articles are studied prospectively in test systems under laboratory conditions to determine their safety… The term does not include basic exploratory studies carried out to determine whether a test article has any potential utility or to determine physical or chemical characteristics of a test article.” [11].
5. INVIVO STUDIES:
In vivo studies, in comparison to in vitro, take place within a living organism. In preclinical trials, this happens within animal subjects. In clinical trials, in vivo studies can use either humans or animals as subjects,In vivo studies are able to address the major limitation of in vitro studies, they are able to demonstrate the impact of a pharmaceutical on the body as a whole, rather than how it impacted isolated cells. This allows in vivo studies to better visualize potential interactions, which can improve its predictions of safety, toxicity, and efficacy. This helps scientists predict the impact of candidate drugs on human disease.
However, while in vivo studies address the drawback of in vitro studies, they have their own major setback. In vivo studies face significant ethical concerns, particularly for preclinical studies where just animal models are permitted. The debate over the ethics of animal testing has raged for decades.
Currently, the regulations and laws governing animal testing are tightening, and preclinical in vivo studies scientists wishing to conduct preclinical studies with animals are required to demonstrate that no other alternative methodology can be used to conduct the experiment. They are also required to demonstrate a sense of balance, that is, the benefits of the study (gain in knowledge) outweigh the drawbacks (suffering caused to the animals).
Like in vitro studies, in vivo studies are also undergoing a technological transformation. Emerging technologies such as crisper will make complex animal models increasingly simple to conduct, cheaper, and faster.
While in vivo studies face significant ethical considerations, it is likely that they will remain a fundamental part of preclinical studies. The future is predicted to bring significant advances in preclinical technologies, both in vitro and in vivo, that should facilitate the gathering of more accurate data, as well as faster and simpler methodologies. It is expected that these advances will improve the quality of preclinical data, as well as reduce the reliance on traditional animal models.[12]
6. CLINICAL TRIALS:
A clinical trial is a prospective biomedical or behavioral research study of human subjects that is designed to answer specific questions about biomedical or behavioral interventions (vaccines, drugs, treatments, devices, or new ways of using known drugs, treatments, or devices).
Clinical trials are used to determine whether new biomedical or behavioral interventions are safe, efficacious, and effective.
Clinical trials include behavioral human subject’s research involving an intervention to modify behavior (increasing the use of an intervention, willingness to pay for an intervention, etc.)
Human subjects research to develop or evaluate clinical laboratory tests (e.g. Imaging or molecular diagnostic tests) might be considered to be a clinical trial if the test will be used for medical decision-making for the subject or the test itself imposes more than minimal risk for subjects.
Biomedical clinical trials of experimental drug, treatment, device or behavioral intervention may proceed through four phases:
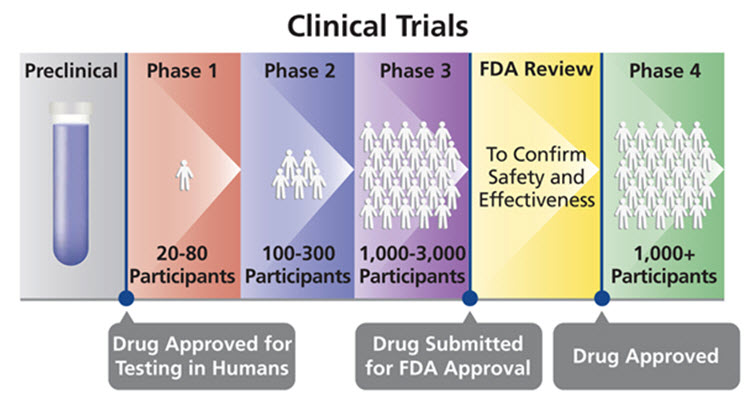
Phase I : clinical trials test a new biomedical intervention in a small group of people (e.g., 20- 80) for the first time to evaluate safety (e.g., to determine a safe dosage range, and to identify side effects).
Phase II : clinical trials study the biomedical or behavioral intervention in a larger group of people (several hundred) to determine efficacy and to further evaluate its safety.
Phase III studies investigate the efficacy of the biomedical or behavioral intervention in large groups of human subjects (from several hundred to several thousand) by comparing the intervention to other standard or experimental interventions as well as to monitor adverse effects, and to collect information that will allow the intervention to be used safely.
Phase IV : studies are conducted after the intervention has been marketed. These studies are designed to monitor effectiveness of the approved intervention in the general population and to collect information about any adverse effects associated with widespread use.[13]
CONCLUTION:
The drug discovery and development of new medicines is a long completed process. research based pharmaceutical companies are committed to advancing science and bringing new medicines to patients. Increased support from government and organization may helps in development safer and cost effective medicines.s
REFERENCE:
1. Shayne CG. Introduction: drug Discovery in the 21stCentury. Drug Discovery Handbook, Wiley Press, 2005; 1-10.
2. Smith GC, OíDonnel JT. The Process of New Drug Discovery and Development, Eds., 2nd edition, Informa Healthcare, New York 2006.
3. Moffat J, Vincent F, Lee J, Eder J, Prunotto M. Opportunities and challenges in phenotypic drug discovery: an industry perspective. Nature Reviews Drug Discovery, 2017; 16(8):531-543.
4. DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. Journal of Health Economics, 2003; 151-185.
5. Gashaw I, Ellinghaus P, Sommer A, Asadullah K. What makes a good drug target. Drug Discovery Today, 2012; 17:S24-S30.
6. https://www.visualsonics.com/application/preclinical/drug-discovery-development.
7. https://www.slideshare.net/rahul_pharma/drug-discovery-and-development-10698574.
8. https://www.pharmatutor.org/articles/review-lead-optimization-techniques
9. https://www.nature.com/articles/nrd1523
10. Yuan, R.; Parmelee, T.; Balian, J.D.; Uppoor, R.S.; Ajayi, F.; Burnett, A.; Lesko, L.J.; Marroum, P. Pharmacokinetics and Drug Disposition In vitro metabolic interaction studies: Experience of the Food and Drug Administration. Clin. Pharm. Ther. 1999, 66, 9-15.
11. Good Laboratory Practice For Nonclinical Studies, U.S. Code of Federal Regulations, Title 21, Chapter I, Part 58 April 1, 2000.
12.INVITRO AND INVIVO STUDIES:
• Gargiulo, G., 2018. Next-Generation in vivo Modeling of Human Cancers. Frontiers in Oncology, 8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6192385/
• Højelse, F., 2000. Preclinical Safety Assessment: In vitro - in vivo Testing. Pharmacology & Toxicology, 86, pp.6-7. https://pubmed.ncbi.nlm.nih.gov/10905745/
• Lorian, V., 1988. Differences between in vitro and in vivo studies. Antimicrobial Agents and Chemotherapy, 32(10), pp.1600-1601. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC175930/
13. https://docs.gatesfoundation.org/documents/clinical_trials.pdf
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT admin@pharmatutor.org
FIND OUT MORE ARTICLES AT OUR DATABASE









