About Authors
Vivekanandan Kalaiselvan, Shatrunajay Shukla, Santhanakrishnan Ramesh Kumar,
Nikita Mishra, Pawan Kumar and Rajeev Singh Raghuvanshi
Abstract
The pharmacovigilance has been evolved as a professional and ethical practice in ensuring the safety of medicines. The Adverse Drug Reactions (ADRs) associated with the use of medicines including Anti-Tuberculous Therapy (ATT) through a robust system of pharmacovigilance helps in promoting the safety of patients at large. The occurrence of ADRs associated with the use of ATT is expected, a large number of medicines are combined and used for prolonged duration. The suspected ADRs associated with first line ATT are well documented. However, the drugs used in second line or multidrug resistant to tuberculosis (TB), namely bedaquiline, reported to cause QT prolongation in electrocardiogram reading as one of the most common ADRs. Therefore, early identification and prevention of ADRs during ATT is essential for promoting the rational use and reduce the burden of anti-microbial resistance, besides achieving better treatment outcomes.
Introduction
The unfortunate tragedy of thalidomide, in 1962, triggered the emergence and implementation of pharmacovigilance across the globe [1]. Thalidomide was introduced in Germany in 1957 and was widely prescribed for the treatment of morning sickness and nausea in pregnant women. Later it was found that babies were born with shortened or absence of limbs (medically known as phocomelia). In 1962, thalidomide was discontinued from the market due to the increased number of scientific reports describing numerous cases of phocomelia [2]. This tragedy led to the creation of the World Health Organization (WHO) pilot research project for International Drug Monitoring in 1968, with the purpose to develop a system and tools applicable internationally, for detecting previously unknown or poorly understood adverse drug reactions (ADRs) of medicines [3]. Currently, this network has been expanded to more than 140 developed, low- and middle-income countries. These 140 countries participated in the WHO programme for international drug monitoring as member states, and 31 countries have also joined as associate member New Insights into the Future of Pharmacoepidemiology and Drug Safety 2 states. These countries have established pharmacovigilance system at their capacity, to monitor the medication safety. WHO and its collaborating centres are continuously providing technical support for capacity building and strengthening of these pharmacovigilance systems [4].
As per WHO, Pharmacovigilance is defined as a “science of detection, assessment, understanding and prevention of ADRs or any other drug related problems” [5]. This enables the scope of clinical practice of monitoring & reporting of ADRs, analyses the information and sharing the learnings with healthcare providers for prevention of such ADRs, for better patient’s safety and outcomes. Pharmacovigilance and its concepts are evolving as one of the most important components in contemporary clinical and regulatory practice. In clinical trials, most medicines will only be tested for short-term safety and efficacy on a limited number of carefully selected individuals (excluding pregnant women, children and elderly). In some cases, as few as 500, and rarely more than 5000, subjects receive the investigational new drug prior to its release [6]. It is not possible to identify and record many ADRs in such a shorter duration, protected environment and restricted population in trials. After stage three of clinical trial, the medicine is available to be launch in the market and is legally set free for consumption by the general population. Post market experience has shown that many adverse effects, interactions (i.e. with foods or other medicines), and risk factors may come to light even after several years of introducing the medicine into the market [7]. Moreover, many studies have shown that an ADR may result into a significantly decrease in the quality of life, increased hospitalizations, prolonged hospital stay and mortality [8]. Therefore, monitoring the safety of the medicines throughout its life period is pivotal, as most of the ADRs are usually reported during prolonged use.
The pharmacovigilance practice applies equally to medicines used in public health programs, including medicines used in Anti-Tubercular Therapy (ATT). As the management of tuberculosis (TB) involves longer duration of therapy and also multiple drugs, these arise as predisposing factors for the occurrence of ADRs [9]. Such ADRs pose a challenge in the management of TB. Though it is a prolonged treatment, medication must be continued in order to ensure the compliance, otherwise it will end with treatment failure or developing antimicrobial resistance [10]. Generally, patients discontinue the medication due to the emergence of ADRs resulting from the administration of first-line anti-TB drugs. During the course of TB treatment, there may be a risk of morbidity and mortality, particularly with drug-induced hepatitis. Therefore, there are public health program in various countries that systematically monitor, prevent and manage ADRs encountered during the treatment of TB, in order to achieve maximum treatment outcomes [11].
TB is a chronic infection caused primarily by Mycobacterium tuberculosis. The lung is generally the first affected organ, as the infection is usually due to inhalation of infected droplet nuclei. Approximately 80% of the TB cases are pulmonary TB [12]. Around 30% patients who are infected with Human Immune Deficiency Virus (HIV) will also develop active tuberculosis. Factors, such as HIV, Resistant TB, drug–drug interactions raise the complexity of problem. As per the WHO strategy, directly observed treatment short-course (DOTS) therapy for the duration of 6–8 months is one of the important components for the treatment of TB. The short-course therapy is usually performed in 2 phases: the initial phase (2 months) involves the concurrent use of at least 3 drugs to rapidly reduce the bacterial population and prevent emergence of drug-resistant bacteria. The second, continuation phase, (4–6 months) involves fewer drugs and is used to eliminate any remaining bacteria and prevent recurrence. Worldwide, HIV infection has been identified as an important predisposing factor of immune-suppression leading to TB [13]. It increases the susceptibility to primary infection and increases the reactivation rate of TB [14]. Although this regimen is effective in treating active TB, it is associated with many ADRs and poses a significant challenge to completion of treatment. Recommended treatment regimens for TB are given in Table 1.

Importance of ADR reporting in tuberculosis
Multiple types of drug therapy are given for TB, and even new TB patients (sensitive to first-line drugs), are receiving a treatment regimen with a combination of four drugs [15]. There is a chance for developing ADR either for one or the combination of drugs, and that has to be identified for ensuring a sustained treatment compliance, till the completion of ATT. When treatment is given to patients with TB-associated drug resistance, either ionized resistance, multidrug resistance or rifampicin resistance, pre-extensively drug resistance or extensively drug resistance TB, the number of drugs given could be higher, and it becomes imperative to identify the resulting/associated ADRs. In case any ADR takes place, the treatment management has to be done appropriately [16]. For TB patients having HIV co-infection, the treatment given for HIV infection, including the antiretroviral therapy, and/or the medication given for the associated conditions, may overlap with the ADR presented, and so it becomes very important to monitor this group of population for efficient management. In addition, also in TB patients with special medical conditions associated, like associated diabetes mellitus, liver, renal or seizure disorders, and psychosis, the treatment should be done cautiously, by closely observing the progress and monitoring all the ADRs encountered. Furthermore, when new drugs like Bedaquiline (BDQ), Delamanid (DLM) and Pretomanid are initiated at TB programs, it is essential that the associated ADRs are captured promptly for effective management of TB [17].
ADRs associated with first-line anti-TB drugs
The ATT is expected to cause more ADRs, because it involves combination of several medicines and is used for a longer duration [9]. One of the most common ADRs observed with the administration of ATT is gastrointestinal symptoms, such as nausea, vomiting etc. These ADRs could be symptomatically managed without the need for a change in the dosage of drugs. The hepatotoxicity is also a risk associated with ATT, and its frequency can range from 2–39% in different countries [18]. As compared to Western population, Indian sub-population studies reported high incidence of hepatotoxicity with ATT [19].
Isoniazid
Isoniazid has been shown to be well tolerated at recommended dose. However, systemic or cutaneous hypersensitivity reactions can occasionally occur during the first weeks of treatment [15]. By daily supplementary dose of pyridoxine in vulnerable patients, the risk of peripheral neuropathy can be excluded. In the later stages of treatement, some susceptible patients can develop neurological disturbance, encompassing optic neuritis, toxic psychosis and generalized convulsions. This may require the discontinuation of isoniazid. An uncommon but potentially serious reaction is symptomatic hepatitis, which could be precluded by prompt withdrawal of treatment. Asymptomatic rise in serum concentrations of hepatic transaminases at the beginning of treatment has very low clinical significance. The same resolves spontaneously as the treatment carry on. Other rare adverse effects linked with isoniazid are lupus-like syndrome, pellagra, anemia, and arthralgias [20].
Rifampicin
At currently recommended doses, this drug has been shown to be well tolerated by most of the patients. Occasionally it may cause gastrointestinal reactions including abdominal pain, nausea, vomiting and pruritus with or without rash [21]. With an intermittent drug administration, adverse effects, such as fever, influenza-like syndrome and thrombocytopenia may occur. In HIV-positive TB patients, exfoliative dermatitis is more common. Patients taking the drug 3 times a week, adverse effects including temporary oliguria, dyspnoea and haemolytic anemia have been reported. If the regimen is changed to daily dosage these reactions usually subsided. In the beginning of treatment, moderate rises in serum concentrations of bilirubin and transaminases are common adverse effects are often transient and not clinical significant. A potentially fatal condition is dose-related hepatitis, it is therefore important to not exceed the maximum recommended daily dose of 600 mg.
Pyrazinamide
This drug has been reported to cause various skin reactions, like maculopapular rash, erythema multiforme, exfoliative dermatitis and drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Among the first-line drugs, pyrazinamide has shown to be the most common drug to cause cutaneous ADRs [22]. Pyrazinamide may cause gastrointestinal intolerance. Hypersensitivity reactions are rare, but have been reported in some patients with modest flushed skin. During the early phases of the treatment, moderate rises in serum transaminase concentrations are common. A rare complication is severe hepatotoxicity. A degree of hyperuricaemia may also occur asymptomatically as a result of inhibition of renal tubular secretion [15]. The treatment may also result into gout, which can be treated with allopurinol. Arthralgia, especially of the shoulders, may occur which can be treated with simple analgesics (especially aspirin). By prescribing regimens with intermittent administration of pyrazinamide, hyperuricaemia and arthralgia may be eliminated. Sideroblastic anemia and photosensitive dermatitis are some of the rare ADRs associated with this drug [7, 8].
Streptomycin
Streptomycin injections are painful, and rash, induration, or sterile abscesses can be formed at injection sites. Numbness and tingling around the mouth occur immediately after injection and cutaneous hypersensitivity reactions can occur. The incidence of ototoxicity associated with the use of ATT may be as high as 25% [23]. With currently recommended doses, the complications like impairment of vestibular function are uncommon. Vertigo is more common than hearing loss. Indications of injury at the 8th cranial (auditory) nerve include ringing in the ears, ataxia, vertigo and deafness. The damage is impermanent and can be reversed by reducing in dosage, or the stopping the treatment with this drug. This damage is commonly occurs within the first 2 months of treatment. More commonly, the other aminoglycoside antibiotics e.g. kanamycin, amikacin and capreomycin are more nephrotoxic than streptomycin. If urinary output falls, albuminuria occurs, or tubular casts are detected in the urine, streptomycin should be stopped, and renal function should be evaluated. Though WHO’s recommendation is not to use injectable streptomycin, we should take into consideration that other recommended treatments with aminoglycosides may cause similar types of ADRs [17].
Ethambutol
Dose-dependent optic neuritis caused by Ethambutol can result in impairment of visual acuity and color vision in one or both eyes. Early changes are usually reversible, but blindness can occur if treatment is not discontinued promptly. Ocular toxicity is rare when ethambutol is used for 2–3 months at recommended doses. Peripheral neuropathy has been reported in approximately 20% of patients treated with ethambutol. Other rare adverse events include generalized cutaneous reaction, arthralgia and, very rarely, hepatitis [24]. Several studies have reported that the drugs used to treat TB may cause ADRs. Management and prevention of such ADRs are important measures to be adopted to increase tolerance. Generally, with non-serious ADRs, the drugs do not need to be stopped, while with serious ADRs, the drugs often have to be stopped and a modified regimen has to be implemented [9].
Capreomycin
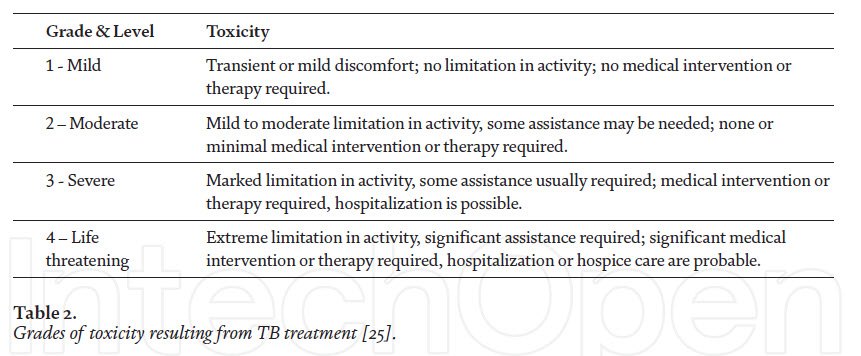
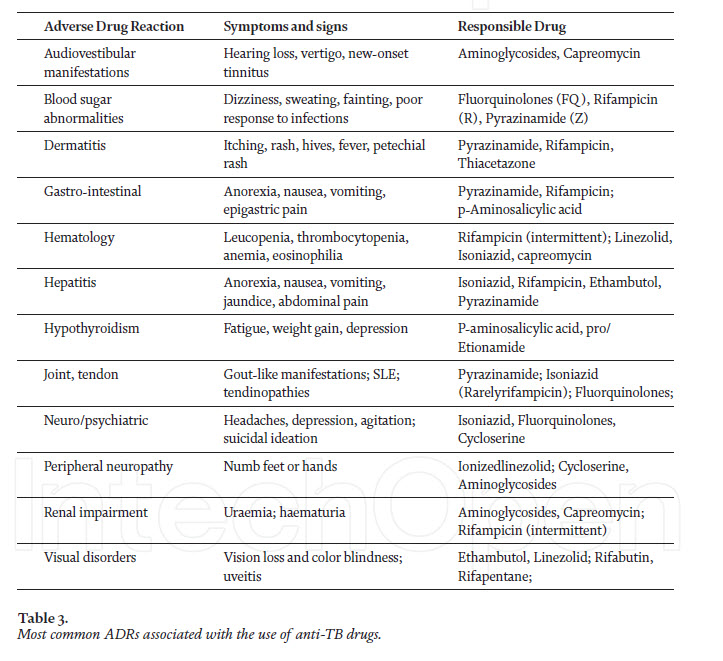
This drug is administered in combination with other first-line drugs. The common ADRs reported are hypersensitivity reactions, including urticaria and rashes, nephrotoxicity, electrolyte disturbance, hearing loss wit tinnitus and vertigo [11]. Grading of toxicity associated with drugs used for TB treatment and the ADRs associated with the anti-TB drugs used for therapy are given in Tables 2 and 3, respectively.
ADRs associated with second-line anti-TB drugs Resistant -TB is usually treated with a combination of drugs that are more toxic than isoniazid and rifampicin. These drugs include fluoroquinolones, aminoglycosides, ethionamide, cycloserine, aminosalicyclic acid, linezolid and clofazimine, among others [26]. The main ADRs associated with the use of cycloserine are reported as neurological disorders, including headache, dizziness, vertigo, drowsiness, tremor, convulsions, confusion, psychosis, depression, rashes, allergic dermatitis, megaloblastic anemia, and changes in liver function tests [27]. Minor adverse effects are relatively common, and they can be easily managed with symptomatic treatment. However, some adverse effects can be life-threatening, for example, nephrotoxicity due to aminoglycosides, cardiotoxicity due to fluoroquinolones, gastrointestinal toxicity due to ethionamide or para-amino-salicylic acid, central nervous system toxicity due to cycloserine, etc. [17].
Multi Drug-resistant
TB (MDR-TB) MDR-TB is caused by organisms that are resistant to isoniazid and rifampicin. As per the WHO reports, an estimated 480 000 worldwide patients developed MDR-TB in 2015, in addition to the 100 000 patients with rifampicin-resistant TB that were newly eligible for MDT-TB treatment [22]. Again, according to WHO, the second highest MDR-TB incident country in the world, China, accounted for 45% of the 580 000 cases, together with Indian and the Russian Federations, with 6.6% of new TB cases and 30% of previously treated cases having MDR/Rifampicin resistant TB. The novel anti tubercular drugs, namely BDQ and DLM, now included in WHO second-line treatment [28], as well as in some countries, have received conditional approval for use in adults with MDR-TB. BDQ, a new anti TB- drug, has been given approval by the United States Food and Drug Administration in 2012 [29], and by the European Medicines Agency in 2014. In India, BDQ was introduced under the conditional access program in 2015. The safety profile and tolerability of a BDQcontaining treatment regimen used in India has been established. QT prolongation in electrocardiogram reading has been reported as one of the most common ADRs with the use of BDQ; the others include peripheral neuropathy, vomiting, breathlessness and thrombocytopenia [30].
Prevalence of adverse events associated with second-line anti-TB drugs in children Children, especially those under 10 years old, can tolerate second-line combination of anti-TB drugs better than adults. In children, the higher rate of ADRs has been observed in those having HIV as comorbid infection, as compared to TB infection alone [14]. Several studies have also revealed that the majority of the adverse events found in children are mild to moderate, thus not requiring interruption or complete cessation of treatment. Moreover, even with the occurrence of few severe adverse events, permanent discontinuation of drugs is rarely necessary [14]. The second–line drugs are generally found to cause more ADRs, as compared to the first-line drugs [31]. The healthcare workers treating children should be aware of this fact and should thus be able to manage such ADRs. Healthcare workers, care givers or parents are required to be trained accordingly, because most of the children may not be able to report the drug-associated ADRs. The MDR-TB treatment outcomes in children are well achieved in many countries by using the currently available drugs [32, 33]. However, the improvement of the MDR-TB treatment programme can be achieved by: (1) implementing targeted or cohort event monitoring of adverse events, with the use of MDR-TB drugs in children; and (2) healthcare works training for a timely ADRs reporting, aiming to achieve the maximum treatment outcomes.
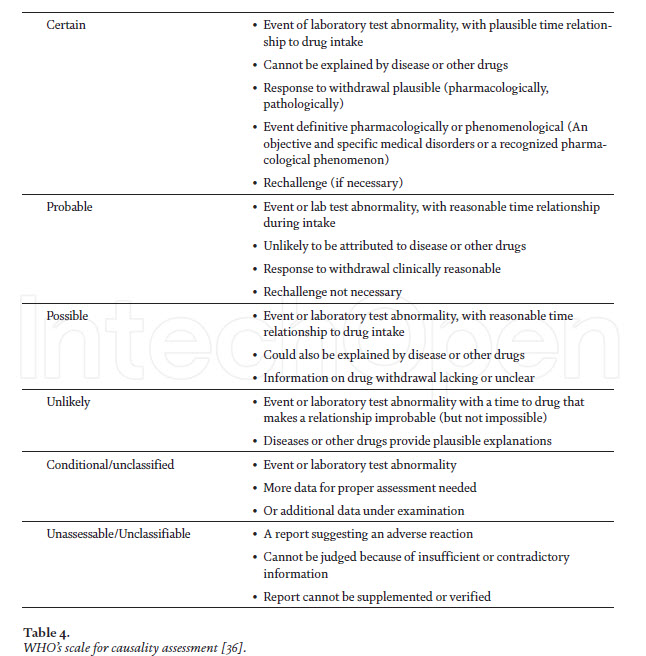
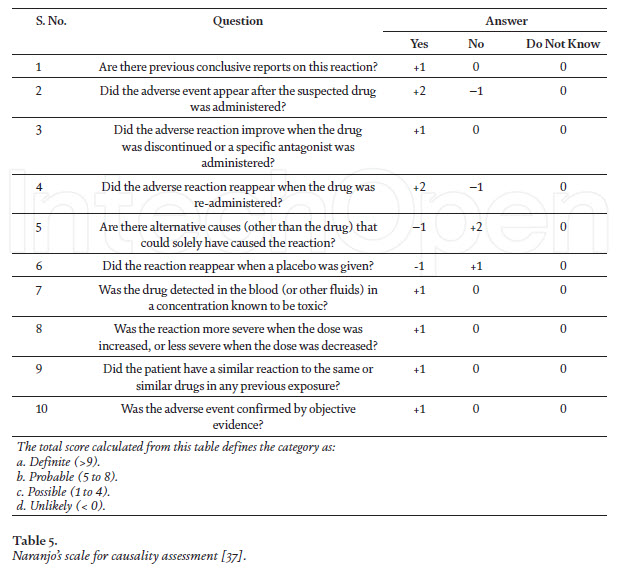
Causality and severity assessment of anti-TB drugs-associated adverse events After determining the adverse events (suspected) of anti-TB drugs, the very next step is to establish the causal or temporal relationship between the drug and the event, i.e., is the drug actually causing the event? It is possible that the administered drug and the occurrence of an adverse event may have a close temporal relationship, but still not be a reaction [34]. Having considered the parameters in assessing the temporal relationship, the next step is to address the following question: “Did these medicines actually cause the event?” In other words, “Is the event a reaction?” It is conceivable/acceptable that the administration of a medicine and the occurrence of an event may have a close relationship, but still not be a reaction, for example, death from myocardial infarction. In actual practice, the assessment of the relationship and causality frequently merge, particularly when an event is a well-known reaction and the relationship is close. The two phases occur without conscious deliberation, but should be there nevertheless. However, it is often necessary to gather other knowledge about the medicine, the patient and the event, in order to undertake a deliberate evaluation of these factors, which are actually external to the drug–event association that has occurred. Causality assessment is the methodological approach for evaluating a signal (identification of new safety alert) [35]. As per WHO, the causality assessment scale is the estimated strength of the relationship between the drug and the ADR can be classified as certain, probable, possible, unlikely, conditional/unclassified,unassessable/unclassifiable (Table 4). The Naranjo scale can also be applied for causality assessment, and is algorithm-based (Table 5) [38]. The severity assessment of ADRs can also be categorized in to into seven levels of severity level 1 and 2 are considered less severe or mild, levels 3 and 4 are moderate, and levels 5, 6 and 7 are classified as severe [39]. Severe level of ADRs includes all potentially life threatening ADRs, and the ones causing permanent damage or requiring intensive medical care. Even some other assessment scales classify severe and lethal.
Conclusions
The emergence of ADRs continues to remain an important public health issue worldwide, as it is among the ten leading causes of mortality. Early identification and prevention of ADRs during TB treatment will lead to the rational use of medicines and to a reduce burden of antimicrobial resistance. Better adherence within the target population will reassure that monitoring and good communication on risks and benefits provide favorable implications for decisions on medicine procurement. Safety monitoring of medicines is thus a vital and crucial element of any health system. As TB treatment relies on a multi-drug therapy for long duration, the emergence of ADRs is inevitable. Therefore, ADR reporting is essential as it will strengthen the evidence, maximize the benefits and minimize the risks.
References
[1] N. Vargesson, “Thalidomide Embryopathy: An Enigmatic Challenge,” ISRN Developmental Biology, Oct. 31, 2013. https://www.hindawi.com/ journals/isrn/2013/241016/ (accessed Feb. 24, 2021).
[2] N. Vargesson, “Thalidomide-induced teratogenesis: history and mechanisms,” Birth Defects Res C Embryo Today, vol. 105, no. 2, pp. 140-156, Jun. 2015, doi: 10.1002/bdrc.21096.
[3] W. H. Organization, Handbook of resolutions and decisions of the World Health Assembly and the Executive Board. World Health Organization, 1973.
[4] D. N. Iessa, “Pharmacovigilance: New Challenges for WHO,” p. 52.
[5] “Pharmacovigilance - an overview | ScienceDirect Topics.” https://www. sciencedirect.com/topics/pharmacologytoxicology- and-pharmaceutical-science/ pharmacovigilance (accessed Feb. 24, 2021).
[6] J. A. Berlin, S. C. Glasser, and S. S. Ellenberg, “Adverse Event Detection in Drug Development: Recommendations and Obligations Beyond Phase 3,” Am J Public Health, vol. 98, no. 8, pp. 1366- 1371, Aug. 2008, doi: 10.2105/AJPH. 2007.124537.
[7] N. Raj, S. Fernandes, N. R. Charyulu, A. Dubey, R. G. S., and S. Hebbar, “Postmarket surveillance: a review on key aspects and measures on the effective functioning in the context of the United Kingdom and Canada,” Ther Adv Drug Saf, vol. 10, Jul. 2019, doi: 10.1177/2042098619865413. [8] R. I. Thomas, D. J. Cameron, and M. C. Fahs, “A Prospective Study of Delirium and Prolonged Hospital Stay: Exploratory Study,” Archives of General Psychiatry, vol. 45, no. 10, pp. 937-940, Oct. 1988, doi: 10.1001/ archpsyc.1988.01800340065009.
[9] “A practical handbook on the pharmacovigilance of medicines used in the treatment of tuberculosis.” Accessed: Mar. 17, 2021. [Online]. Available: https://www.who.int/docs/defaultsource/ documents/tuberculosis/apractical- handbook-on-thepharmacovigilance- of-medicines-usedin- the-treatment-of-tuberculosis. pdf?sfvrsn=6e5fc0cf_5.
[10] F. Imam et al., “Adverse drug reaction prevalence and mechanisms of action of first-line anti-tubercular drugs,” Saudi Pharmaceutical Journal, vol. 28, no. 3, pp. 316-324, Mar. 2020, doi: 10.1016/j.jsps.2020.01.011.
[11] “Pivotal role of Pharmacovigilance Programme of India in containment of antimicrobial resistance in India Agrawal V, Shrivastava TP, Adusumilli PK, Vivekanandan K, Thota P, Bhushan S - Perspect Clin Res.” https://www.picronline.org/article. asp?issn=2229-3485;year=2019;volume= 10;issue=3;spage=140;epage=144;aulast =Agrawal (accessed Mar. 17, 2021).
[12] C. Hoffmann, “Pulmonary tuberculosis in adults,” in Tuberculosis, Elsevier, 2009, pp. 332-341.
[13] T. T. Todorova, G. Tsankova, and N. L. and T. Kostadinova, “Tuberculosis and HIV — Doubling the Fatality,” Immunopathology and Immuno modulation, Nov. 2015, doi: 10.5772/61138.
[14] J. Bruchfeld, M. Correia-Neves, and G. Källenius, “Tuberculosis and HIV Coinfection,” Cold Spring Harb Perspect Med, vol. 5, no. 7, Jul. 2015, doi: 10.1101/ cshperspect.a017871.
[15] World Health Organization and Stop TB Initiative (World Health Organization), Eds., Treatment of tuberculosis: guidelines, 4th ed. Geneva: World Health Organization, 2010.
[16] “WHO_HTM_TB_2014.23_eng.pdf.” Accessed: Mar. 17, 2021. [Online]. Available: https://apps.who.int/iris/ bitstream/handle/10665/137334/ WHO_HTM_TB_2014.23_eng.pdf;jsessi onid=3F28C9E7FB3CC604788D92E5CE B971F8?sequence=1.
[17] World Health Organization, WHO consolidated guidelines on drug-resistant tuberculosis treatment. 2019.
[18] “Risk Factors of Hepatotoxicity During Anti-tuberculosis Treatment.” https://www.ncbi.nlm.nih.gov/pmc/ articles/PMC4923276/ (accessed Feb. 24, 2021).
[19] P. R et al., “Hepatic toxicity in South Indian patients during treatment of tuberculosis with short-course regimens containing isoniazid, rifampicin and pyrazinamide,” Tubercle, Jun. 1986. https://pubmed. ncbi.nlm.nih.gov/3775870/ (accessed Feb. 24, 2021).
[20] “WHO | Toman’s tuberculosis: case detection, treatment and monitoring: questions and answers (2nd edition),” WHO. https://www.who.int/tb/ publications/toman/en/ (accessed Feb. 24, 2021).
[21] “Treatment of Tuberculosis American Thoracic Society, CDC, and Infectious Diseases Society of America.” https://www.cdc.gov/mmwr/preview/ mmwrhtml/rr5211a1.htm (accessed Feb. 24, 2021).
[22] “Prevalence of adverse drug reaction with first-line drugs among patients treated for pulmonary tuberculosis - Clinical Epidemiology and Global Health.” https://cegh.net/ article/S2213-3984(15)00073−1/fulltext (accessed Feb. 24, 2021).
[23] M. Rd, S. Cr, and L. Ps, “Risk factors for the development of auditory toxicity in patients receiving aminoglycosides,” The Journal of infectious diseases, Jan. 1984. https://pubmed.ncbi.nlm.nih. gov/6693788/ (accessed Feb. 24, 2021). [24] X. Lv et al., “Adverse Reactions Due to Directly Observed Treatment Strategy Therapy in Chinese Tuberculosis Patients: A Prospective Study,” PLOS ONE, vol. 8, no. 6, p. e65037, Jun. 2013, doi: 10.1371/journal. pone.0065037. [25] C. Sekaggya-Wiltshire et al., “Anti-TB drug concentrations and drug-associated toxicities among TB/ HIV-coinfected patients,” J Antimicrob Chemother, vol. 72, no. 4, pp. 1172-1177, Apr. 2017, doi: 10.1093/jac/dkw534.
[26] K. E. Dooley et al., “Old Drugs, New Purpose: Retooling Existing Drugs for Optimized Treatment of Resistant Tuberculosis,” Clinical Infectious Diseases, vol. 55, no. 4, pp. 572-581, Aug. 2012, doi: 10.1093/cid/cis487.
[27] “National Formulary of India 5th Edition, 2016, Page 208.” Indian Pharmacopoeia Commission.
[28] M. Grzemska, “Updated WHO MDR-TB treatment guidelines and the use of new drugs in children,” Resid Pediatr, vol. 7, no. Supl, pp. 7-10, Oct. 2017, doi: 10.25060/residpediatr-2017. v7s1-03.
[29] S. Yadav, G. Rawal, and M. Baxi, “Bedaquiline: A Novel Antitubercular Agent for the Treatment of Multidrug- Resistant Tuberculosis,” J Clin Diagn Res, vol. 10, no. 8, pp. FM01–FM02, Aug. 2016, doi: 10.7860/ JCDR/2016/19052.8286.
[30] “Effectiveness and safety of bedaquiline under conditional access program for treatment of drug-resistant tuberculosis in India: An interim analysis - PubMed.” https://pubmed. 13 .
[31] “First– and Second–Line Drugs and Drug Resistance | IntechOpen.” https:// www.intechopen.com/books/ tuberculosis-current-issues-indiagnosis- and-management/first-andsecond- line-drugs-and-drug-resistance (accessed Feb. 24, 2021).
[32] H. H. Tola, K. J. Khadoura, W. Jimma, S. Nedjat, and R. Majdzadeh, “Multidrug resistant tuberculosis treatment outcome in children in developing and developed countries: A systematic review and meta-analysis,” International Journal of Infectious Diseases, vol. 96, pp. 12-18, Jul. 2020, doi: 10.1016/j.ijid.2020.03.064.
[33] H. S. Schaaf, S. Thee, L. van der Laan, A. C. Hesseling, and A. J. Garcia- Prats, “Adverse effects of oral secondline antituberculosis drugs in children,” Expert Opin Drug Saf, vol. 15, no. 10, pp. 1369-1381, Oct. 2016, doi: 10.1080/14740338.2016.1216544.
[34] H. S. Rehan, D. Chopra, and A. K. Kakkar, “Physician’s guide to pharmacovigilance: Terminology and causality assessment,” European Journal of Internal Medicine, vol. 20, no. 1, pp. 3-8, Jan. 2009, doi: 10.1016/j. ejim.2008.04.019.
[35] “UMC | What is a signal?” https:// www.who-umc.org/research-scientificdevelopment/ signal-detection/what-isa- signal/ (accessed Mar. 17, 2021).
[36] S. A. Zaki, “Adverse drug reaction and causality assessment scales,” Lung India, vol. 28, no. 2, pp. 152-153, 2011, doi: 10.4103/0970-2113.80343.
[37] “ADVERSE DRUG REACTIONCAUSALITY ASSESSMENT.” Accessed: Mar. 17, 2021. [Online]. Available: http://www.ijrpc.com/files/00050.pdf.
[38] R. P. Naidu, “Causality assessment: A brief insight into practices in pharmaceutical industry,” Perspect Clin Res, vol. 4, no. 4, pp. 233-236, 2013, doi: 10.4103/2229-3485.120173.
[39] S. C. Hartwig, J. Siegel, and P. J. Schneider, “Preventability and severity assessment in reporting adverse drug reactions,” Am J Hosp Pharm, vol. 49, no. 9, pp. 2229-2232, Sep. 1992.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT admin@pharmatutor.org
FIND OUT MORE ARTICLES AT OUR DATABASE









