{ DOWNLOAD AS PDF }
ABOUT AUTHORS
M. Manasa Rekha1*, T.Mubeena2,
1*,2 Doctor of pharmacy,
Department of Pharmacy Practice,
Annamacharya college of Pharmacy,
Rajampet, Y.S.R kadapa district, Andhra Pradesh, India.
* manasarekharoyal@gmail.com
ABSTRACT:
The area of the pharmacy concerned with science and practice of rational usage of the drugs. The clinical pharmacist is the one of the member in the health care team. clinical pharmacists provide care to their patients and that this practice can occur in any practice setting. Drug Utilization Reviews (DUR), also referred to as Drug Utilization Evaluations (DUE) or Medication Utilization Evaluations (MUE), are defined as an authorized, structured, ongoing review of healthcare provider prescribing, pharmacist dispensing, and patient use of medication. DURs involve a comprehensive review of patients' prescription and medication data before, during, and after dispensing to ensure appropriate medication decision making and positive patient outcomes.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2529
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 5, Issue 10 Received On: 07/06/2017; Accepted On: 20/06/2017; Published On: 01/10/2017 How to cite this article: Rekha MM, Mubeena T; A study on role of Doctor of Pharmacy in Drug Utilization Evaluation Pattern Analysis in inpatient units and reporting its comorbidities in a tertiary care teaching hospital; PharmaTutor; 2017; 5(10); 55-62 |
INTRODUCTION:
DOCTOR OF PHARMACY AS CLINICAL PHARMACIST:
The area of the pharmacy concerned with science and practice of rational usage of the drugs. The clinical pharmacist is the one of the member in the health care team. clinical pharmacists provide care to their patients and that this practice can occur in any practice setting. The clinical pharmacist’s application of evidence and evolving sciences the application of legal, ethical, social, cultural, and economic principles in the practice.

DRUG UTILIZATION : Drug Utilization Reviews (DUR), also referred to as Drug Utilization Evaluations (DUE) or Medication Utilization Evaluations (MUE), are defined as an authorized, structured, ongoing review of healthcare provider prescribing, pharmacist dispensing, and patient use of medication. DURs involve a comprehensive review of patients' prescription and medication data before, during, and after dispensing to ensure appropriate medication decision making and positive patient outcomes.
TYPES OF DRUG UTILIZATION :
DURs are classified into three categories:
• Prospective - evaluation of a patient's therapy before medication is dispensed.
• Concurrent - ongoing monitoring of drug therapy during the course of treatment.
• Retrospective - review of therapy after the patient has received the medication.
PROSPECTIVE DUR:
A Prospective DUR involves evaluating a patient's planned drug therapy before a medication is dispensed. This process allows the pharmacist to identify and resolve issues before the patient actually receives the medication. Pharmacists routinely perform prospective reviews in their daily practice by assessing a prescription Medication's dosage and directions and reviewing patient information for possible drug interactions or duplicate therapy.
ISSUES COMMONLY ADDRESSED BY PROSPECTIVE DUR:
• Drug-disease contraindications.
• Therapeutic interchange.
• Generic substitution.
• Incorrect drug dosage.
• Inappropriate duration of drug treatment.
• Drug-allergy interactions.
• Clinical abuse/misuse.
CONCURRENT DUR:
A Concurrent DUR is performed during the course of treatment and involves the ongoing monitoring of drug therapy to ensure positive patient outcomes. Some refer to this as case management or health management. It presents pharmacists with the opportunity to alert prescribers to potential problems and to intervene in areas such as drug-drug interactions, duplicate therapy, over or under utilization, and excessive or insufficient dosing. This type of review allows therapy for a patient to be altered if necessary. Concurrent DURs often occurs in institutional settings.
ISSUES COMMONLY ADDRESSED BY CONCURRENT DUR:
• Drug-drug interactions.
• Excessive doses.
• High or low dosages.
• Duplicate therapy.
• Drug-disease interactions.
• Over and underutilization.
• Drug-age precautions.
• Drug-gender precautions.
• Drug-pregnancy precautions.
RETROSPECTIVE DUR:
In retrospective DUR, patient medical charts or computerized records are screened to determine whether the drug therapy met approved criteria and aids prescribers in improving care for their patients, individually and within groups of patients, such as those with diabetes, asthma, or high blood pressure.
ISSUES COMMONLY ADDRESSED BY RETROSPECTIVE DUR:
• Therapeutic appropriateness.
• Over and underutilization.
• Appropriate generic use.
• Therapeutic duplication.
• Drug-disease contra indications .
• Drug-drug interactions .
• Incorrect drug dosage.
• Inappropriate duration of treatment .
• Clinical abuse/misuse .
WHY DUR’S ARE IMPORTANT?
DUR programs play a key role in helping managed health care systems understand, interpret, and improve the prescribing, administration, and use of medications. Employers and health plans find DUR programs valuable because the results are used to foster more efficient use of scarce health care resources. Clinical Pharmacists play a key role in this process because of their expertise in the area of pharmaceutical care. DURs afford the managed care pharmacist the opportunity to identify trends in prescribing within groups of patients such as those with Chronic Diseases such as HIV, Cancer, asthma, diabetes, or high blood pressure etc. Pharmacists can then, in collaboration with other members of the health care team, initiate action to improve drug therapy for both individual patients and covered populations. DURs serve as a means of improving the quality of patient care, enhancing therapeutic outcomes, and reducing inappropriate pharmaceutical expenditures, thus reducing overall health care costs.
VALUES OF DUR PROGRAM:
Managed health care systems and pharmacy benefit management companies (PBMs) have the responsibility of managing the medication use in the client's membership. DUR programs are integral in helping to understand, interpret, and improve the prescribing, administration, and use of medications. DUR programs are able to provide physicians with feedback on their performance and prescribing behaviors as compared to pre-set criteria, accepted standards-of-practice or treatment protocols such as those established by national organizations such as the National Institutes of Health or the American Heart Association. DUR information also allows for the compare and contrasting of healthcare providers in order to evaluate a particular provider's approach to treating certain diseases against their peers. These comparisons are useful in stimulating physicians to change their prescribing habits in an effort to improve care. DUR information also assists managed health care systems and PBMs in designing educational programs that improve rational prescribing, formulary compliance, and patient compliance. These educational programs may take the form of face-to-face education of physicians and patients by clinical pharmacists, telephone calls, letters, newsletters, and educational symposia.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
AIM:
The study aims to assess the role of Doctor Of Pharmacy in drug utilization pattern analysis in inpatient units and reporting its comorbidities in a tertiary care teaching hospital.
OBJECTIVES:
The Main Objective Of The Present Study Is To Prevent The Following During Medication Therapy:
• Drug-disease contraindications.
• Therapeutic interchange.
• Generic substitution.
• Incorrect drug dosage.
• Inappropriate duration of drug treatment.
• Drug-allergy interactions.
• Clinical abuse/misuse
• Therapeutic appropriateness.
• Over and underutilization.
• Appropriate generic use.
• Therapeutic duplication.
• Drug-drug interactions .
METHODOLOGY:
Study Design: It is a observational and interventional study.
Study Period: The Present study was conducted for a period of 12 months from june 2016 to june 2017.
Study site : The Present study was conducted in GENERAL MEDICINE, PEDIATRICS, ,OPHTHALMIC, SURGERY, ORTHOPEDICS, ENT, ART, STD Departments at Rajiv Gandhi Institute of Medical Sciences ( RIMS), Kadapa.
Sample size: The Patients admitted in hospital with multiple diseases with poly pharmacy during the study period of 12 months and a total of 700 prescriptions were collected for this study
Source of Data: All the patients satisfying the inclusion criteria were selected from GENERAL MEDICINE, PEDIATRICS, OPHTHALMIC, SURGERY, ORTHOPEDICS, ENT, ART, STD department in Rajiv Gandhi institute of medical sciences (RIMS) Government Hospital, Kadapa. All the required data was collected from patients through Patient representative interview and case sheets and treatment charts.
Inclusion criteria:
• Prescriptions of Patients with aging above 18 years with multiple diseases with poly pharmacy, in GENERAL MEDICINE, PEDIATRICS, OPHTHALMIC, SURGERY, ORTHOPEDICS, ENT, ART,STD inpatient wards.
• Prescriptions of Patients having previous history of medical, medication problems
• The Patients who are willing to participate in the study.
Exclusion criteria:
• Prescriptions of Patients who are not willing to Participate in the study.
• Prescriptions of Patients below 18 years of age group.
• Prescriptions Other than GENERAL MEDICINE, PEDIATRICS, OPHTHALMIC, SURGERY, ORTHOPEDICS, ENT, ART, STD all the remaining wards are excluded.
RESULTS:
Table1.1DISTRIBUTION OF MALE AND FEMALE PATIENTS IN DIFFERENT WARDS:
|
NAME OF THE INPATIENT UNIT. |
NUMBER OF FEMALE POPULATION |
NUMBER OF MALE POPULATION |
TOTAL |
|
GENERAL MEDICINE
|
70
|
70
|
140
|
|
PAEDIATRICS
|
24 |
32 |
56 |
|
OPHTHALMIC
|
31 |
28 |
59 |
|
SURGERY |
45
|
61 |
106 |
|
ORTHOPAEDICS |
25 |
31 |
56 |
|
ENT |
43 |
62 |
105 |
|
ART |
37 |
60 |
97 |
|
STD |
54
|
27 |
81 |
|
TOTAL |
329 |
371 |
700 |
Fig 1.1DIAGRAMMATIC DISTRIBUTION OF MALE AND FEMALE PATIENTS IN DIFFERENT WARDS:

Table 1.2: DISTRIBUTION OF PRESCRIPTIONS WITH MUTILPLE DISEASES AND WITH POLYPHARMACY IN DIFFERENT WARDS:
|
NAME OF INPATIENT UNIT |
NUMBER OF PRESCRIPTIONS |
|
GENERAL MEDICINE |
140
|
|
PAEDIATRICS
|
56 |
|
OPHTHALMIC
|
59 |
|
SURGERY |
106 |
|
ORTHOPAEDICS |
56 |
|
ENT |
105 |
|
ART |
97 |
|
STD |
81 |
|
TOTAL |
700 |
Fig.1.2: DIAGRAMMATIC DISTRIBUTION OF PRESCRIPTIONS WITH MUTILPLE DISEASES AND WITH POLYPHARMACY IN DIFFERENT WARDS:
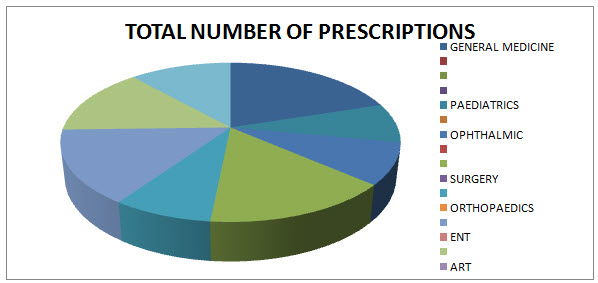
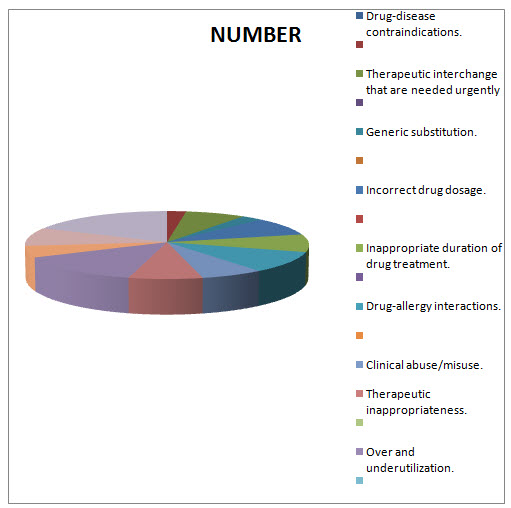
Table.1.3 DISTRIBUTION OF MEDICATION THERAPY RELATED PROBLEMS THAT ARE OVERCOMED BT DOCTOR OF PHARMACY DURING DUE STUDY/PATTERN ANALYSIS:
|
Types of Medication Therapy Related Problems that are Overcomed During DUE Analysis. |
Total number of Medication Therapy RelatedProblems that are Overcomed During DUE Analysis. |
||
|
Drug-disease contraindications.
|
NUMBER |
PERCENTAGE |
|
|
24 |
245.28 |
||
|
Generic substitution.
|
27 |
275.94 |
|
|
Incorrect drug dosage.
|
90 |
919.8 |
|
|
Inappropriate duration of drug treatment.
|
86 |
878.92 |
|
|
Drug-allergy interactions.
|
102 |
1042.44 |
|
|
Clinical abuse/misuse. |
68 |
694.96 |
|
|
Therapeutic inappropriateness.
|
79 |
807.38 |
|
|
Over and underutilization.
|
141 |
1441.02 |
|
|
In Appropriate generic use.
|
56 |
572.32 |
|
|
Therapeutic duplication.
|
89 |
909.58 |
|
|
Drug-drug interactions |
184 |
1880.48 |
|
|
|
Total=1022 |
Total percentage= 12706.3216 |
|
FIG.1.3 DIAGRAMATIC PERCENTAGE DISTRIBUTION OF MEDICATION THERAPY RELATED PROBLEMS THAT ARE OVERCOMED BT DOCTOR OF PHARMACY DURING DUE STUDY/PATTERN ANALYSIS:
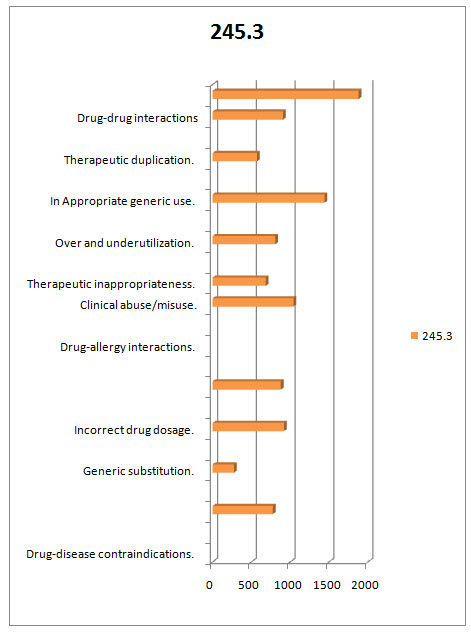
FIG.1.4 DIAGRAMATIC DISTRIBUTION OF MEDICATION THERAPY RELATED PROBLEMS THAT ARE OVERCOMED BT DOCTOR OF PHARMACY DURING DUE STUDY/PATTERN ANALYSIS:
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
DISCUSSION:
In the present study a total of 700 prescriptions were analyzed for DUE among them 329 prescriptions belongs to female patients and 371 prescriptions belongs to male patients. After the DUE Analysis the following medication therapy related problems were overcomed Drug-disease contraindications 24(245.28). Generic substitution27(275.94) Incorrect drug dosage90(919.8) Inappropriate duration of drug treatment86(878.92) Drug-allergy interactions102(1042.44) Clinicalabuse / misuse79(807.38) Therapeuticappropriateness141(1441.02) Over and underutilization56(572.32) Inappropriate generic use 56(572.32) Therapeutic duplication89 (909.58) Drug-drug interactions 184 91880.48) . And a total of 1022 medication therapy related problems were resolved and their overall percentage is 12706.3266. Prism Graph Pad software is used for this study and the P-Value Is < 0.001 which concludes the present study was highly significant.
COCLUSION:
The present study concludes that DUE analysis should carry out frequently in order to minimize the medication therapy related problems during the course of treatment and Doctor Of Pharmacy professionals are those whose main objective is to provide overall health care to the society, in U.S.A and other western countries the prescribing pattern of drugs is under the control of Pharma doctors i.e., doctor of pharmacy professionals who are well trained in medication usage and are involved in promotion of appropriate medication usage ,so in those countries there are less medication therapy related problems but in India as the entire prescribing pattern is under the control of physicians who are less familiar with pharmacology of drugs when compared with pharmd no one is superior or no one is inferior in the field of health care everyone has their priorities , so it is the duty of Indian government to get involved the Doctor Of Pharmacy in the actual patient care in Indian hospitals where they can prove themselves
REFERENCES:
1. *M. Manasa rekha ,A. Bharath kumar ---a prospective study on implementation of clinical pharmacy services to general medicine department in a teritary care teaching hospital published in journal of pharma research in ISSN: 2319-5622.
2. American College of Clinical Pharmacy. Pharmacotherapy 2008-28 (6), 816–817.
3. American College of Clinical Pharmacy.The Definition of Clinical Pharmacy. Pharmacotherapy 2008; 28(6):816-817.
4. Kucukarslan SN, Peters M, Mlynarek M, Nafziger DA. Dopharmacists’ presences on rounding teams reduce preventable adverse drug events in hospital general medical units.CMAJ 2004 Feb; 170(3):333.
5. Roberts MS , Stokes JA , King MA, Lynne TA, Purdie DM.Outcomes of a randomized controlled trial of a clinical pharmacy intervention in 52 nursing homes. Br J Clin Pharmacol 2001; 51:257-265.
6. Langebrake C, Hilgarth H. Clinical Pharmacist intervention in German University Hospital. Pharm World Sci 2010; 32:194-199.
7. srinivasan r, ramya g. Adverse drug reaction-causality assessment. international journal of research in pharmacy and chemistry.2011,1(3).
8. dr. rajaram, g., dr. sugirda, p. and dr. lenin, r.Pattern of adverse cutaneous drug reactions presenting to general practitioners in a semi urban area.asian journal of science and technology vol. 6, issue 09, pp. 1788-1790, september, 2015.
9. Jean F Kozak and Akber Mithani.Prevalence of Adverse Drug Events in Long Term Care: Variations in Screening Between Nurses and Physician-Pharmacist Reviewers. HSOA Journal of Gerontology & Geriatric Medicine.2015, 1: 007.
10. American College of Clinical Pharmacy Board of Regents on April 8, 2005. 13000 West 87th Street Parkway, Suite100, Lenexa, KS 66215-4530; e-mail: accp@accp.com, or download from http://www.accp.com.11.. Chacko Jiyo1, Satish D2. Suthar3,etal Drug utilization study of HIV positive patients registered with antiretroviral therapy centre of a tertiary care hospital.
12.National AIDS Control Organization, Govt of India,New Delhi. www.nacoonline.org.
13.Jigar D Kapadia1, Chetna K Desai2, A study of utilization pattern, efficacy and safety ofdrugs prescribed for opportunistic infections in HIV infected patients.
14. Ragesh G., Sindhubharathi A, Ushasri M, Srinivasulu A.A Study On Assessment Of Clinical Pharmacy Services To Cardiology Department In Tertiary Care Teaching Hospital.Int J Pharm Pharm Sci, Vol 6, Issue 2, 192-195.2015.
15. Satish Kumar BP, Prasanna Dhalal, Rajesh Venkataraman. Assessment of Clinical Pharmacist Intervention in Tertiary Care Teaching Hospital of southern India, Asian journal of pharmaceutical and clinical research.,6, 2013, 258-261.









