About Authors:
Jaideep Sharma
Guru jambheshwar university of science & technology
Hisar,
Haryana
Cancer, known medically as a malignant neoplasm is a term for a large group of different disease, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumours, and invade nearby parts of the body. The cancer may also spread to more distant parts of the body through the lymphatic system or bloodstream. Not all tumours are cancerous. Benign tumours do not grow uncontrollably, do not invade neighbouring tissues, and do not spread throughout the body.
Healthy cells control their own growth and will destroy themselves if they become unhealthy. Cell division is a complex process that is normally tightly regulated. Cancer occurs when problems in the genes of a cell prevent these controls from functioning properly. These problems may come from damage to the gene or may be inherited, and can be caused by various sources inside or outside of the cell. Faults in two types of genes are especially important: ontogenesis, which drive the growth of cancer cells, and tumour suppressor genes, which prevent cancer from developing.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1201
What causes cancer?
Many things are known to increase the risk of cancer, including tobacco use, infection, radiation, lack of physical activity, poor diet and obesity, and environmental pollutants.[1] These can directly damage genes or combine with existing genetic faults within cells to cause the disease.[2] A small percentage of cancers, approximately five to ten percent, are entirely hereditary.
Cancers are primarily an environmental disease with 90-95% of cases attributed to environmental factors and 5-10% due to genetics.[1] Environmental, as used by cancer researchers, means any cause that is not genetic, not merely pollution. Common environmental factors that contribute to cancer death include tobacco (25-30%), diet and obesity (30-35%), infections (15-20%), radiation (both ionizing and non-ionizing, up to 10%), stress, lack of physical activity, and environmental pollutants.[2]
Path physiology of cancer:[5]
Cancer is fundamentally a disease of failure of regulation of tissue growth. In order for a normal cell to transform into a cancer cell, the genes which regulate cell growth and differentiation must be altered.
The affected genes are divided into two broad categories .Oncogenes are genes which promote cell growth and reproduction. Tumours suppression genes are genes which inhibit cell division and survival. Malignant transformation can occur through the formation of novel oncogenes, the inappropriate over-expression of normal oncogenes, or by the under-expression or disabling of tumour suppressor genes. Typically, changes in many genes are required to transform a normal cell into a cancer cell.
Genetic changes can occur at different levels and by different mechanisms. The gain or loss of an entire chromosome can occur through errors in mitosis More common are mutations, which are changes in the nucleotide sequence of genomic DNA.
Large-scale mutations involve the deletion or gain of a portion of a chromosome. Genomic application occurs when a cell gains many copies (often 20 or more) of a small chromosomal locus, usually containing one or more oncogenes and adjacent genetic material. Translocation occurs when two separate chromosomal regions become abnormally fused, often at a characteristic location. A well-known example of this is the Philadelphia chromosome, or translocation of chromosomes 9 and 22, which occurs in chronic myeloid leukaemia, and results in production of theBCR abl fusion protein , an oncogenic tyrosine kinase .
Small-scale mutations include point mutations, deletions, and insertions, which may occur in the promoter region of a gene and affect its expression, or may occur in the gene's coding genes and alter the function or stability of its protein product. Disruption of a single gene may also result from integration of genomic material from a DNA virus or retrovirus, and resulting in the expression of viral oncogenes in the affected cell and its descendants.
Replication of the enormous amount of data contained within the DNA of living cells will probabilistically result in some errors (mutations). Complex error correction and prevention is built into the process, and safeguards the cell against cancer. If significant error occurs, the damaged cell can "self-destruct" through programmed cell death, termed apoptosis. If the error control processes fail, then the mutations will survive and be passed along to daughter cells.
Some environments make errors more likely to arise and propagate. Such environments can include the presence of disruptive substances called carcinogens, repeated physical injury, heat, ionising radiation, or hypoxia .
The errors which cause cancer are self-amplifying and compounding, for example:
· A mutation in the error-correcting machinery of a cell might cause that cell and its children to accumulate errors more rapidly.
· A further mutation in an oncogene might cause the cell to reproduce more rapidly and more frequently than its normal counterparts.
· A further mutation may cause loss of a tumour suppressor gene, disrupting the apoptosis signalling pathway and resulting in the cell becoming immortal.
· A further mutation in signalling machinery of the cell might send error-causing signals to nearby cells.
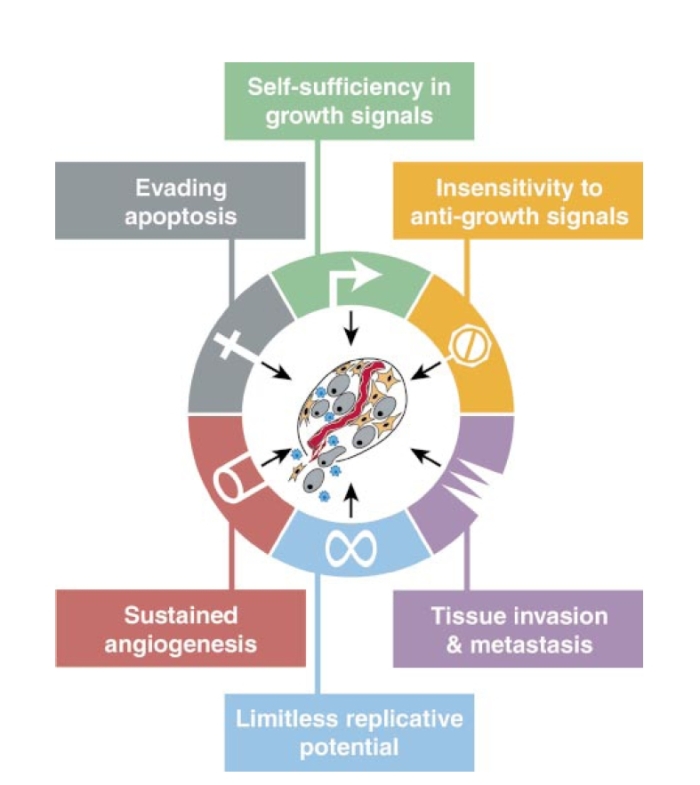
Figure 1. Acquired Capabilities of Cancer
Types of cancer
Cancers are classified by the type of cell that the tumour resembles and is therefore presumed to be the origin of the tumour. These types include:
[adsense:468x15:2204050025]
- Carcinoma: Cancers derived from epithelial cells. This group includes many of the most common cancers, particularly in the aged, and include nearly all those developing in the breast, prostate, lung, pancreas, and colon.
- Sarcoma: Cancers arising from connective tissue (i.e. bone, cartilage, fat, nerve), each of which develop from cells originating in mesenchymal cells outside the bone marrow.
- Lymphoma and leukaemia: These two classes of cancer arise from hematopoietic (blood-forming) cells that leave the marrow and tend to mature in the lymph nodes and blood, respectively.
- Germ cell tumour: Cancers derived from pluripotent cells, most often presenting in the testicle or the ovary (seminoma and dysgerminoma, respectively.
- Blastoma: Cancers derived from immature "precursor" cells or embryonic tissue. These are also most common in children.
EPIDEMIOLOGY
In 2008 approximately 12.7 million cancers were diagnosed (excluding non-melanoma skin cancers and other non-invasive cancers) and 7.6 million people died of cancer worldwide.[3] Cancers as a group account for approximately 13% of all deaths each year with the most common being: lung cancer (1.3 million deaths), stomach cancer (803,000 deaths), colorectal cancer (639,000 deaths), liver cancer (610,000 deaths), and breast cancer (519,000 deaths). this makes invasive cancer the leading cause of death in the developed world and the second leading cause of death in the developing world.[3] Over half of cases occur in the developing world.[3]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Types of treatment
Chemotherapy
Most commonly, chemotherapy acts by killing cells that divide rapidly, one of the main properties of most cancer cells. This means that it also harms cells that divide rapidly under normal circumstances: cells in the bone marrow, digestive tract and hair follicles.
This results in the most common side effects of chemotherapy: myelosuppression (decreased production of blood cells, hence also immunosuppression), mucositis (inflammation of the lining of the digestive tract), and alopecia (hair loss).
The majority of chemotherapeutic drugs can be divided in to alkylating agents, antimetabolites, anthracyclines, plant alkaloids, topoisomerase inhibitors, and other antitumor’s agents. All of these drugs affect cell division or DNA synthesis and function in some way.
Alkylating agents
Alkylating agents are so named because of their ability to alkylate many nucleophilic functional groups under conditions present in cells. Cisplatin and carboplatin, as well as oxaliplatin, are alkylating agents. They impair cell function by forming covalent bonds with the amino, carboxyl, sulfhydryl, and phosphate groups in biologically important molecules.
Other agents are mechlorethamine, cyclophosphamide, chlorambucil, ifosfamide. They work by chemically modifying a cell's DNA.
Anti-metabolites
Anti-metabolites masquerade as purines ((azathioprine, mercaptopurine)) or pyrimidines—which become the building blocks of DNA. They prevent these substances from becoming incorporated in to DNA during the "S" phase (of the cell cycle), stopping normal development and division. They also affect RNA synthesis. Due to their efficiency, these drugs are the most widely used cytostatics.
Plant alkaloids and terpenoids
These alkaloids are derived from plants and block cell division by preventing microtubule function. Microtubules are vital for cell division, and, without them, cell division cannot occur. The main examples are vinca alkaloids and taxanes.
Vinca alkaloids
Vinca alkaloids bind to specific sites on tubulin, inhibiting the assembly of tubulin into microtubules (M phase of the cell cycle). They are derived from the Madagascar periwinkle, Catharanthus roseus (formerly known as Vinca rosea). The vinca alkaloids include:
- Vincristine
- Vinblastine
- Vinorelbine
- Vindesine
Podophyllotoxin
Podophyllotoxin is a plant-derived compound which is said to help with digestion as well as used to produce two other cytostatic drugs, etoposide and teniposide. They prevent the cell from entering the G1 phase (the start of DNA replication) and the replication of DNA (the S phase). The exact mechanism of its action is not yet known.
The substance has been primarily obtained from the American Mayapple (Podophyllum peltatum). Recently it has been discovered that a rare Himalayan Mayapple (Podophyllum hexandrum) contains it in a much greater quantity, but, as the plant is endangered, its supply is limited. Studies have been conducted to isolate the genes involved in the substance's production, so that it could be obtained recombinantly.
Taxanes
The prototype taxane is the natural product paclitaxel, originally known as Taxol and first derived from the bark of the Pacific Yew tree. Docetaxel is a semi-synthetic analogue of paclitaxel. Taxanes enhance stability of microtubules, preventing the separation of chromosomes during anaphase.
Topoisomerase inhibitors
Topoisomerases are essential enzymes that maintain the topology of DNA. Inhibition of type I or type II topoisomerases interferes with both transcription and replication of DNA by upsetting proper DNA supercoiling.
- Some type I topoisomerase inhibitors include camptothecins: irinotecan and topotecan.
- Examples of type II inhibitors include amsacrine, etoposide, etoposide phosphate, and teniposide. These are semisynthetic derivatives of epipodophyllotoxins, alkaloids naturally occurring in the root of American Mayapple (Podophyllum peltatum).
Cytotoxic antibiotics
These include:
· actinomycin
· anthracyclines
o doxorubicin
o daunorubicin
o valrubicin
o idarubicin
o epirubicin ( which also inhibit topoisomerase II)
· other cytotoxic antibiotics
o Bleomycin acts in unique way through oxidation of a DNA-bleomycin-Fe(II) complex and forming free radicals, which induce damage and chromosomal aberrations.
o plicamycin
o mitomycin
Radiotherapy
Radiation therapy is commonly applied to the cancerous tumours because of its ability to control cell growth. Ionizing radiation works by damaging the DNA of exposed tissue. It is believed that cancerous cells may be more susceptible to death by this process as many have turned off their DNA repair ability during the process of becoming cancerous. To spare normal tissues (such as skin or organs which radiation must pass through in order to treat the tumours), shaped radiation beams are aimed from several angles of exposure to intersect at the tumours, providing a much larger absorbed dose there than in the surrounding, healthy tissue. Besides the tumour itself, the radiation fields may also include the draining lymph nodes if they are clinically or radio logically involved with tumours, or if there is thought to be a risk of subclinical malignant spread. It is necessary to include a margin of normal tissue around the tumours to allow for uncertainties in daily set-up and internal tumours motion. These uncertainties can be caused by internal movement (for example, respiration and bladder filling) and movement of external skin marks relative to the tumour position.
It is also common to combine radiation therapy with surgery, chemotherapy, hormone therapy, Immunotherapy or some mixture of the four. Most common cancer types can be treated with radiation therapy in some way. The precise treatment intent (curative, adjuvant, neoadjuvant, therapeutic, or palliative) will depend on the tumour type, location, and stage, as well as the general health of the patient. Total body irradiation (TBI) is a radiation therapy technique used to prepare the body to receive a bone marrow transplant.
Brachytherapy, in which a radiation source is placed inside or next to the area requiring treatment, is another form of radiation therapy that minimizes exposure to healthy tissue during procedures to treat cancers of the breast, prostate and other organs.
Radiation therapy has several applications in non-malignant conditions, such as the treatment of trigeminal neuralgia, severe thyroid eye disease, pterygium, pigmented villonodular synovitis, and prevention of keloid scar growth, vascular restenosis, and heterotopic ossification. The use of radiation therapy in non-malignant conditions is limited partly by worries about the risk of radiation-induced cancers.
Mechanism of action
Radiation therapy works by damaging the DNA of cancerous cells. This DNA damage is caused by one of two types of energy, photon or charged particle. This damage is either direct or indirect ionization of the atoms which make up the DNA chain. Indirect ionization happens as a result of the ionization of water, forming free radicals, notably hydroxyl radicals, which then damage the DNA. In the older, most common form of radiation therapy, intensity-modulated radiation therapy (IMRT) (photons), most of the radiation effect is through free radicals. Because cells have mechanisms for repairing single-strand DNA damage, double-stranded DNA breaks prove to be the most significant technique to cause cell death. Cancer cells are generally undifferentiated and stem cell-like; they reproduce more than most healthy differentiated cells, and have a diminished ability to repair sub-lethal damage. Single-strand DNA damage is then passed on through cell division; damage to the cancer cells' DNA accumulates, causing them to die or reproduce more slowly.
Surgery
In theory, non-hematological cancers can be cured if entirely removed by surgery, but this is not always possible. When the cancer has metastasized to other sites in the body prior to surgery, complete surgical excision is usually impossible. In the Halstedian model of cancer progression, tumours grow locally, and then spread to the lymph nodes, then to the rest of the body. This has given rise to the popularity of local-only treatments such as surgery for small cancers. Even small localized tumours are increasingly recognized as possessing metastatic potential.
Examples of surgical procedures for cancer include mastectomy for breast cancer, prostatectomy for prostate cancer, and lung cancer surgery for non-small cell lung cancer. The goal of the surgery can be either the removal of only the tumour, or the entire organ. A single cancer cell is invisible to the naked eye but can regrow into a new tumour, a process called recurrence. For this reason, the pathologist will examine the surgical specimen to determine if a margin of healthy tissue is present, thus decreasing the chance that microscopic cancer cells are left in the patient.
In addition to removal of the primary tumour, surgery is often necessary for staging, e.g. determining the extent of the disease and whether it has metastasized to regional lymph nodes. Staging is a major determinant of prognosis and of the need for adjuvant therapy.
If surgery is possible and appropriate, it is commonly performed before other forms of treatment, although the order does not affect the outcome. In some instances, surgery must be delayed until other treatments are able to shrink the tumour.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Other Treatment Method
Angiogenesis inhibitors
Angiogenesis inhibitors prevent the extensive growth of blood vessels (angiogenesis) that tumours require to survive. Some, such as bevacizumab, have been approved and are in clinical use. One of the main problems with anti-angiogenesis drugs is that many factors stimulate blood vessel growth in cells normal or cancerous. Anti-angiogenesis drugs only target one factor, so the other factors continue to stimulate blood vessel growth.
Immuno therapy
Cancer immunotherapy refers to a diverse set of therapeutic strategies designed to induce the patient's own immune system to fight the tumours. Contemporary methods for generating an immune response against tumours include intravesical BCG immunotherapy for superficial bladder cancer, and use of interferon’s and other cytokines to induce an immune response in renal cell carcinoma and melanoma patients.
Vaccines to generate specific immune responses are the subject of intensive research for a number of tumours, notably malignant melanoma and renal cell carcinoma. Sipuleucel-T is a vaccine-like strategy in late clinical trials for prostate cancer in which dendritic cells from the patient are loaded with prostatic acid phosphatase peptides to induce a specific immune response against prostate-derived cells.
Allogeneic hematopoietic stem cell transplantation ("bone marrow transplantation" from a genetically non-identical donor) can be considered a form of immunotherapy, since the donor's immune cells will often attack the tumour in a phenomenon known as graft-versus-tumour effect. For this reason, allogeneic HSCT leads to a higher cure rate than autologous transplantation for several cancer types, although the side effects are also more severe.
Hormonal therapy
Hormonal therapy is one of the major modalities of medical treatment for cancer, others being cytotoxic chemotherapy and targeted therapy (bio therapeutics). It involves the manipulation of the endocrine system through exogenous administration of specific hormones, particularly steroid hormones, or drugs which inhibit the production or activity of such hormones (hormone antagonists). Because steroid hormones are powerful drivers of gene expression in certain cancer cells, changing the levels or activity of certain hormones can cause certain cancers to cease growing, or even undergo cell death. Surgical removal of endocrine organs, such as orchiectomy and oophorectomy can also be employed as a form of hormonal therapy.
Hormonal therapy is used for several types of cancers derived from hormonally responsive tissues, including the breast, prostate, endometrium, and adrenal cortex. Hormonal therapy may also be used in the treatment of paraneoplastic syndromes or to ameliorate certain cancer- and chemotherapy-associated symptoms, such as anorexia.
Perhaps the most familiar example of hormonal therapy in oncology is the use of the selective estrogen-response modulator tamoxifen for the treatment of breast cancer, although another class of hormonal agents, aromatase inhibitors, now have an expanding role in that disease.
Hyperthermia therapy
Hyperthermia therapy is a type of medical treatment in which body tissue is exposed to slightly higher temperatures to damage and kill cancer cells or to make cancer cells more sensitive to the effects of radiation and certain anti-cancer drugs. When combined with radiation therapy, it is called thermoradiotherapy.
Local hyperthermia for certain small tumours is generally accepted, similar to surgically removing a tumour. Whole-body hyperthermia is generally considered to be a promising experimental cancer treatment.
Hyperthermia is only useful for certain kinds of cancer, and is not in widespread use. Hyperthermia is most effective when used alongside conventional therapies, so it is normally used as an adjuvant therapy. The most effective uses are currently being studied.
Mechanism of action
Hyperthermia may kill or weaken tumour cells, and is controlled to limit effects on healthy cells. Tumour cells, with a disorganized and compact vascular structure, have difficulty dissipating heat. Hyperthermia may therefore cause cancerous cells to undergo apoptosis in direct response to applied heat, while healthy tissues can more easily maintain a normal temperature. Even if the cancerous cells do not die outright, they may become more susceptible to ionizing radiation therapy or to certain chemotherapy drugs, which may allow such therapy to be given in smaller doses.
Intense heating will cause denaturation and coagulation of cellular proteins, rapidly killing cells within a tumour. More prolonged moderate heating to temperatures just a few degrees above normal can cause more subtle changes. A mild heat treatment combined with other stresses can cause cell death by apoptosis. There are many biochemical consequences to the heat shock response within in cell, including slowed cell division and increased sensitivity to ionizing radiation therapy.
Hyperthermia can kill cells directly, but it’s more important use is in combination with other treatments for cancer. Hyperthermia increases blood flow to the warmed area, perhaps doubling perfusion in tumours, while increasing perfusion in normal tissue by ten times or even more. This enhances the delivery of medications. Hyperthermia also increases oxygen delivery to the area, which may make radiation more likely to damage and kill cells, as well as preventing cells from repairing the damage induced during the radiation session.
Cancerous cells are not inherently more susceptible to the effects of heat. When compared in in vitro studies, normal cells and cancer cells show the same responses to heat. However, the vascular disorganization of a solid tumour results in an unfavourable microenvironment inside tumours. Consequently, the tumour cells are already stressed by low oxygen, higher than normal acid concentrations, and insufficient nutrients, and are thus significantly less able to tolerate the added stress of heat than a healthy cell in normal tissue.
Mild hyperthermia, which provides temperatures equal to that of a naturally high fever, may stimulate natural immunological attacks against the tumour. However it is also induces a natural physiological response called thermo tolerance, which tends to protect the treated tumour.
Moderate hyperthermia, which heats cells in the range of 40 to 42 °C, damages cells directly, in addition to making the cells radiosensitive and increasing the pore size to improve delivery of large-molecule chemotherapeutic and immunotherapeutic agents (molecular weight greater than 1,000 Daltons), such as monoclonal antibodies and liposome-encapsulated drugs. Cellular uptake of certain small molecule drugs is also increased. Most local and regional cancer treatments are in this temperature range.
Very high temperatures, above 50 °C (122 °F), are used for ablation (direct destruction) of some tumours.[3] This generally involves inserting a metal tube directly into the tumour, and heating the tip until the tissue next to the tube has been killed.
Laser in cancer treatment
Laser therapy uses high-intensity light to treat cancer and other illnesses. Lasers can be used to shrink or destroy tumours or precancerous growths. Lasers are most commonly used to treat superficial cancers (cancers on the surface of the body or the lining of internal organs) such as basal cell skin cancer and the very early stages of some cancers, such as cervical, penile, vaginal, vulvar, and non-small cell lung cancer.
Lasers also may be used to relieve certain symptoms of cancer, such as bleeding or obstruction. For example, lasers can be used to shrink or destroy a tumours that is blocking a patient’s trachea (windpipe) or oesophagus. Lasers also can be used to remove colon polyps or tumours that are blocking the colon or stomach.
Laser therapy can be used alone, but most often it is combined with other treatments, such as surgery, chemotherapy, or radiation therapy. In addition, lasers can seal nerve endings to reduce pain after surgery and seal lymph vessels to reduce swelling and limit the spread of tumour cells.
Laser therapy is often given through a flexible endoscope (a thin, lighted tube used to look at tissues inside the body). The endoscope is fitted with optical fibres (thin fibres that transmit light). It is inserted through an opening in the body, such as the mouth, nose, anus, or vagina. Laser light is then precisely aimed to cut or destroy a tumour.
Laser-induced interstitial thermotherapy (LITT), or interstitial laser photocoagulation, also uses lasers to treat some cancers. LITT is similar to a cancer treatment called hyperthermia, which uses heat to shrink tumours by damaging or killing cancer cells.
Photodynamic therapy
Photodynamic therapy(PDT) is used clinically to treat a wide range of medical conditions, including malignant cancers, and is recognised as a treatment strategy which is both minimally invasive and minimally toxic. While the applicability and potential of PDT has been known for over a hundred years, the development of modern PDT has been a gradual one, involving scientific progress in the fields of photobiology and cancer biology, as well as the development of modern photonic devices, such as lasers and LEDs.
Most modern PDT applications involve three key components : a photosensitizer, a light source and tissue oxygen. The wavelength of the light source needs to be appropriate for exciting the photosensitizer to produce reactive oxygen species. The combination of these three components leads to the chemical destruction of any tissues which have either selectively taken up the photosensitizer or have been locally exposed to light. In understanding the mechanism of PDT it is important to distinguish it from other light-based and laser therapies such as laser wound healing and rejuvenation which do not require a photosensitizer.
In order to achieve the selective destruction of the target area using PDT while leaving normal tissues untouched, either the photosensitizer can be applied locally to the target area or photosensitive targets can be locally excited with light. For instance, in the treatment of skin conditions, including acne, psoriasis, and also skin cancers, the photosensitizer can be applied topically and locally excited by a light source. In the local treatment of internal tissues and cancers, after photosensitizers have been administered intravenously, light can be delivered to the target area using endoscopes and fiber optic catheters .
Compared to normal tissues, most types of cancers are especially active in both the uptake and accumulation of photosensitizer’s agents, which makes cancers especially vulnerable to PDT. Since photo sensitizers can also have a high affinity for vascular endothelial cells, PDT can be targeted to the blood carrying vasculature that supplies nutrients to tumours, increasing further the destruction of tumours.
Photosensitizers can also target many viral and microbial species, including HIV and MRSA.Using PDT, pathogens present in samples of blood and bone marrow can be decontaminated before the samples are used further for transfusions or transplants. PDT can also eradicate a wide variety of pathogens of the skin and of the oral cavities. Given the seriousness that drug resistant pathogens have now become, there is increasing research into PDT as a new antimicrobial therapy.
Over the last thirty years, PDT has seen considerable development in a wide range of medical applications. At the cutting edge of new PDT developments, many scientists worldwide are exploring ways of enhancing photosensitizer efficacy and targeting, while new research in Russia looks to use PDT to kill internal pathogens such as mycobacterium tuberculosis, and a significant development in Asia involves whole body Next Generation PDT (NGPDT) using a tumour-specific chlorophyll-based photosensitizer to treat a wide variety of solid cancers, including deep tissue and multisite cancers.
Mechanism of action
The basis of PDT is the interaction of light with photosensitive agents to produce an energy transfer and a local chemical effect. This is broadly similar to what is seen in photosynthesis, although in this case, many photosensitizers work together to harvest light energy to produce chemical reactions. Of the many photosensitizers that have been used in PDT, each has its own unique excitation properties. Usually, the photosensitizer is excited from a ground singlet state to an excited singlet state. It then undergoes intersystem crossing to a longer-lived excited triplet state.
One of the few chemical species present in tissue with a ground triplet state is molecular oxygen. When the photosensitizer and an oxygen molecule are in proximity, an energy transfer can take place that allows the photosensitizer to relax to its ground singlet state, and create an excited singlet state oxygen molecule. Singlet oxygen is a very aggressive chemical species and will very rapidly react with any nearby bio molecules. Ultimately, these destructive reactions will kill cells through apoptosis or necrosis. PDT can be considered a form of targeted singlet oxygen chemotherapy, where the targeting is achieved with the combination of the photosensitizer (functioning as a catalyst) and intense light.
A similar example is that cattle may become photosensitive if they graze on plants that contain photosensitizing toxins, such as marigold (Tagetes)
Targeted cancer therapy
Targeted therapy, which first became available in the late 1990s, has had a significant impact in the treatment of some types of cancer, and is currently a very active research area. This constitutes the use of agents specific for the deregulated proteins of cancer cells. Small molecule targeted therapy drugs are generally inhibitors of enzymatic domains on mutated, overexpressed, or otherwise critical proteins within the cancer cell. Prominent examples are the tyrosine kinase inhibitors imatinib (Gleevec/Glivec) and gefitinib (Iressa).
Monoclonal antibody therapy is another strategy in which the therapeutic agent is an antibody which specifically binds to a protein on the surface of the cancer cells. Examples include the anti-HER2/neu antibody trastuzumab (Herceptin) used in breast cancer, and the anti-CD20 antibody rituximab, used in a variety of B-cell malignancies.
Targeted therapy can also involve small peptides as "homing devices" which can bind to cell surface receptors or affected extracellular matrix surrounding the tumours. Radionuclides which are attached to these peptides (e.g. RGDs) eventually kill the cancer cell if the nuclide decays in the vicinity of the cell. Especially oligo- or multimers of these binding motifs are of great interest, since this can lead to enhanced tumours specificity and avidity.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Types
The main categories of targeted therapy are small molecules and monoclonal antibodies.
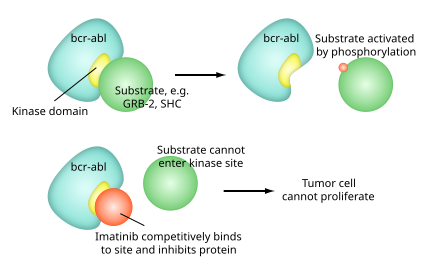
Small molecules
Mechanism of imatinib
- Imatinib mesylate (Gleevec, also known as STI–571) is approved for chronic myelogenous leukaemia, gastrointestinal stromal tumours and some other types of cancer. Early clinical trials indicate that imatinib may be effective in treatment of dermatofibrosarcoma protuberans.
- Gefitinib (Iressa, also known as ZD1839), targets the epidermal growth factor receptor (EGFR) tyrosine kinase and is approved in the U.S. for non small cell lung cancer. EGFR is also over expressed in the cells of other solid tumours, such as lung and breast cancers. This leads to inappropriate activation of the apoptotic Ras signal transduction cascade, eventually leading to uncontrolled cell proliferation.Gefitinib inhibits EGFR tyrosine kinase by binding to the adenosine triphosphate (ATP)-binding site of the enzyme. Thus the function of the EGFR tyrosine kinase in activating the Ras signal transduction cascade is inhibited; and malignant cells are inhibited.
- Erlotinib (marketed as Tarceva). Erlotinib inhibits epidermal growth factor receptor,[5] and works through a similar mechanism as gefitinib. Erlotinib has been shown to increase survival in metastatic non small cell lung cancer when used as second line therapy. Because of this finding, erlotinib has replaced gefitinib in this setting.
- Bortezomib (Velcade) is an apoptosis-inducing proteasome inhibitor drug that causes cancer cells to undergo cell death by interfering with proteins. It is approved in the U.S. to treat multiple myeloma that has not responded to other treatments.
- The selective estrogen receptor modulator tamoxifen has been described as the foundation of targeted therapy.
- Bcl-2 inhibitors (e.g. obatoclax in clinical trials, ABT-263, and Gossypol.
- PARP inhibitors (e.g. Iniparib, Olaparib in clinical trials)
- Janus kinase inhibitors
- PI3K inhibitors
- Apatinib is a selective VEGF Receptor 2 inhibitor which has shown encouraging anti-tumours activity in a broad range of malignancies in clinical trials.[8] Apatinib is currently in clinical development for metastatic gastric carcinoma, metastatic breast cancer and advanced hepatocellular carcinoma.
- AN-152, (AEZS-108) doxorubicin linked to [D-Lys(6)]- LHRH, Phase II results for ovarian cancer
- Salinomycin has demonstrated potency in killing cancer stem cells in both laboratory-created and naturally occurring breast tumours in mice.
Monoclonal antibodies
Several are in development and a few have been licenced by the FDA. Examples of licensed monoclonal antibodies include:
- Rituximab (marketed as MabThera or Rituxan) targets CD20 found on B cells. It is used in non Hodgkin lymphoma
- Trastuzumab (Herceptin) targets the Her2/neu (also known as ErbB2) receptor expressed in some types of breast cancer
- Cetuximab (marketed as Erbitux) targets the epidermal growth factor receptor. It is used in the treatment of colon cancer and non-small cell lung cancer.
- Bevacizumab (marketed as Avastin) targets circulating VEGF ligand. It is approved for use in the treatment of colon cancer, breast cancer, non-small cell lung cancer, and is investigational in the treatment of sarcoma. Its use for the treatmentof brain tumours has been recommended
Anticancer agents from plant source [4,8]
Cancer is a major public health burden in both developed and developing countries. Plant derived agents are being used for the treatment of cancer.
Plant derived anticancer agents in clinical use
The isolation of the vinca alkaloids, vinblastine and vincristine from the Madagascar periwinkle, Catharanthus roseus G. Don. (Apo-cynaceae) introduced a new era of the use of plant material as anticancer agents. They were the first agents to advance into clinical use for the treatment of cancer .
Vinblastine and vincristine are primarily used in combination with other cancer chemothe-rapeutic drugs for the treatment of a variety of cancers, including leukaemia’s, lymphomas, advanced testicular cancer, breast and lung cancers, and Kaposi’s sarcoma .
The discovery of paclitaxel (Taxol®, ) from the bark of the Pacific Yew, Taxus brevifolia Nutt. (Taxaceae) is another evidence of the success in natural product drug discovery. Various parts of Taxus brevifolia and other Taxus species (e.g., Taxus Canadensis Marshall, Taxus baccata L.) have been used by several Native American Tribes for the treatment of some non-cancerous cases while Taxus baccata was reported to use in the Indian Ayurvedic medicine for the treatment of cancer
Paclitaxel is significantly active against ovarian cancer, advanced breast cancer, small and non-small cell lung cancer.
Camptothecin, isolated from the Chinese ornamental tree Camptotheca acuminate Decne (Nyssaceae), was advanced to clinical trials by NCI in the 1970s but was dropped because of severe bladder toxicity .
Topotecan and irinotecan are semi-synthetic derivatives of camptothecin and are used for the treatment of ovarian and small cell lung cancers, and colo-rectal cancers, respectively
. Epipodophyllotoxin is an isomer of podophyllotoxin which was isola-ted as the active anti-tumours agent from the roots of Podophyllum species, Podophyllum peltatum Linnaeus and Podophyllum emodi Wallich (Berberidaceae) .
Etoposide and teniposide are two semi-synthetic deriva-tives of epipodophyllotoxin and are used in the treatment of lymphomas and bronchial and testicular cancers.
Homoharringtonine isolated from the Chinese tree Cephalotaxus harringtonia var. drupacea (Sieb and Zucc.) (Cephalotaxaceae), is another plant-derived agent in clinical use.
A racemic mixture of harringtonine and homoha-rringtonine has been used successfully in China for the treatment of acute myelogenous leukaemia and chronic myelogenous leukaemia .
Ellipti-nium , a derivative of ellipticine, isolated from a Fijian medicinal plant Bleekeria vitensis A.C. Sm., is marketed in France for the treatment of breast cancer.
Plant-derived anticancer agents for future development
Numerous types of bioactive compounds have been isolated from plant sources. Several of them are currently in clinical trials or preclinical trials or undergoing further investigation.
Flavopiridol is a synthetic flavone, derived from the plant alkaloid rohitukine, which was isolated from Dysoxylum binectariferum Hook. f. (Meliaceae). It is currently in phase I and phase II clinical trials against a broad range of tumours, including leukaemia, lymphomas and solid tumours.
Synthetic agent roscovitine which is derived from natural product olomucine, originally isolated from Raphanus sativus L. (Brassicaceae), is in Phase II clinical trials in Europe .
Combre-tastatins were isolated from the bark of the South African tree Combretum caffrum (Eckl. & Zeyh.) Kuntze (Combretaceae) . Combretastatin A-4 is active against colon, lung and leukaemia cancers and it is expected that this molecule is the most cytotoxic phyto-molecule isolated so far Betulinic acid , a pentacyclic triterpene, is a common secondary metabolite of plants, primarily from Betula species (Betulaceae). Betulinic acid was isolated from Zizyphus species, e.g. mauritiana, rugosa and oenoplia and displayed selective cytotoxicity against human melanoma cell lines. The development of systemic and topical formulations of the agent for potential clinical trials by the NCI is ongoing .
Pervilleine A was isolated from the roots of Erythroxylum pervillei Baill. (Erythroxylaceae). Pervilleine A was selectively cytotoxic against a multidrug resistant (MDR) oral epidermoid cancer cell line (KB-V1) in the presence of the anticancer agent vinblastine . Pervilleine A is currently in preclinical development .
Silvestrol was first isolated from the fruits of Aglaila sylvestre Merrill (Meliaceae). Silvestrol exhibited cytotoxicity against lung and breast cancer cell lines . Biological studies are ongoing to determine the mechanism(s) of action for silvestrol.
Two novel alkaloids, schischkinnin and montamine have been isolated from the seeds of Centaurea schischkinii and Centaurea montana. Both of the alkaloids exhibited significant cytotoxicity against human colon cancer cell lines. The uni-que structural features of schischkinninand montamine can be exploited as a template for generating com-pounds with enhanced anticancer activity. However, further investigations are necessary for their use as anticancer agents.
References
1. Anand P, Kunnumakkara AB, Kunnumakara AB, et al. (September 2008).”Cancer is a preventable disease that requires major lifestyle changes “harm. Res. 25 (9): 2097–116.
2. Kinzler, Kenneth W.; Vogelstein, Bert (2002).”Introduction “The genetic basis of human cancer (2nd, illustrated, revised Ed.). New York: McGraw-Hill, Medical Pub. Division. p. 5
3. Jemal, A; Bray, F, Centre, MM, Ferlay, J, Ward, E, Forman, D (2011-02-04). "Global cancer statistics". CA: a cancer journal for clinicians 61 (2): 69–90.
4. mohammad shoeb “anticancer agents from medicinal plants “Bangladesh J pharmacol 2006;1;35-41
5. Douglas hanahan ,Robert a. Weinberg “the hallmark of cancer “cell vol(2007).100.57-70
6. cancer.gov National cancer institute NIH USA.
7. IAEA report on radiotherapy
8. Rajandeep, karan, harpreet J. Nat. Prod. Plant Resour,2011,1(1);119-124
9. cancerquest.com
10. biomedcentral.com
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









