 About Authors: Shouvik Bhattacharya1, Tapas Kumar Pal2
About Authors: Shouvik Bhattacharya1, Tapas Kumar Pal2
NSHM College Of Pharmaceutical Technology, West Bengal (NCPT)
1 - Student of B.pharm, 4th year,
2 - Assistant Professor of NCPT
Reference ID: PHARMATUTOR-ART-1075
Introduction
Recent trends in Pharmaceutical formulation development technology have presented viable dosage alternatives for patients who may have difficulty swallowing tablets or liquids. Traditional tablets and capsules administered with an 8-oz. (One glass) of water may be inconvenient or impractical for some patients. However, some patients, particularly pediatric and geriatric patients, have difficulty swallowing or chewing solid dosage forms. Many pediatric and geriatric patients are unwilling to take these solid preparations due to fear of choking. For example, a very elderly patient may not be able to swallow a daily dose of antidepressant in the form of a Caplet shaped Tablet. An eight-year-old with allergies could use a more convenient dosage form than antihistamine syrup. A schizophrenic patient in the institutional setting can hide a conventional tablet under his or her tongue to avoid their daily dose of an atypical antipsychotic. A middle-aged woman undergoing radiation therapy for breast cancer may be too nauseous to swallow her H2-blocker. Fast-dissolving tablets (FDTs) / Orally disintegrating tablets (ODTs) are a perfect fit for all of these patients. Fast-dissolving drug delivery systems have rapidly gained acceptance as an important new way of administering drugs. There are multiple fast-dissolving OTC and Rx products on the market worldwide, most of which have been launched in the past 3 to 4 years. There have also been significant increases in the number of new chemical entities under development using a fast-dissolving drug delivery technology.
[adsense:336x280:8701650588]
DEFINITION
The Center for Drug Evaluation and Research (CDER), US FDA defined Oral Disintegrating Tablets (ODT) as “A solid dosage form containing medicinal substances, which disintegrates rapidly, usually within a matter of seconds, when placed upon the tongue.”
FDTs disintegrate and/or dissolve rapidly in the saliva without the need for water. Some tablets are designed to dissolve in saliva remarkably fast, within a few seconds, and are true fast-dissolving tablets. Others contain agents to enhance the rate of tablet disintegration in the oral cavity, and are more appropriately termed fast-disintegrating tablets, as they may take up to a minute to completely disintegrate. When put on tongue, this tablet disintegrates instantaneously, releasing the drug, which dissolves or disperses in the saliva. Some drugs are absorbed from the mouth, pharynx and oesophagus as the saliva passes down into the stomach. In such cases, bioavailability of drug is significantly greater than those observed from conventional tablet dosage form. Their growing importance was underlined recently when European Pharmacopoeia adopted the term “Orodispersible Tablet” as a tablet that to be placed in oral cavity where it disperses rapidly before swallowing.
Salient Features of Fast Dissolving Drug Delivery System
1. Ease of administration for patients who are mentally ill, disabled and uncooperative.
2. Requires no water.
3. Quick disintegration and dissolution of the dosage form.
4. Overcomes unacceptable taste of the drugs.
5. Can be designed to leave minimal or no residue in the mouth after administration and also to provide a pleasant mouth feel.
6. Allows high drug loading.
7. Ability to provide advantages of liquid medication in the form of solid preparation. Adaptable and amenable to existing processing and packaging machinery.
8. Cost-effective.
Significance of Oral Disintegrating Tablets
Oral Disintegrating Tablets offer dual advantages of solid dosage forms and liquid dosage forms along with special features which include:
Accurate dosing
Being unit solid dosage forms, provide luxury of accurate dosing, easy Portability and manufacturing, good physical and chemical stability and an ideal alternative for pediatric and geriatric patients.
Enhanced bioavailability
Bioavailability of drugs is enhanced due to absorption from mouth, pharynx and esophagus.
Rapid action
Fast onset of therapeutic action as tablet gets disintegrated rapidly along with quick dissolution and absorption in oral cavity.
Patient compliance
No need of water to swallow the dosage form. Hence, it is convenient for patients who are traveling and do not have immediate access to water.
Ease of administration
Convenient to administer specially for geriatric, pediatric, mentally disabled and bed ridden patients who have difficulty in swallowing.
Obstruction free
No risk of suffocation in airways due to physical obstruction when swallowed, thus providing improved safety and compliance.
Enhanced palatability
Good mouths feel, especially for pediatric patients as taste masking technique is used to avoid the bitter taste of drug.
Simple packaging
No specific packaging required. It can be packaged in push through blisters.
Business avenue
Provide new business opportunities in the form of product differentiation, line extension, uniqueness and life cycle management.
Cost effective
Conventional processing and packaging equipments allow the manufacturing of tablets at low cost.
Characteristics of Fast Dissolving Delivery Systems
1. Ease of administration: Fast Dissolving Delivery Systems are easy to administer and handle hence, leads to better patient compliance. Usually, elderly people experience difficulty in swallowing the conventional dosage forms (tablets, capsules, solutions and suspensions) because of tremors of extremities and dysphasia.
2. Taste of the medicament: As most drugs are unpalatable, mouth dissolving delivery systems usually contain the medicament in taste masked form. Delivery systems dissolve or disintegrate in patient’s mouth, thus releasing the active ingredients which come in contact with the taste buds and hence, taste masking of the drugs becomes critical to patient compliance.
3. Hygroscopicity: Several fast dissolving dosage forms are hygroscopic and cannot maintain physical integrity under normal condition from humidity which calls for specialized product packaging.
4. Friability: In order to allow fast dissolving tablets to dissolve in the mouth, they are made of either very porous and soft-molded matrices or compressed into tablets with very low compression force, which makes the tablets friable and/or brittle which are difficult to handle, often requiring specialized peel-off blister packaging.
5. Mouth feel: Mouth feel is critical, and patients should receive a product that feels pleasant. Any large particles from the disintegrating tablet that are insoluble or slowly soluble in saliva would lead to an unpleasant gritty feeling. This can be overcome by keeping the majority of the particles below the detectable size limit. In some cases, certain flavors can imbibe an improved mouth feel perception, resulting in a product that is perceived as being less gritty, even if the only change is the flavor. Effervescence can be added to aid disintegration and improve mouth feel by reducing the “dryness” of a product.
Conventional Techniques Used in the Preparation of Fast Dissolving Drug Delivery Systems
Various technologies used in the manufacture of Fast dissolving tablets include:
Freeze drying or lyophilization
Tablet Molding
Direct compression
Spray drying
Sublimation
Taste masking
Mass extrusion
Cotton Candy process
Melt Granulation
Phase Transition
Nanonization
Fast Dissolving Films
Freeze drying or Lyophilization
A process in which water is sublimated from the product after freezing. Lyophilization is a pharmaceutical technology which allows drying of heat sensitive drugs and biological at low temperature under conditions that allow removal of water by sublimation. The ideal drug characteristics for this process are relative water insolubility with fine particle size and good aqueous stability in suspensions. Primary problems associated with water-soluble drugs are formation of eutectic mixture, because of freezing point depression and formation of glassy solid on freezing, which might collapse on sublimation. The addition of mannitol or crystal forming materials induces crystallinity and imparts rigidity to amorphous material Lyophilization results in preparations, which are highly porous, with a very high specific surface area, which dissolve rapidly and show improved absorption and bioavailability. Jaccard and Leyder used lyophilization to create an oral pharmaceutical preparation that not only dissolves rapidly but also improved the bioavailability of several drugs such as spironolactone and trolendomycin. Corveleyn and Remon studied various formulation and process parameters by using hydrochlorothiazide as a model drug on the basis of which US Patent 6,010,719 was granted. Tablets prepared by lyophilization, are fragile and possess low mechanical strength, which make them difficult to handle and they also exhibit poor stability on storage under stressed conditions. Glassy amorphous porous structure of excipients as well as the drug substance produced with freeze drying results in enhanced dissolution
Freeze drying process normally consists of three steps:
• Material is frozen to bring it below the eutectic point.
• Primary drying to reduce the moisture around 4% w/w of dry product.
• Secondary drying to reduce the bound moisture up to required final volume.
[adsense:336x280:8701650588]
Entire freeze drying process is carried out at non elevated temperature; therefore, nullifying adverse thermal effects that may affect drug stability during processing. R.P. Scherer patented Zydis technology utilizing lyophilization or freeze drying process in development of mouth dissolving tablets on the basis of patents issued to Gregory.
Excipients Used in the Manufacture of FDT Using Freeze Drying Technique
|
Excipients |
Main Purpose |
Examples |
|
Polymer |
Strength and rigidity |
Gelatin, alginate, dextrin, hydrolyzed dextran, polyvinyl alcohol, polyvinyl pyrrolidone |
|
Polysaccharides |
Crystallinity, hardness and palatability |
Mannitol and sorbitol |
|
Collapse protectants |
Prevention of shrinking |
Glycerin |
|
Flocculating Agents |
Uniform dispersion |
Xanthan and acacia gum |
|
Preservatives |
Prevention of microbial growth |
Parabens |
|
Permeation enhancer |
Transmucosal permeability enhancer |
Sodium lauryl sulphate |
|
pH adjusters |
Chemical stability |
Citric acid and sodium hydroxide |
|
Flavours & sweeteners |
Patient compliance |
Aspartame, orange flavor |
|
Water |
Porous unit formation |
------- |
Molding
Tablet produced by moulding are solid dispersion. Moulded tablets disintegrate more rapidly and offer improved taste because the dispersion matrix is in general made from water soluble sugars. The active ingredients in most cases are absorbed through the mucosal lining of the mouth. The manufacturing process of molding tablets involves moistening the powder blend with a hydro alcoholic solvent followed by pressing into mold plates to form a wetted mass (compressing molding). The solvent is then removed by air drying. Thus the process is similar to what is used in the manufacture of tablet triturates. Such tablets are less compact than compressed tablets and possess a porous structure that hastens dissolution.
Molded forms are also prepared using a heat-molding process that involves setting the molten mass that contains a dispersed drug. The heat-molding process uses an agar solution as a binder and a blister packaging well as a mold to manufacture a tablet. The process involves preparing a suspension that contains a drug, agar, and sugar (e.g., mannitol or lactose), pouring the suspension into the blister packaging well, solidifying the agar solution at room temperature to form a jelly, and drying at -30oC under vacuum.
Another process used is called no-vacuum lyophilization, which involves the evaporation of a solvent from a drug solution or suspension at standard pressure. Pebley et al., evaporated a frozen mixture containing a gum (e.g., acacia, carageenan, guar, tragacanth, or xanthan), a carbohydrate (e.g., dextrose, lactose, maltose, mannitol, or maltodextrin), and a solvent in a tablet shaped mould. Moulded tablets typically do not possess great mechanical strength. Erosion and breakage of the moulded tablet often occur during handling and opening of blister packs.
Direct compression
Direct compression is one of the popular techniques for preparation of these dosage forms. The advantages of this method include easy implementation, use of conventional equipments along with commonly available excipients, limited number of processing steps and cost effectiveness.
Disintegration and solubilization of directly compressed tablets depend on single or combined action of disintegrants, water-soluble excipients and effervescent agents. The basic principle involved in development of these dosage forms using this technique is addition of superdisintegrants in optimum concentrations so as to achieve rapid disintegration along with pleasant mouth feel. It is considered as the best method to prepare orally disintegrating dosage forms since the prepared tablets offer higher disintegration due to absence of binder and low moisture contents. This approach is also considered as disintegrant addition technology.
Bi et al and Watanabe et al developed fast-dissolving tablets using microcrystalline cellulose and low substituted Hydroxypropyl cellulose as disintegrating agents in the range of 8:2-9:1. Shu et al also prepared rapid oral disintegrating tablets by direct compression using co-ground mixture of D-Mannitol and Crosspovidone.
Ideal Requirements, Advantages and Limitations of Direct Compression
|
S. No |
Ideal requirements |
Advantages |
Limitations |
|
1 |
Flowability |
Cost effective production |
Segregation |
|
2. |
Compressibility |
Better stability of API |
Variation in functionality |
|
3. |
Dilution Potential |
Faster dissolution |
Low dilution potential |
|
4. |
Reworkability |
Less wear and tear of punches |
Reworkability |
|
5. |
Stability |
Simple validation |
Poor compressibility of API |
|
6. |
Controlled Particle Size |
Low microbial contamination |
Lubricant sensitivity |
Spray drying
Spray drying is a process by which highly porous, fine powders can be produced. Spray-dryers are invariably used in the pharmaceutical industry to produce highly porous powders. Allen et al. have reported applying this process to the production of fast dissolving tablet. The formulations that were produced contained hydrolyzed and unhydrolyzed gelatin as a support agent for the matrix, Mannitol as a bulking agent, and sodium starch glycolate or crosscarmellose as a disintegrant. Disintegration and dissolution was further enhanced by adding an acid (e.g., citric acid) or an alkali (e.g., sodium bicarbonate). The formulation was spray dried to yield a porous powder. Tablets manufactured from this powder disintegrated in less than 20 s in an aqueous medium.
A typical procedure involved in the manufacturing of ODT using this technique is mentioned here. The active drug is dissolved or dispersed in an aqueous solution of a carrier/polymer. The mixture is dosed by weight and poured in the wells of preformed Blister Packs. The trays holding the blister packs are passed through liquid Nitrogen freezing tunnel to freeze the drug solution or dispersion. Then the frozen blister packs are placed in refrigerated cabinets to continue the freeze drying. After Freeze drying aluminum foil backing is applied on a blister sealing machine. Finally the blisters are packed and shipped. The main drawbacks of lyophilization technique are that it is time consuming and expensive. Fragility makes conventional packaging unsuitable for these products and poor stability under stressed conditions.
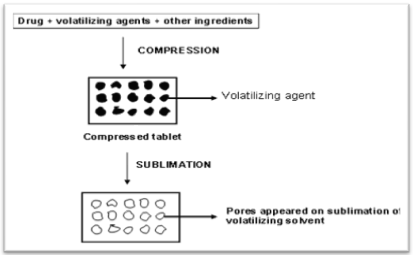
Sublimation
The key to rapid disintegration for mouth dissolving tablets is the presence of a porous structure in the tablet matrix. Conventional compressed tablets that contain highly water-soluble ingredients often fall to dissolve rapidly because of low porosity of the matrix. Hence to generate porous matrix, volatile ingredients are used that are later subjected to a process of sublimation. In studies conducted by Heinemann and Rothe, Knitsch and Roser and Blair, inert solid ingredients that displayed high volatility (e.g., ammonium bicarbonate, ammonium carbonate, benzoic acid, camphor, Hexamethonium tetramine, naphthalene, Phthalic anhydride, urea, and urethane were compressed along with other excipients into a table. The volatile material was then removed by sublimation, leaving behind a porous matrix.
Solvents such as cyclohexane and benzene were also suggested for the generation of porosity in the matrix. Koizumi et al. applied sublimation technology to manufacture tablets that rapidly dissolve in saliva. Mannitol is used as a matrix former, and camphor was used as a sublimating agent. The tablets dissolved in 10-20 s and displayed satisfactory handling properties. Makino et al. reported a method using water as pore-forming material. A mixture of drug and a carbohydrate (e.g., erythritol, glucose, maltitol, sucrose, xylitol). The water was then removed, yielding highly porous tablets with satisfactory mechanical strength and a high dissolution rate. Gohel M. et al prepared mouth dissolving tablets of Nimesulide using vacuum drying technique and found that it would be an effective alternative approach compared to the use of more expensive adjuvants in the formulation of these dosage forms.
Mass-Extrusion (Melt-Extrusion)
Preparation of ODTs of NSAID and paracetamol by melt extrusion method was patented by Sherry et al. (2008). The method involved dry blending of sugar alcohol and drugs with other excipients that may be present in the granular component. This powder mixture was heated at a temperature of 100 to 165°C in an extruder in order to completely melt the sugar alcohol. This resultant mass consisting of fully or partially molten sugar alcohol (xylitol, sorbitol, mannitol, etc.) and non-molten (NSAID (ibuprofen, naproxen, diclofenac) or paracetamol) and other optional excipients was poured on cooled stainless steel trays or a cooled moving belt (10°C) and allowed to cool The molten mixture typically solidified within 60 sec. The solid mass thus formed was milled by passing through a cone mill fitted. with a screen with a round hole of 1 mm diameter. The resulting granules were blended with extra-granular components namely, colloidal silicon dioxide, magnesium stearate, stearic acid, lactose, dicalcium phosphate and microcrystalline cellulose in a blender. The blended material was fed to a rotary tableting machine and compressed into tablets under compaction force ranging from 4 kN to 14 kN. This technology involves softening the active blend using the solvent mixture of water-soluble polyethylene glycol and methanol and subsequent expulsion of softened mass through the extruder or syringe to get a cylinder of the product into even segments using heated blade to form tablets. The dried cylinder can also be used to coat granules for bitter drugs and thereby achieve taste masking.
Melt Extrusion Temperature Conditions of Sugar Alcohols
|
Sugar alcohols |
Melting Point (° C) |
Melt extrusion range (° C) |
|
D-sorbitol |
98 to 100 |
108 -132 |
|
Xylitol |
95 to 97 |
102 -127 |
|
Adonitol |
102 to 104 |
112 – 134 |
|
Arabitol |
101 to 104 |
111 -134 |
|
Mannitol |
167 to 170 |
177 – 200 |
|
meso-Erythritol |
120 to 123 |
130-153 |
Cotton candy process
This process is so named as it utilizes an inimitable spinning mechanism to produce floss like crystalline structure, which mimics cotton candy. This technique involves formation of matrix of polysaccharides or saccharides by simultaneous action of flash melting and spinning. The matrix formed is partially recrystallized to have better flow properties and compressibility. This matrix is milled and blended with active ingredients as well as excipients and subsequently compressed to ODTs. This process can accommodate high doses of drug and offers improved mechanical strength. However, high process temperature limits the use of this process.
Phase transition
Kuno et al proposed a novel method to prepare ODTs with sufficient hardness by involving the phase transition of sugar alcohol. In this technique, ODTs are produced by compressing and subsequently heating tablets that contain two sugar alcohols, one with high and other with a low melting point. Heating process enhances the bonding among particles leading to sufficient hardness of tablets which was otherwise lacking owing to low/little compatibility.
Melt granulation
Melt granulation is a process in which pharmaceutical powders are efficiently agglomerated by the use of binder which can be a molten liquid, a solid or a solid that melts during the process. For accomplishing this process, high shear mixers are utilized, where the product temperature is raised above the melting point of binder by a heating jacket or by the heat of friction generated by impeller blades. Perissutti et al prepared carbamazepine fast-release tablets by melt granulation technique using polyethylene glycol 4000 as a melting binder and lactose monohydrate as hydrophilic filler.
Nanonization
A recently developed Nanomelt technology involves reduction in the particle size of drug to nano size by wet-milling technique. Surface adsorption of the nano crystals of the drug is done on selected stabilizers for stabilizing them against agglomeration, which are then incorporated into MDTs. This technique is mainly advantageous for poor water soluble drugs and also for a wide range of doses (up to 200 mg of drug per unit).
Fast Dissolving Films
It is a newer developing front in MDDDS that provides a very convenient means of taking medications and supplements. In this technique, water soluble film forming polymer (pullulan, CMC, HPMC, HEC, HPC, PVP, PVA etc.), drug and other taste masking ingredients are dissolved in non-aqueous solvent to prepare non-aqueous solution, which on evaporation of solvent forms a film. Resin adsorbate or coated micro particles of the drug can be incorporated into the film if the drug is bitter. This film when placed in mouth, melts or dissolves rapidly and release the drug in solution or suspension form. This system forms the thin films of size less than 2 X 2 inches which dissolves within 5 sec with instant drug delivery and flavored taste.
PATENTED TECHNOLOGIES OF FAST DISSOLVING TABLET TECHNOLOGY
Currently, four fast-dissolving/disintegrating technologies have reached the U.S. market:
* Zydis (R.P. Scherer, Inc.),
* WOWTAB (Yamanouchi Pharma Technologies,Inc.),
* OraSolv (Cima Labs, Inc.).
* DuraSolv (Cima Labs, Inc.).
Three others are available outside the U.S.:
* FlashDose (Fuisz Technologies, Ltd.),
* Flashtab (Prographarm Group),
* OraQuick (KV Pharmaceutical Co., Inc.)
Zydis Technology
Zydis formulation is a unique freeze dried tablet in which drug is physically entrapped or dissolved within the matrix of fast-dissolving carrier material. When Zydis units are put into the mouth, the freeze-dried structure disintegrates instantaneously and does not require water to aid swallowing. The Zydis matrix is composed of many materials designed to achieve a number of objectives. To impart strength and resilience during handling, polymers such as gelatin, dextran or alginates are incorporated. These form a glossy amorphous structure, which imparts strength. To obtain crystallinity, elegance and hardness, saccharides such as Mannitol or sorbitol are incorporated. Water is used in the manufacturing process to ensure production of porous units to achieve rapid disintegration. Various gums are used to prevent sedimentation of dispersed drug particles in the manufacturing process. Collapse protectants such as glycine prevent the shrinkage of Zydis units during freeze drying process or long term storage. Zydis products are packed in blister packs to protect the formulation from moisture in the environment.
Limitations:
* The amount of drug could be incorporated should generally be less than 400mg for insoluble drugs and less that 60mg for soluble drugs.
* The particle size of the insoluble drugs should not be less than 50μm and not more than 200μm to prevent sedimentation during processing.
Advantages:
* Buccal pharyngeal and gastric regions are all areas of absorption from this formulation. Any pre-gastric absorption avoids first-pass metabolism and can be an advantage in drugs that undergo a great deal of hepatic metabolism.
* The Zydis formulation self-preserving because the final water concentration in the freeze-dried product is too low to allow for microbial growth.
Disadvantages:
* The process of freeze-drying is a relatively expensive manufacturing process.
* The formulation is very lightweight and fragile, and therefore should not be stored in backpacks or the bottom of purses.
* It has poor stability at higher temperatures and humidities.
* The freeze-drying is time consuming process.
* It has poor physical resistance.
* Loading of high dose of water-soluble drugs is not possible.
OraSolv Technology
Orasolv Technology has been developed by CIMA labs. In this system active medicament is taste masked. It also contains effervescent disintegrating agent. Tablets are made by direct compression technique at low compression force in order to minimize oral dissolution time. Conventional blenders and tablet machine is used to produce the tablets. The tablets produced are soft and friable and packaged in specially designed pick and place system.
Advantages:
* The Orosolv formulations are not very hygroscopic
* The formulation can accommodate high doses.
* It also provides a distinct, pleasant sensation of effervescence in the mouth.
Disadvantages
* A weaker and more brittle tablet in comparison with conventional tablets.
* Poor mechanical strength.
* The cost of fast dissolving tablets is higher than the cost of standard tablets made by direct compression
* Manufacturing requires a controlled environment at low relative humidity.
List of US FDA Approved Products Available in the Market
|
Patented Technology |
Products® |
Name of the Company |
Composition |
|
Zydis |
Claritin, Reditab |
R. P. Scherer / Schering Plough, Kenilworth, USA. |
Micronized loratidine (10mg), citric acid, mannitol, gelatin, mint flavor |
|
|
Feldene Melt |
Pfizer Inc, NY, USA |
Piroxicam (10 or 20 mg), mannitol, gelatin, aspartame, citric anhydrous |
|
|
Maxalt-MLT |
R.P.Scherer / Merck & Co., NY, USA. |
Rizatriptan (5 or 10 mg), mannitol, gelatin, aspartame, peppermint flavor |
|
|
Pepcid RPD |
Merck & CO., NY, USA. |
Famotidine (20 or 40 mg), mannitol, gelatin,aspartame |
|
|
Zyprexa Zydis |
R.P.Scherer/Eli Lilly, Indianapolis, USA |
Olanzapine (5, 10, 15 or 20 mg), mannitol, gelatin, aspartame, methyl paraben sodium, propyl paraben sodium |
|
|
Zofran ODT |
R.P.Scherer/Glaxo Wellcome, Middlesex, UK. |
Ondansetron (4 or 8 mg), mannitol, gelatin, aspartame, methyl paraben sodium, propyl paraben sodium, strawberry flavor |
|
Orasolv |
Remeron Soltab |
CIMA / Organon, Glaxo Wellcome, Middlesex, UK. |
Mirtazepine (15,30 or 45 mg), mannitol, aspartame, citric acid, crosspovidone, Avicel, NaHCO3, HPMC, magnesium stearate povidone, PMA, starch, sucrose, orange flavor |
|
|
Tempra First Tabs |
CIMA / Mead Johnson, Bristol Myers Squibb, NY, USA. |
Acetaminophen (80 or 160 mg), mannitol (currently available in Canada) |
|
Durasolv |
Nulev |
CIMA/Schwarz Pharma. |
Hyoscyamine sulphate (0.125mg), aspartame, colloidal silicon dioxide, crospovidone, mint flavor, magnesium stearate, mannitol, Avicel |
|
|
Zoming ZMT |
CIMA / AstraZeneca, Wilmington, USA. |
Zolmitriptan (2.5mg), mannitol, aspartame, citric acid anhydrous, crospovidone, Avicel, sodium bicarbonate, magnesium stearate colloidal silicon dioxide, orange flavor |
Wowtab Technology
Wowtab Technology is patented by Pharmaceutical Co. WOW means "Without Water ". In this process, combination of low mouldability saccharides and high mouldability saccharides is used to obtain a rapidly melting strong tablet. The active ingredient is mixed with low mouldability saccharides and granulated with high mouldability saccharides and compressed into tablet.
Advantages:
* Offers Superior mouth feel due to the smooth melt action
* It is suitable for both conventional bottle and blister packaging
* Bit more stable to the environment than the Zydis and orasolv.
Flash Dose Technology
Flash dose technology has been patented by Fuisz. Nurofen meltlet, a new form of ibuprofen as melt-in-mouth tablets, prepared using flash dose technology is the first commercial product launched by Biovail Corporation. Flash dose tablets consists of self binding shearform matrix termed as "floss". Shearform matrices are prepared by flash heat processing.
Flashtab Technology
Prographarm laboratories have patented the Flashtab technology. Tablets prepared by this system consist of an active ingredient in the form of microcrystals. Drug microgranules may be prepared by using the conventional techniques like coacervation, microencapsulation, and extrusion- spheronisation. All the processing utilized conventional tableting technology.
Oraquick Technology
The Oraquick fast dissolving/disintegrating tablet formulation utilizes a patented taste masking technology. KV Pharmaceutical claims its microsphere technology, known as Micro Mask, has superior mouth feel over taste-masking alternatives. The taste masking process does not utilize solvents of any kind, and therefore leads to faster and more efficient production. Also, lower heat of production than alternative fast-dissolving/disintegrating technologies make Oraquick appropriate for heat-sensitive drugs. KV Pharmaceutical also claims that the matrix that surrounds and protects the drug powder in microencapsulated particles is more pliable, meaning tablets can be compressed to achieve significant mechanical strength without disrupting taste-masking Oraquick claims quick dissolution in a matter of seconds, with good taste-masking. There are no products using the Oraquick technology currently on the market, but KV pharmaceutical has products in development such as analgesics, scheduled drugs, cough and cold, psychotropics, and anti-infectives.
Each technology has a different mechanism, and each fast-dissolving / disintegrating dosage form varies regarding the following:
* Mechanical strength of final product;
* Drug and dosage form stability;
* Mouth feel;
* Taste;
* Rate of dissolution of drug formulation in saliva;
* Swallow ability;
* Rate of absorption from the saliva solution;
* Overall bioavailability.
POTENTIAL CANDIDATES FOR FAST DISSOLVING TABLETS (FDT)
Several factors must be considered while selecting an appropriate drug candidate for development of orally disintegrating dosage forms. The ultimate characteristics of a drug for dissolution in the mouth and pregastric absorption from ODTs include:
• Free from bitter taste.
• Dose lower than 20 mg.
• Small to moderate molecular weight.
• Good solubility in water and saliva.
• Partially nonionized at the oral cavity's pH.
• Ability to diffuse and partition into the epithelium of the upper GIT (log P >1, or preferably >2).
• Ability to permeate oral mucosal tissue.
In contrast, the following characteristics may render a drug unsuitable for delivery as an orally disintegrating dosage form:
• Short half-life and frequent dosing.
• Very bitter or unacceptable taste because taste masking cannot be successfully achieved.
• Require controlled or sustained release.
• Combination with anticholinergics.
Analgesics and Anti-inflammatory Agents:
Aloxiprin, auranofin,azapropazone, benorylate, diflunisal, etodolac, fenbufen, fenoprofen calcim, Flurbiprofen, ibuprofen, indomethacin, ketoprofen,meclofenamic oxaprozin acid, mefenamic acid, nabumetone, naproxen, oxyphenbutazone,phenylbutazone,piroxicam, sulindac.
Anthelmintics:
Albendazole, Bephenium Hydroxynaphthoate,cambendazole ,Dichlorophen, ivermectin, mebendazole, oxamniquine, oxfendazole, oxantel embonate, praziquantel, pyrantel embonate, thiabendazole. Anti-Arrhythmic Agents: Amiodarone HCl, Disopyramide, Flecainide acetate, quinidine sulphate.
Anti-bacterial Agents:
Benethamine penicillin, cinoxacin, ciprofloxacin HCl, clarithromycin,clofazimine, cloxacillin, demeclocycline, Doxycycline, erythromycin, ethionamide, imipenem, nalidixic acid, nitrofurantoin, rifampicin, spiramycin, sulphabenzamide, sulphadoxine, sulphamerazine, sulphacetamide, sulphadiazine, sulphafurazole, Sulphamethoxazole, sulphapyridine, tetracycline, trimethoprim.
Anti-coagulants: Dicoumarol, Dipyridamole, Nicoumalone, Phenindione.
Anti-depressants:
Amoxapine, ciclazindol, maprotiline HCl, Mianserin HCl, Nortriptyline HCl, Trazodone HCl, Trimipramine maleate.
Anti-diabetics:
Acetohexamide, chlorpropamide, glibenclamide, gliclazide, glipizide, tolazamide, tolbutamide.
Anti-epileptics:
Beclamide, carbamazepine, clonazepam, ethotoin, methoin, methsuximide, methylphenobarbitone, oxcarbazepine, paramethadione, phenacemide, phenobarbitone, phenytoin, phensuximide, primidone, sulthiame, valproic acid.
Anti-fungal:
Amphotericin, butoconazolenitrate, clotrimazole, Terconazole, tioconazole, undecenoic acid natamycin, nystatin, econazolenitrate, fluconazole, flucytosine, griseofulvin, Itraconazole, ketoconazole, miconazole, sulconazole nitrate, terbinafine HCl.
Anti-gout Agents: Allopurinol,probenecid,sulphinpyrazone.
Anti-hypertensive Agents:
Amlodipine, carvedilol, benidipine, darodipine, dilitazem HCl, diazoxide, felodipine, guanabenz acetate, indoramin, isradipine, minoxidil, nicardipine HCl, nifedipine, nimodipine, phenoxybenzamine HCl, prazosin HCL, reserpine, terazosin HCl.
Anti-malarials:
Amodiaquine, chloroquine, chlorproguanil HCl, halofantrine HCl, mefloquine HCl, proguanil HCl, Pyrimethamine, quinine sulphate.
Anti-migraine Agents:
Dihydroergotamine mesylate, ergotamine tartrate, methysergidemaleate, pizotifen maleate, sumatriptan succinate.
Anti-muscarinicAgents:
Atropine, benzhexol HCl, biperiden, ethopropazine HCl, hyoscine butyl bromide, hyoscyamine, mepenzolate Bromide, orphenadrine, oxyphencylcimine HCl, tropicamide.
Anti-neoplasticAgents and Immunosuppressants:
Aminoglutethimide, amsacrine, azathioprine, busulphan, chlorambucil, cyclosporin, dacarbazine, estramustine, etoposide, lomustine, melphalan,mercaptopurine, methotrexate, mitomycin, mitotane, mitozantrone, procarbazine HCl, tamoxifen citrate, testolactone.
Anti-protozoal Agents:
Benznidazole, clioquinol, decoquinate, diiodohydroxyquinoline, Diloxane furoate, dinitolmide, furzolidone, metronidazole, nimorazole, nitrofurazone, omidazole, tinidazole.
Anti-thyroid Agents: Carbimazole, propylthiouracil.
Anxiolytic, Sedatives, Hypnotics and Neuroleptics:
Alprazolam, amylobarbitone, barbitone, bentazepam, bromazepam, bromperidol, brotizolam, butobarbitone, carbromal, chlordiazepoxide, chlormethiazole, chlorpromazine, clobazam, clotiazepam, clozapine, diazepam, droperidol, ethinamate, flunanisone, flunitrazepam, fluopromazine, flupenthixol decanoate, fluphenazine decanoate, flurazepam, haloperidol,
Cardiac Inotropic Agents: Amrinone, digitoxin, digoxin, enoximone, lanatoside C, medigoxin.
Corticosteroids:
Beclomethasone, betamethasone, budesonide, cortisone acetate, desoxymethasone, dexamethasone, fludrocortisoneacetate, flunisolide, flucortolone, fluticasone propionate, hydrocortisone, methylprednisolone, prednisolone, prednisone, triamcinolone.
Diuretics:
Acetazolamide, amiloride, bendrofluazide, bumetanide, chlorothiazide, chlorthalidone, ethacrynic acid,frusemide, metolazone, spironolactone, triamterene.
Anti-parkinsonian Agents: Bromocriptine mesylate, lysuride maleate.
Gastro-intestinal Agents:
Bisacodyl, cimetidine, cisapride, diphenoxylate HCl, domperidone, famotidine, loperamide, mesalazine,nizatidine, omeprazole, ondansetron HCL,ranitidine HCl, sulphasalazine
Histamine H,-Receptor Antagonists:
Acrivastine, astemizole, cinnarizine, cyclizine, cyproheptadine HCl, dimenhydrinate, flunarizine HCl, loratadine, meclozine HCl, oxatomide, terfenadine,triprolidine.
Lipid Regulating Agents: Bezafibrate, clofibrate, fenofibrate, gemfibrozil,probucol.
Local Anaesthetics: Lidocaine
Neuro-muscular Agents: Pyridostigmine.
Nitrates and otherAnti-anginal Agents:
Amyl nitrate, glyceryltrinitrate, isosorbide dinitrate, isosorbide mononitrate, pentaerythritol tetranitrate.
NutritionalAgents:
Betacarotene, vitamin A, vitamin B2, vitamin D, vitamin E, vitamin K. Opioid Analgesics: codeine, morphine Dextropropyoxyphene, diamorphine, dihydrocodeine, meptazinol, methadone, nalbuphine, pentazocine.
OralVaccines:
Vaccines designed to prevent or reduce the symptoms of diseases of which the following is a representative:
Influenza, Tuberculosis, Meningitis, Hepatitis, Whooping Cough, Polio, Tetanus, Diphtheria, Malaria, Cholera, Herpes, Typhoid, HIV, AIDS, Measles, Lyme disease, Travelers’ Atrophic rhinitis, Erysipelas, Foot and Mouth disease, Swine, pneumonia, auto-immune disease conditions affecting companion and farm animals etc.
Proteins, Peptides and Recombinant drugs:
Insulin, glucagon, growth hormone (somatotropin),polypeptides or their derivatives, calcitonins and synthetic modifications thereof, enkephalins, interferons, LHRH and analogues (nafarelin, buserelin, zolidex), GHRH, secretin, bradykin antagonists, GRF, THF, TRH, ACTH analogues, IGF (insulin like growth factors), CGRP (calcitoningene related peptide), atrial natriurectic peptide, vasopressin and analogues (DDAVP, lypressin), factor VIII, G-CSF (granulocyte-colony stimulating factor), EPO (erythropoitin).
SexHormones:
Clomiphenecitrate, danazol, ethinyloestradiol, medroxyprogesterone acetate, mestranol, tibolone. Methyltestosterone, norethisterone, norgestrel, oestradiol, conjugated oestrogens, progesterone, stanozolol, Stiboestrol, testosterone
Spermicides: Nonoxynol.
Stimulants: Amphetamine, dexamphetamine, dexfenfluramine, fenfluramine, mazindol, pemoline
OTHER EXCIPIENTS USED IN FAST DISSOLVING TABLETS
Excipients balance the properties of the actives in fast-melting tablets. This demands a thorough understanding of the chemistry of these excipients to prevent interaction with the actives. Determining the cost of these ingredients is another issue that needs to be addressed by formulators. The role of excipients is important in the formulation of fast-dissolving tablets. These inactive food-grade ingredients, when incorporated in the formulation, impart the desired organoleptic properties and product efficacy. Excipients are general and can be used for a broad range of actives, except some actives that require masking agents.
BULKING MATERIALS:
Bulking materials are significant in the formulation of fast-dissolving tablets. The material contributes functions of a diluents, filler and cost reducer. Bulking agents improve the textural characteristics that in turn enhance the disintegration in the mouth, besides adding bulk also reduces the concentration of the active in the composition. The recommended bulking agents for this delivery system should be more sugar-based such as mannitol, polydextrose, lactitol, DCL (direct compressible lactose) and starch hydrolystate for higher aqueous solubility and good sensory perception. Mannitol in particular has high aqueous solubility and good sensory perception. Bulking agents are added in the range of 10 percent to about 90 percent by weight of the final composition.
The excipients could be ranked in descending order in terms of their brittleness:
microcrystalline cellulose > spray-dried lactose > beta lactose > alpha lactose > alpha lactose monohydrate > dicalcium phosphate dihydrate.
The sugar based excipients which are commonly used are especially bulking agents (like dextrose, fructose, lactilol, maltilol, maltose, mannitol, sorbitol,starch hydrolysate, polydextrose and xylitol) which display high aqueous solubility and sweetness, and hence impart taste masking property and provide pleasing mouth feel.
Mizumitoet al classified sugar-based excipients into two types on the basis of molding and dissolution rate:
Type 1 saccharides (lactose and mannitol) exhibit low mouldability but high dissolution rate.
Type 2 saccharides (maltose and maltilol) exhibit high mouldability but low dissolution rate.
Properties of Modified Starches/Celluloses Used in ODTs
|
S. No. |
Superdisintegrant |
Properties
|
|
1. |
Croscarmellose sodium |
High swelling capacity, effective at low concentration (0.5-2.0%), can be used up to 5%. |
|
2. |
Crospovidone |
Completely insoluble in water. Rapidly disperses and swells in water, but does not gel even after prolonged exposure. Greatest rate of swelling compared to other disintegrants. Greater surface area to volume ratio than other disintegrants. Effective concentration (1-3%). Available in micronized grades if needed for improving state of dispersion in the powder blend. |
|
3. |
Sodium starch glycolate |
Absorbs water rapidly, resulting in swelling up to 6%. High concentration causes gelling and loss of disintegration |
EMULSIFYING AGENTS:
Emulsifying agents are important excipients for formulating fast-melting tablets they aid in rapid disintegration and drug release without chewing, swallowing or drinking water. In addition, incorporating emulsifying agents is useful in stabilizing the immiscible blends and enhancing bioavailability. A wide range of emulsifiers is recommended for fast-dissolving tablet formulation, including alkyl sulfates, propylene glycol esters, lecithin, sucrose esters and others. These agents can be incorporated in the range of 0.05 percent to about 15 percent by weight of the final composition.
LUBRICANTS:
Lubricants, though not essential excipients, can further assist in making these tablets more palatable after they disintegrate in the mouth. Lubricants remove grittiness and assist in the drug transport mechanism from the mouth down into the stomach.
FLAVOURS AND SWEETENERS:
Flavours and taste-masking agents make the products more palatable and pleasing for patients. The addition of these ingredients assists in overcoming bitterness and undesirable tastes of some active ingredients. Both natural and synthetic flavours can be used to improve the organoleptic characteristic of fast-melting tablets. Formulators can choose from a wide range of sweeteners including sugar, dextrose and fructose, as well as non-nutritive sweeteners such as aspartame, sodium saccharin, sugar alcohols and sucralose. The addition of sweeteners contributes a pleasant taste as well as bulk to the composition.
SUPERDISINTEGRANTS:
Disintegrants are substances routinely included in tablet formulations and in some hard shell capsule formulations to promote moisture penetration and dispersion of the matrix of dosage form in dissolution fluids. An oral solid dosage form should ideally disperse into the primary particles from which it was prepared. Superdisintegrants are generally used at a low concentration, typically 1-10% by weight relative to total weight of dosage unit. Generally employed superdisintegrants are crosscarmellose sodium (Ac-Di-Sol), Crosspovidone (CP), sodium starch glycolate (SSG) etc. which represent example of crosslinked cellulose, crosslinked polymer and crosslinked starch respectively.
Selection of appropriate formulation excipients and manufacturing technology is necessary for obtaining the optimized design features of orally disintegrating dosage forms. Ideally, superdisintegrants should cause the tablet to disrupt, not only into the granules from which it was compressed but also into powder particles from which the granules were prepared.
SELECTION OF SUPERDISINTEGRANTS:
Although superdisintegrants primarily affect the rate of disintegration, but when used at high levels they can also affect mouth feel, tablet hardness and friability. Hence, various ideal factors to be considered while selecting an appropriate superdisintegrants for a particular formulation should:
• Produce rapid disintegration, when tablet comes in contact with saliva in the mouth/oral cavity.
• Be compactable enough to produce less friable tablets.
• Produce good mouth feel to the patients. Thus, small particle size is preferred to achieve patient compliance.
• Have good flow, since it improves the flow characteristics of total blend.
Mechanism of action of disintegrant:
Various mechanisms proposed in this concern include water wicking, swelling, deformation recovery, repulsion and heat of wetting. It seems likely that no single mechanism can explain the complex behavior of the disintegrants. However, each of these proposed mechanisms provides some understanding of different aspects of disintegrant action.
Water wicking:
The ability of disintegrant to draw water into the porous network of tablet is essential for effective disintegration. On keeping the tablet into suitable aqueous medium, the medium enters into tablet and replaces the air adsorbed on the particles which weakens the intermolecular bonds and breaks the tablet into fine particles. Water uptake by tablet depends upon hydrophilicity of the drug/excipients and on tableting conditions. Unlike swelling, which is mainly a measure of volume expansion with accompanying force generation, water wicking is not necessarily accompanied by a volume increase. The ability of a system to draw water can be summarized by Washburn’s equation:
L2 = (γ Cosθ/2η) × rt
The Washburn equation is too simplistic to apply to a dynamic tablet-disintegration process, but it does show that any change in the surface tension (γ), pore size (r), solid-liquid contact angle (θ) or liquid viscosity (η) could change the water wicking efficiency. L is the length of water penetration in the capillary and t is the time. This process is also considered as capillary action method.
Swelling:
Although water penetration is a necessary first step for disintegration, swelling is probably the most widely accepted mechanism of action for tablet disintegrants. For swelling to be effective as a mechanism of disintegration, there must be a superstructure against which disintegrant swells. Figure below represents the disintegration of tablet by wicking and swelling. Swelling of the disintegrant against the matrix leads to development of a swelling force. A large internal porosity in the dosage form in which much of the swelling can be accommodated reduces the effectiveness of the disintegrant. On the other hand, sufficient swelling force is exerted in the tablet with low porosity. It is worthwhile to note that if packing fraction is very high, fluid is unable to penetrate in the tablet and disintegration is again slowed down.
Heat of wetting:
When disintegrants with exothermic properties get wetted, localized stress is created due to capillary air expansion, which aids in disintegration of tablet. This explanation, however, is limited to only a few types of disintegrants and cannot describe the action of most modern disintegrating agents.
Due to release of gases:
Carbon dioxide gets released within tablets on wetting due to interaction between bicarbonate and carbonate with citric acid or tartaric acid. The tablet disintegrates due to generation of pressure within the tablet. This effervescent mixture is used when pharmacist needs to formulate very rapidly dissolving tablets or fast disintegrating tablet. As these disintegrants are highly sensitive to small changes in humidity level and temperature, strict control of environment is required during preparation of the tablets. The effervescent blend is either added immediately prior to compression or can be added into two separate fractions of formulation.
Particle repulsive forces:
This is another mechanism of disintegration that attempts to explain the swelling of tablet made with non-swellable disintegrants. Guyot-Hermann proposed a particle-particle repulsion theory to explain the observation that particles which do not swell extensively such as starch, could still disintegrates tablets. According to this theory, water penetrates into tablet through hydrophilic pores and a continuous starch network is created that can convey water from one particle to the next, imparting a significant hydrostatic pressure. The water then penetrates between starch grains because of its affinity for starch surfaces, thereby breaking hydrogen bonds and other forces holding the tablet together. The electric repulsive forces between particles are the mechanism of disintegration and water is required for it.
Deformation recovery:
Deformation recovery theory implies that the shape of disintegrant particles is distorted during compression and the particles return to their precompression shape upon wetting, thereby causing the tablet to break apart. Such a phenomenon may be an important aspect of the mechanism of action of disintegrants such as Crosspovidone and starch that exhibit little or no swelling.
By enzymatic reaction:
Enzymes present in the body also act as disintegrants. These enzymes dearth the binding action of binder and helps in disintegration. Due to swelling, pressure is exerted in the outer direction that causes the tablet to burst or the accelerated absorption of water leads to an enormous increase in the volume of granules to promote disintegration.
Application of Various Commercially Used Combinations of Modified Cellulose/Starch Used in ODTs
|
Superdisintegrant and Disintegrants |
Applications |
|||||
|
Brand Name |
Common Name |
Classification |
Functional Category |
Properties |
EMC at 25ºC/ 90%RH |
Typical Uses |
|
CL-Kollidon |
Crospovidone |
Polyvinyl-pyrrolidone |
Tablet super disintegrant |
Swelling (18% in 10s), (45% in 20s) |
62% |
Disintegrant (Dry and Wet granulation) |
|
Ac-DiSol |
Croscarmellose Sodium |
Cellulose, carboxy-methyl ether, sodium salt crosslinked |
Tablet and capsule disintegrant |
Wicking and swelling (12% in 10s), (23% in 20s) |
88% |
Disintegrant for capsules, tablets and granules |
|
Explotab Primojel |
Sodium starch glycolate |
Sodium carboxymethyl starch |
Tablet and capsule super disintegrant |
Swelling capacity (300 times) |
------ |
Disintegrant (Dry and Wet granulation) |
|
Explotab V17 |
Sodium starch glycolate |
(Cross linked substituted Carboxy-methyl ether) sodium carboxymethyl starch |
Super disintegrant |
More swelling than Explotab |
|
Disintegration & dissolution aid. Not for use in wet granulation |
|
Explotab CLV |
Sodium starch glycolate |
(Cross linked low substituted Carboxy-methyl ether) Sodium carboxymethyl starch |
Super disintegrant |
Swelling |
|
Use in wet granulation and high shear equipment |
|
L-HPC |
Hydroxypropyl cellulose(low substituted) |
Cellulose, 2-hydroxypropyl ether |
Tablet and capsule super disintegrant |
Swelling (13% in 10s), (50% in 20s) |
37% |
Disintegrant and Binder in wet granulation |
|
Starch 1500 |
Starch, Pre-gelatinized |
Pregelatinized starch |
Diluent , binder and disintegrant |
Hygroscopic |
22% |
Binder/diluent & disintegrant |
|
Avicel |
Microcrystalline cellulose |
Cellulose |
Tablet & capsule diluent, Tablet disintegrant |
Hygroscopic, swelling- (12% in 10s), (18% in 20s) |
18% |
Binder/diluent, lubricant and disintegrant |
TASTE MASKING TECHNOLOGIES
Taste masking is an essential requirement for fast dissolving tablets for commercial success. Taste masking of the active ingredients can be achieved by various techniques. Drugs with unacceptable bitter taste can be microencapsulated into pH sensitive acrylic polymers. Cefuroxime axetil is microencapsulated in various types of acrylic polymers (e.g., Eudragit E, Eudragit L-55 and Eudragit RL) by solvent evaporation and solvent extraction techniques. These polymer microspheres showed efficient taste masking and complete dissolution in a short period. Fine granules of drug and disintegrant (e.g. low substituted Hydroxypropyl cellulose) when coated with a water insoluble polymer (e.g. ethylcellulose) masked the bitter taste of sparfloxacin. The addition of low substituted Hydroxypropyl cellulose as disintegrant to the drug in cores resulted in increased dissolution rate and bioavailability of sparfloxacin compared to its conventional tablets.
Ozer and Hincal reported a simple coacervation method using gelatin, and anhydrous sodium sulphate as coacervating agent for taste making of beclamide. Beclamide is an anti-epileptic drug with unpleasant taste. It is microencapsulated into gelatin, with sodium sulphate as coacervating agent, and glutaraldehyde as hardening agent. The microcapsules after formation are dehydrated using alcohol. The core: wall substance ratio was 1:1, and the taste could be successfully masked.
A novel technique for taste masking of macrolides (e.g. erythromycin and clarithromycin) is reported by Yajima . Monoglycerides having a low melting point which can form good elaborate film, and easily soluble in intestine, and polymers which are insoluble in the mouth (pH 5-8), but are freely soluble in stomach (pH 1-4), are selected for taste masking of drugs with unpleasant taste. The polymer is dissolved or dispersed in monoglyceride, and the drug is granulated with above mixture and the resultant granules are cooled.
Traditional taste masking techniques in oral pharmaceuticals
Taste masking using flavours and sweeteners: Artificial sweeteners and flavours are generally being used along with other taste-masking techniques to improve the efficiency of these techniques in dentifrices, mouthwashes and cough drops. The examples are given in Table no. 1.
Taste masking using Lipophilic Vehicles: - It is the property of oils, surfactants, polyalcohols and lipids to increase the viscosity in the mouth and to coat the taste buds and therefore they are potential taste masking agents. Formulations with a large excess of lecithin or lecithin like substances are claimed to control bitter taste in pharmaceuticals. Examples are given in Table no. 2.
Taste masking by Coating with Hydrophilic Vehicles: - Carbohydrates can be used as a coating material to mask the taste of orally administered drugs. Various forms of proteins have been used extensively for taste masking. Some examples are given in Table no. 3
Taste masking by Ion-Exchange Resins (IERs):- To stabilize the sensitive components, to sustain the drug release, to disintegrate tablets, and to mask taste, ion-exchange resins are used in formulations. Some examples of drugs and taste masking agents and ion exchange resins are given in Table no.4.
Table. 1: Taste masking using flavours and sweeteners
|
SL No |
Drug(s) |
Taste masking agent(s) |
|
1. |
Aspirin |
Sodium phenolate |
|
2. |
Chlorpheniramine, Phenyl propanolamine |
Sod. bicarbonate, citric acid, orange/cream flavour |
|
3. |
Famotidine |
Sod. bicarbonate, citric acid, lemon flavour |
|
4. |
Ibuprofen |
Sod. citrate dihydrate, sod. saccharin, refined sugar |
|
5. |
Theophylline |
D-sorbitol, sodium saccharin, sodium glutamate, and vanilla essence |
|
6. |
Acetaminophen |
Sod. bicarbonate, citric acid, cherry flavour |
|
7. |
Caffeine |
Starch, lactose, and mannitol |
Table. 2: Taste masking using lipophilic vehicles
|
S. No |
Drug(s) |
Taste masking agent(s) |
|
1. |
Isoprothiolane |
Hydrogenated oil and HPMC |
|
2. |
Acetaminophen |
Molten stearyl stearate |
|
3. |
Talampicillin HCl |
Magnesium aluminum silicate & soyabean lecithin |
|
4. |
Clarithromycin |
Glyceryl monostearate and AMCE |
|
5. |
Indeloxazine HC |
Hydrogenated oil and surfactants |
HPMC=Hydroxypropyl methyl cellulose; AMCE=Aminoalkyl methacrylate copolymer E
Table. 3: Taste masking using polymer coating
|
S.No |
Drug(s) |
Polymer(s) used |
|
|
Pinaverium bromide |
Cellulose or shellac |
|
|
Ibuprofen |
Methacrylic acid copolymer (Eudragit) |
|
|
Amoxycillin trihydrate |
MCC, L-HPC |
|
|
Clarithromycin |
Carbopol, PVP |
|
|
Roxithromycin |
PEG, Eudragit L 100–55 |
|
|
Cefuroxime axetil |
Eudragit L-55 and RL |
|
|
Pirenzepine & Oxybutynin |
Eudragit E-100, MCC, HPC |
|
|
Levofloxacin |
Eudragit E100, cellulose acetate |
HPMC= Hydroxypropyl methyl cellulose; HEC= Hydroxyethyl cellulose; HPC=Hydroxypropyl cellulose; L-HPC=
Low substituted hydroxypropyl cellulose; CMC= Carboxy methyl cellulose; PVP= Polyvinyl pyrollidone; EC=Ethyl
cellulose; MCC= Microcrystalline cellulose; PEG= Polyethylene glycol; Tio2=Titanium dioxide.
Table. 4: List of drugs and taste masking ion exchange resins
|
S. No |
Drug(s) |
Resin/complexing agent |
|
1 |
Carbetapentane citrate |
Cyclodextrin |
|
2 |
Ibuprofen |
Hydroxypropyl b-cyclodextrin |
|
3 |
Diphenhydramine HCl |
Indion CRP 244, indion CRP 254 |
|
4 |
Buflomedil
|
Amberlite IRP 69 |
|
5 |
Orbifloxacin |
Amberlite IRP 69 |
EVALUATION OF BLEND
The prepared blend was evaluated by following tests.
Angle of repose
Bulk density
Tapped density
Carr’s index
Hauser’s ratio
Angle of repose
Angle of repose was determined by using funnel method. The accurately weighed blend was taken in a funnel. The height of the funnel was adjusted in such a way that the tip of the funnel just touches the apex of the heap of blend. The drug (as solid dispersion)-excipients blend was allowed to flow through the funnel Freely on to the surface. The diameter of the powder cone was measured and angle of repose was calculated using the following equation: Tan q = h/r
Where h and r are the height and radius of the powder conc.
Bulk density
Apparent bulk density was determined by pouring a weighed quantity of blend into graduated cylinder and measuring the volume and weight.
BD =Weight of the powder / Volume of the packing.
Tapped Density
It was determined by placing a graduated cylinder, containing a known mass of drug-excipients blend. The cylinder was allowed to fall under its own weight onto a hard surface from the height of 10cm at 2- second intervals. The tapping was continued until no further change in volume was noted.
TBD =Weight of the powder / volume of the tapped packing.
Compressibility Index
The Compressibility Index of the blends was determined by Carr’s compressibility index.
Carr’s compressibility index (%) = [(TBD-LBD) X 100] / TBD
A similar index has been defined by Hausner ---Hauser’s ratio = Tapped density/ Poured density
Hausner’s ratio<1.25 – Good flow = 20% Carr
Hausner’s ratio >1.25 – Poor flow = 33% Carr
Commercially available fast dissolving tablets in India
|
Trade Name |
Active Drug |
Manufacturer |
|
Cefadur DT |
Cefadroxil |
Cipla (protec) |
|
Cefinar DT |
Cefixime |
Zydus Alidac |
|
Zofran ODT; Vomokind MD |
Ondansetron |
Glaxo Wellcome; Mankind |
|
Torrox MT; Dolib MD |
Rofecoxib |
Torrent pharmaceuticals; Panacea |
|
Acivir DT |
Acyclovir |
Cipla |
|
Dom DT; Domestal DT |
Domperidone |
Dr. Morepen; Torrent Pharma |
|
Nexus MD; Nimex MD;Nimed MD; Nimulid MD |
Nimesulide |
Lexus; Mexon Health Care; Zota Pharma;Panacea Biotech |
|
Mosid MT |
Mosapride |
Torrent Pharma |
|
Allegra ODT |
Fexofenadine |
Sanofi Aventis |
|
Benadryl Allergy Fast Melt |
Diphenhydramine |
Pfizer Consumer Healthcare |
|
Cibalginadue FAST |
Ibuprofen |
Novartis Consumer Health |
|
Pepcid RPD |
Famotidine |
Merck & Co. |
|
Claratin RediTabs |
Loratadine |
Schering-Plough Corporation |
|
Maxalt-MLT |
Rizatriptan |
Merck & Co |
|
Mirtazapine ODT |
Mirtazapine |
Teva Pharmaceuticals |
|
Zotacet MD |
Cetrizine HCl |
Zota Pharma |
|
Romilast |
Montelukast |
Ranbaxy |
CONCLUSION
The innovations in the arena of formulating Fast Dissolving Tablets are aimed at both increasing the performance of the dosage form by decreasing the disintegration time and increasing the patient compliance by masking the objectionable taste of the active ingredients. FDT need to be formulated for pediatric, geriatric, bedridden, psychotic patients, for those patients who are busy in traveling, has difficulty in swallowing and may not have access to water. FDT offers the combined advantages of ease of dosing and convenience of dosing in the absence of water or fluid. These achievements require constant up gradation of formulation variables as well as technologies involved in the production of dosage forms. In this article, I have attempted to unveil the strategies that have been used by inventors for improving the performance and acceptability of Fast Dissolving Tablets. The use of superdisintegrants for achieving these aims is not new. However, with the improvement design of new techniques, it has become possible to develop FDTs with reduced content of superdisintegrants and with better mouth feel.
The use of techniques like freeze drying, direct compression and effervescence are highly suitable for formulating stable and acceptable dosage forms of vitamins, enzymes and thermolabile drugs. Which are indeed highly acceptable means of delivery drugs to especially, pediatric and geriatric patients. The development of Durasolv and Orasolv technologies are worth mentioning in this regard. Similarly, considerable research towards producing modified microcrystalline cellulose or starch in order to engineer them suitable for direct compression has significantly reduced the product development time for optimizing FDT formulation.
The application of nanotechnology to formulation is expected to further enhance the acceptance and performance of these dosage forms. However, not much work seems to have been done in this particular specialized area. Nevertheless, judicious use of excipients and technology can be expected to make the task of formulating an acceptable and effective FDT easier than before. However, substantial amount of research remains to be conducted for the development of natural polymer based system which is highly site specific. Furthermore, development of such system correlating well with all desired characteristics for effective delivery would nevertheless be an appropriate futuristic endeavor. Therefore in coming era, there is going to be continued interest for the development of natural polymers based orally disintegrating tablets. The future trends in innovations of drug delivery systems will continue to bring together different technological disciplines and formulation aspects to create novel technologies.
REFERENCES
[1] Sharma S. Pharmainfo.net, 2008; 6(5).
Available at: https://www.pharmainfo.net/reviews/ orodispersable-tablet-review.
[2] Fu Y, Yang S, Jeong SH, Kimura S, Park K. Crit Rev Ther Drug Carrier Sys. 2004; 21, 433-475.
[3] Rakesh Pahwa, Mona Piplani, Prabodh C.Sharma, Dhirender Kaushik and Sanju Nanda; Orally Disintegrating Tablets - Friendly to Pediatrics and Geriatrics;
Available online at www.scholarsresearchlibrary.com.
[4] CIMA Labs, Inc. CIMA--Technologies. 2 FEB 2011;
Available at: https://www.cimalabs.com/tech.htm.
[5] Yamanouchi Pharma Technologies, Inc. WOWTAB. 25 JAN 2011;
Available at: https://www.ypharma.com/wowtab.html.
[6] KV Pharmaceutical Company. Drug Delivery Technologies (technical bulletin);
KV Pharmaceutical Company. OraQuick, 2 FEB 2010;
Available at: https://www.kvpharma.com/tech/3_1_oraquick.html
[7] www. ElanNanoCrystal_Technology.html.
[8] Augsburger LL, Brzeczko AW, Shah U. Encyclopedia of Pharmaceutical Technology. 2nd edition.2002; 3, 2623-2638.
[9] Kamal Saroha, Pooja Mathur, Surender Verma, Navneet Syan and Ajay Kumar on Mouth dissolving tablets: An overview on future compaction in oral formulation technologies , Der Pharmacia Sinica, 2010, 1 (1): 179-187; Available at: at www.pelagiaresearchlibrary.com.
[10] Saxena V et al IJRAP 2010 1(2),399-407; Orally Disintegrating Tablets-A friendly dosage form.
[11] Honey Goel, Parshuram Rai et al on Orally Disintegrating Systems: Innovations in Formulation and Technology at Recent Patents on Drug Delivery & Formulation 2008, 2, 258-274.
[12] Rajeshree Panigrahi, Saiprasanna Behera on A Review On Fast Dissolving Tablets;
URL: https://www.webmedcentral.com/article_view/809.
[13] Shailendra Kumar Singh et al on Fast Disintegrating Combination Tablets Of Omeprazole And Domperidone; Asian Journal of Pharmaceutical and Clinical Research , Vol.2 Issue 3, July-September 2009.
[14] Mukesh P. Ratnaparkhi et al; Fast Dissolving Tablet at JPR Vol 2, No 1 (2009): January.
[15] D Bhowmik et al; Fast Dissolving Tablet: An Overview at Journal of Chemical and Pharmaceutical Research, 2009, 1(1): 163-177.
[16] Prashant Khemariya et al; Preparation and evaluation of mouth dissolving tablets of meloxicam; International Journal of Drug Delivery 2 (2010) 76-80;
available online at https://www.arjournals.org/ijdd.html.
[17] Bhupendra G Prajapati et al; A Review on Recent patents on Fast Dissolving Drug Delivery System; International Journal of PharmTech Research (IJPRIF) Vol.1, No.3, pp 790-798 , July-Sept 2009.









