About Authors:
S.R.Edwin Singh*
Department of Pharmaceutics, Seshachala College of Pharmacy,
Tirupathi – Chennai High way, Puttur-517 583,
Chittoor (dist), A.P., India.
Abstract:
This article aims to review the recent developments in the buccal adhesive drug delivery systems to provide basic principles to the young scientists, which will be useful to circumvent the difficulties associated with the formulation design. The Rapid developments in the field of molecular biology and gene technology resulted in generation of many macromolecular drugs including peptides, proteins, polysaccharides and nucleic acids in great number possessing superior pharmacological efficacy with site specificity and devoid of unwanted and toxic effects. The oral cavity is highly acceptable by patients. The mucosa is relatively permeable with a rich blood supply, it is robust and shows short recovery times after stress or damage. The virtual lack of Langerhans cells makes the oral mucosa tolerant to potential allergens. Buccal drug delivery leads direct access to the systemic circulation through the internal jugular vein bypasses drugs from the hepatic first pass metabolism leading to high bioavailability. Buccal route is an attractive route of administration for systemic drug delivery. Buccal bioadhesive films, releasing topical drugs in the oral cavity at a slow and predetermined rate, provide distinct advantages over traditional dosage forms for treatment of many diseases.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1319
INTRODUCTION
For many decades treatment of an acute disease or a chronic illness has been mostly accomplished by delivering drugs using various pharmaceutical dosage forms, including tablets, capsules, pills, suppositories, creams, ointments, liquids, aerosols and injectables as carriers. Amongst various routes of drug delivery, oral route is perhaps the most preferred to the patient and the clinician alike However, this route presents some problems for a few drugs1. The enzymes in the GI fluids, GIT-pH conditions, the enzymes bound to GIT membranes are a few factors responsible for the bioavailability problems. The blood that drains the GIT carries the drug directly to the liver leading to first-pass metabolism. Thus drugs, which are susceptible to acid hydrolysis and extensive presystemic metabolism, may exhibit poor bioavailability. The inherent problems associated with the drug, in some cases, can be solved by modifying the formulation or by changing the routes of administration. Parentral, mucosal and transdermal routes circumvent hepatic first-pass metabolism and offer alternative routes for the systemic delivery of drugs2.
A drug administered via parentral route places the drug directly into the systemic circulation and produces effective plasma drug levels. However, this route is associated with pain on administration and formulations need to be sterile.
During past few years, increasing interest has arisen in the use of bioadhesive formulations for the development of novel drug delivery systems. Bioadhesive formulations have a wide scope of applications, for both systemic and local effects. Transmucosal routes of drug delivery (i.e., the mucosal linings of the nasal, rectal, vaginal, ocular and oral cavity) offer distinct advantages over peroral administration for systemic drug delivery.
The oral cavity is highly acceptable by patients. The mucosa is relatively permeable with a rich blood supply, it is robust and shows short recovery times after stress or damage. The virtual lack of Langerhans cells makes the oral mucosa tolerant to potential allergens. The oral transmucosal drug delivery bypasses first pass effect and avoids pre-systemic elimination in the GI tract. These factors make the oral mucosa a very attractive and feasible site for systemic drug delivery within the oral mucosal cavity, delivery of drugs is classified into the three categories: (1) sublingual delivery, which is delivery of drugs through the mucosal membranes lining the floor of the mouth, (2) buccal delivery, which is drug administration through the mucosal membranes lining the cheeks, and (3) local delivery, which is drug delivery into the oral cavity4.
Though the buccal mucosa is less permeable than sublingual mucosa, it is better suited to the use of retentive systems such as mucoadhesive patches or tablets, because mucosa has a smooth and relatively immobile surface for placement of such systems. These attributes make the buccal mucosa more suitable for sustained delivery applications. Buccal mucosa also gives rapid absorption of drugs than oral route. A few drugs have been successfully administered via buccal route. For example, buccal buprenorphine works as rapidly as sublingual buprenorphine. Other examples are nicotine, morphine, propranolol etc.
Advantages of Drug Delivery Through Buccal Mucosa
i) It permits easy accessibility
ii) It is a passive system and does not require activation.
iii) Enzymatic activity is very low as compared to stomach.
iv) It bypasses hepatic first-pass metabolism, prevents gastric acid lability, thus increases bioavailability of drug.
v) Buccal mucosa is highly perfused with blood vessels and offers greater permeability than skin.
Limitations of Drug Delivery Through Buccal Mucosa5
i) Drug that is impermeable to the oral mucosa cannot be used.
ii) Surface area available for absorption is less.
iii) Patient may swallow the patch.
iv) Eating and drinking are restricted until complete absorption has taken place, so these drug delivery systems should be fast releasing systems.
Some simple drug delivery systems to oral mucosa include solutions, mouthwashes, mouth paints, chewable tablets, buccal and sublingual tablets and capsules. Bioadhesive multilayered compacts containing cetylpyridinium chloride have been formulated and evaluated in vitro. The device produced more uniform and effective plasma levels with adequate comfort and less irritancy for 3 hours when compared to a proprietary lozenge formulation.
The first evidence of the oral mucosa as a potential absorptive mucosa was through studies of Brunton. He showed that sublingual therapy with glyceryl nitrate could alleviate symptoms of angina pectoris.
The advances in bioadhesive and controlled release technology have caused much interest in drug delivery through the oral mucosa. A number of compounds have been administered through the oral mucosa like nitroglycerine, ascorbic acid, nicotine, noscapine, timolol, propranolol, astemizole, and chlorpheniramine maleate. Peptides such as insulin have been studied via buccal and sublingual routes
Table – 1 : Commercially available drug delivery systems for systemic delivery by the oral Mucosal Route.
|
Mucosal site |
Drug |
Dosage form |
|
Buccal |
Propranolol |
patch-SR |
|
Lorazepam |
lyophilized tablets |
|
|
Verapamil HCl |
sustain release tablets |
|
|
Oxazepam |
lyophilised tablets |
|
|
Prochlorperazine |
bioadhesive tablets and solutions |
|
|
Nicotine |
chewing gum |
|
|
Sublingual |
Nitroglycerine |
tablets, sprays, bioadhesive tablets |
|
Buprenorphine |
Tablets |
|
|
Nifedipine |
Tablets |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Oral Cavity as a Site for Drug Delivery
Physical Description of Oral Cavity
The oral cavity can be divided into two regions; the outer oral vestibule which is bounded by lips and cheeks and the oral cavity itself. The borders being formed by the hard and soft palates, the floor of the mouth and the pillars of the fauces and tonsils. Virtually all of the membranes that line the oral cavity could potentially be used for systemic drug delivery. However each region possesses different properties and characteristics and therefore requires different approaches in the design and formulation of suitable delivery systems6.
Regional variations in the composition of oral mucosa pertinent to systemic drug delivery
Several membranes line the oral cavity and each offers different problems for its utilization as a portal for drug entry into the systemic circulation. The membranes that line the oral cavity have a total area of approximately 200 cm2 and show differences in structure, thickness and blood flow, depending on their location. Both keratinized and nonkeratinized tissues of varying thickness and composition are found in the oral cavity. In general, nonkeratinized tissue is considerably thicker than keratinized tissue, but the nonkeratinized floor of the mouth is very thin (approximately 100 mm). The keratinized layers of the oral mucosal epithelia form a protective surface, which is mechanically tough and resistant to physical insult and penetration by any foreign substance7.
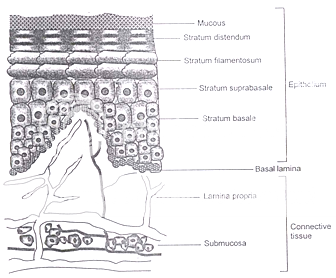
Figure 2: Schematic diagram of cross section of oral mucosa
Probably two most useful mucosal membranes for systemic drug delivery in the oral cavity are the buccal and sublingual membranes. The total area of these membranes is 50.2 cm2 and 26.5 cm2 with the thickness of 500 – 600 mm and 100 – 200 mm, respectively. Both these regions are non-keratinized.
Blood Supply to the Oral Mucosa
The blood supply to the oral cavity tissue is delivered via the external carotid artery, which branches into the maxillary, lingual and facial arteries. Blood from the capillary beds is collected by three main veins that finally flow into the internal jugular vein. Thus, delivery of drugs via the oral mucosa drains directly into the systemic circulation and thus avoids first pass metabolism in the liver. Squier et al (1976) have documented values for blood flow through the oral mucosa of Rhesus Monkey as given in Table 2.
Table 2: Blood flow through oral mucosa of Rhesus Monkey
|
Site |
Blood flow (ml/min/cm2) |
|
Buccal |
2.40 |
|
Sublingual |
0.97 |
|
Gingival |
1.47 |
|
Palatal |
0.89 |
Although blood flow through oral mucosa of humans has not been reported, but it is generally considered that the blood flows through human oral mucosa, even during disease, is sufficiently fast as not to be rate limiting factor in the absorption of drugs via the oral mucosa.
Saliva
There are three major glands supplying saliva to the oral cavity; parotid; sublingual and submaxillary. Saliva is composed of 99% water and is a complex fluid containing organic and inorganic materials. The pH of saliva ranges from 5.8 to 7.4. It has a low buffering capacity and principle buffer of Saliva being bicarbonate8. Saliva is low in enzyme content and its other components such as potassium, calcium and proteins do not appear to adversely affect drug delivery.
Factors Affecting Oral Mucosal Drug Delivery System Design
Many factors affect the successful delivery of a drug molecule into the systemic circulation via oral mucosa. In general, the approach taken in the development of an oral mucosal drug delivery system is to identify suitable drug candidate based on both their physicochemical properties and ability to penetrate the oral mucosa and to optimize their delivery through rational drug delivery system design9.
Biopharmaceutical Properties
Thus far we have considered the physicochemical properties of the drug that affect its selection as a drug candidate to penetrate the oral mucosa.
Other factors include organoleptic properties of the drug and excipients, texture of the delivery system, irritation or allergenic properties, discoloration or erosion of teeth, or the potential to alter the natural microflora. Any of these properties may limit the drug candidate list for this route.
Organoleptic Properties
The organoleptic properties of a drug or the delivery system may result in poor patient compliance or acceptance of the product. The detection of a bad taste would be detrimental to the success of the delivery system. This can be overcome through the formulation of a unidirectional delivery system, which will prevent the release of drug into the oral cavity. The texture of the delivery system may also affect patient compliance or acceptability.
Daily Dose Size
The oral epithelium being an efficient barrier to drug penetration allows only small quantities of drug for penetration even over a period of a day. This means that an upper limit exists on daily delivery of drug. For example, realistically a unidirectional buccal drug deliver system would not cover an area of buccal mucosa larger than 2 cm2 and would be unlikely to be retained on the buccal mucosa for longer than 24 hours. Th total amount of drug that could be systematically delivered across buccal mucosa from a 2 cm2 system in one day has been estimated to be 10-20 mg. Therefore, buccal drug delivery is suitable only for drugs whose daily dose is on the order of a few mg. Clearly the resultant plasma concentration of the drug will depend upon the clearance10.
Toxicity to oral mucosa
If a pharmacologically active material is to be presented to the oral mucosa over an extended period, there is the potential for an irritant or allergic response to the drug. It should be noted that the irritancy/sensitization should not only be limited to the drug but also to the components of the delivery system, which are also in intimate contact with the oral mucosa. Again the toxic effects of excipients, e.g., penetration enhancers, would be enhanced by the occlusive nature of the system and by extended contact times of the system in contact with the mucosa11. Rathbone et al suggested that as a site for drug delivery, the oral cavity offers several advantages over the gastrointestinal route and other alternative routes (Table 3).
Table 3: Comparison of Gastrointestinal route and oral mucosal route for drug delivery.
|
Parameter |
Gastrointestinal |
Oral mucosal |
Nasal |
|
Accessibility |
+ |
++ |
++ |
|
Permeability |
+++ |
++ |
+++ |
|
Reactivity |
++ |
+++ |
+ |
|
Surface area |
+++ |
+++ |
++ |
|
Surface environment |
+ |
+++ |
++ |
|
Vascular drainage |
+++ |
++ |
+++ |
|
First pass clearance |
+ |
+++ |
+++ |
|
Patient acceptability |
++ |
+++ |
++ |
Key : + Poor ; ++ Good. +++ Excellent
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Possible Routes for Drug Transport Across the Oral Mucosa
The cellular structure of the oral mucosa suggests that there are two permeability barriers. The intercellular space and cytoplasm are essentially hydrophilic in character and become a transport barrier for lipophilic compounds mainly because the solubility of a lipophilic compound in this environment is low. In contrast, the cell membrane is lipophilic and the penetration of a hydrophilic compound into the cell membrane is low due to a low partition coefficient12. Thus closely compacted cell membranes become obstacles that hydrophilic compounds have to move around.
The coexistence of the hydrophilic and lipophilic regions in the oral mucosa suggests that there are two routes for drug transport, i.e., the paracellular and the transcellular routes. The paracellular route is the primary route for hydrophilic compounds, because it is difficult for a hydrophilic compound to penetrate into the lipophilic cell membrane and thus, the intercellular space is the preferred route for drug transport. In this case, the limited surface area of the intercellular space and the tortuous pathway within the area are the main limitations for this route.
For lipophilic compounds, the partition coefficient are high, because the surface area for the transcellular route is large and the path length for transcellular movement is relatively short, the permeability of lipophilic compounds across the epithelial cell membrane is typically high.
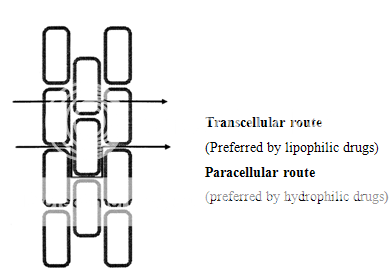
Figure 3. Diagram showing the two possible drug transport routes, in the oral mucosa.
The flux of drug movement through the paracellular route can be written as:
DH e
JH = -------------- CD (2)
hH
Where
e = the fraction of surface area of the paracellular route
DH = the diffusion coefficient in the intercellular spaces
hH = the pathlength of the paracellular route
CD = the donor side drug concentration
The flux of drug in the transcellular route can be expressed as:
(1- e) DL KP
JL = ------------------CD (3)
hL
Where Kp = the partition coefficient between lipophilic region (cell membrane) and hydrophilic region (delivery solution; intercellular space and cytoplasm)
hL = the pathlength of transcellular route
DL = the diffusion coefficient
CD = the donor side drug concentration.
Thus as per equations (2) and (3), if the drug is transported via paracellular route (equation 2), the permeability of the drug is independent on its partition coefficient. Conversely, if the drug is transported via the transcellular route (equation 3), the permeability of the drug is partition coefficient dependent.
The dosage forms described below are generally a combination of those different strategies.
Table 4 Dosage forms developed for the buccal mucosal drug delivery systems and some commercially available bioadhesive buccal formulations
|
Dosage forms developed |
Commercially available bioadhesive buccal delivery systems |
|
Solutions, Tablets, Lyophilized tablets, Chewing gum, Bioadhesive tablets, Solutions - spray, Laminated systems and patches, Hydrogels, Adhesive films, Hollow fibres, Microspheres, Liposomes |
Bioadhesive tablets, Sublingual mucosal delivery of nitroglycerine: Susadrin® Buccal mucosal delivery of Prochloroperazine: Buccastem ® Chewing gum Bucccal mucosal delivery of nicotine: Nicorette ® |
Chewing Gum
Chewing gums are mobile drug delivery systems. The main target mucosa for drug absorption is the sublingual mucosa. However, drug is released into saliva and its subsequent spreading may cause the drug to be absorbed across other mucosae of the oral cavity. Drug release from chewable formulation is generally rapid but not as immediate as in the case for the fast-dissolving tablets. A study by Christrup et al., showed that about 57% of ascorbic acid incorporated in a chewable preparation was released after 5 minutes. This percentage was not significantly increased after 30 minutes of chewing. Similar trends were observed with noscapine derivatives13.
Hollow Fibers
As an alternative to chewable formulations, Burnside et al reported the design of a microporous hollow fiber of polysulfone (molecular weight cutoff 5,00,000 Daltons) intended for the delivery of histrelin, an LHRH agonist. This fiber is intended to be placed in the buccal cavity for oral mucosal drug delivery14.
Bioadhesive Tablets
Bioadhesive tablets are immobilized drug delivery systems. They can be formulated into monolithic, partially coated or multilayered matrices. Monolithic tablets are easy to manufacture by conventional techniques and provide the possibility of holding large amounts of drug. Drugs can be co- formulated with an absorption enhancer if required.
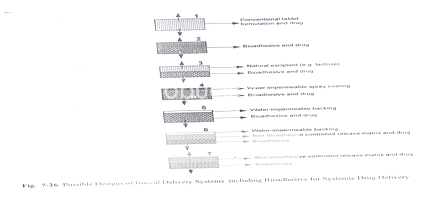
Figure: Designs of buccal delivery systems including bioadhesive for systemic drug delivery
Laminated Systems and Patches
In response to some of the drawbacks of tablets, different flexible, adhesive films and laminated adhesive patches have been developed. Drug loaded adhesive films can be prepared quite easily by using adhesive polymers. Kurosaki et al reported the use of a simple film of hydroxypropyl cellulose for the delivery of propranolol. Rodu et al prepared a simple film by complexing hydroxypropylcellulose with tannic and boric acid.
Adhesive patches can be designed either for unidirectional release into the oral mucosa or for bidirectional release into the oral cavity as well as into the oral mucosa (Figure 6). The adhesive part of the system can be used as a drug carrier or as an adhesive for the retention of a drug loaded non-adhesive layer13. The use of an impermeable backing layer will maximize the drug concentration gradient and prolong adhesion because the system is protected from saliva (Figure 7). Typically the size of such systems would be 1-3 cm2 but may have dimensions as large as 10-15 cm2 depending on the site of administration. Poly (acrylic acid) based patches have been used successfully for the delivery of opioid analgesics.
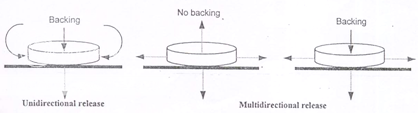
Figure 6: By varying the extent and permeability of backing a patch can be designed for systemic as well as local (oral cavity) drug delivery
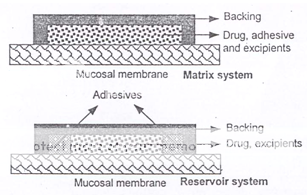
Figure 7: Alternative matrix and reservoir patch designs
Bioadhesion
Adhesion can be defined as the bond produced by contact between a pressure sensitive adhesive and a surface. Good defined bioadhesion as the state in which two materials, at least one of which being of a biological nature, are held together for an extended period of time by interfacial forces. It is also defined as the ability of a material (synthetic or biological) to adhere to a biological tissue for an extended period of time15.
Type-I :Bioadhesion is characterized by adhesion occurring between biological objects without involvement of artificial materials. Cell fusion and cell aggregations are good examples.
Type-II :Bioadhesion can be represented by cell adhesion onto culture dishes or adhesion to a variety of substances including metals, wood and other synthetic materials.
Type-III :Bioadhesion can be described as adhesion of artificial substances to biological substrates such as adhesion of polymer to skin or other soft tissues.
The goal of the development of bioadhesives is to duplicate, mimic or improve biological adhesives, which are both durable where required and degradable where necessary and not toxic at all.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Theories of Bioadhesion
Several theories have been proposed to explain the fundamental mechanisms of adhesion. In a particular system, one or more theories can equally well explain or contribute to the formation of bioadhesive bonds.
A. Electronic theory
According to this theory, electron transfer occurs upon contact of an adhesive polymer with a mucus glycoprotein network because of differences in their electronic structures. This results in the formation of an electrical double layer at the interface. Adhesion occurs due to attractive forces across the double layer16.
B. Absorption theory
According to this theory, after an initial contact between two surfaces, the material adheres because of surface forces acting between the atoms in the two surfaces. Two types of chemical bonds resulting from these forces can be distinguished.
· Primary chemical bonds of covalent nature, which are undesirable in bioadhesion.
· Secondary chemical bonds having many different forces of attraction, including electrostatic forces, vander Waals forces and hydrogen and hydrophobic bonds.
C. Wetting theory
Wetting theory best describes the adhesion of liquid or paste to a biological surface. The wetting theory emphasizes the intimate contact between the adhesive and mucus. The work of adhesion can be expressed in terms of surface and interfacial tension (g), being defined as the energy pre cm2 released when an interface is formed. The work of adhesion is given by,
Wa = gA + gB- gAB
Where ‘A’ and ‘B’ refer to the biological membrane and the bioadhesive formulation respectively.
Adsorption
In the adsorption theory, a bioadhesive polymer adheres to mucus because of secondary surface forces such as van der Walls forces, hydrogen bonds or hydrophobic interactions17.
From a drug delivery point of view, our interest is primarily in understanding the mechanism of bioadhesion, which appears best explained by a combination of wetting, diffusion and electronic theory, although other mechanism many be operative for a given system.
Methods of Determination of Bioadhesion
During the design and development of novel bioadhesive controlled systems, various types of experimental tests must be carried out to assure thermodynamic compatibility, physical and mechanical stability, solute diffusion, release studies, surface analysis and bioadhesive bond strength.
(1) In vitro / Ex vivo methods
a) Methods based on measurement of tensile strength.
b) Methods based on measurement of shear strength.
c) Other in vitro methods.
I. Adhesion weight method
II. Fluorescent probe method
III. Flow channel method
IV. Falling liquid film method
(2) In vivo Methods
The methods are based on the measurement of the residence time of bioadhesives the application site. Ch’ng et al. in order to investigate the GI transit of bioadhesive beads, developed an in vivo methods in rats, inserting Cr labelled bioadhesive material in the stomach and measuring the radioactivity in cut segments of the intestine18.
Duchene et al. described the method used by Davis, which involved the scintigraphic method to study the GI transit of a bioadhesive form.
Bioadhesive Polymers
Bioadhesive polymers are defined as polymers that can adhere onto a biological substrate. Polymers, which can adhere to either hard or soft tissue, have been used for many years in surgery and dentistry. Polymers that adhere to the mucin-epithelial surface can be conveniently divided into three broad categories.
1. Polymers that become sticky when placed in water and owe their bioadhesion to stickiness.
2. Polymers that adhere through non-specific, non-covalent interactions which are primarily electrostatic in nature.
3. Polymers that bind to specific receptor sites on the cell surface.
The bioadhesive properties of a polymer is affected by the following factors.
A. Molecular Weight and Polymer Conformation
The optimum molecular weight for maximum bioadhesion depends on
the type of bioadhesive polymer at issue. It is generally understood that the threshold required for successful bioadhesion is at least 100,000 molecular weight. It is obvious that the polymer molecule must have an adequate length to allow chain interpenetration. It is also necessary to consider the size and configuration of the polymer molecule. For example, with polyethylene oxide the adhesive strength increases even upto molecular weights of 40,00,000. These polymers are well known to contain molecules for highly linear configuration which contribute to interpenetration with dextran.
Cross-linking density
An increase in cross linking is found to decrease the strength of mucoadhesion, due to decreasing diffusion coefficient, chain segment flexibility and mobility. Therefore, the extent of interpenetration was reduced.
B. Charge and Ionization
Using a cell culture fluorescent probe technique, polymer were studied for their bioadhesive potential19. It appears that charge density is an important element for bioadhesion. Carboxylated polyanions are good potential bioadhesives for drug delivery.
C. Concentration of Polymer
There is an optimum concentration of polymer corresponding to the best bioadhesion. In highly concentrated systems, the adhesive strength drops significantly. In concentrated solutions, the coiled molecules become solvent poor and the chains available for interpenetration are not numerous.
D. pH
pH was found to have a significant effect on mucoadhesion as observed in studies of polyacrylic polymers cross linked with COOH groups. pH influences the charge on the surface of both mucus and the polymers.
Applied strength
To place a solid bioadhesive system, it is necessary to apply a defined strength. Whatever the polymer, poly (acrylic acid/divinyl benzene poly (HEMA) or carbopol 934, the adhesion strength increases with the applied strength or with the duration of its application, upto an optimum21. The pressure initially applied to the mucoadhesive tissue contact site can affect the depth of interpenetration. If high pressure is applied for a sufficiently long period of time, polymers become mucoadhesive even thought they do not have attractive interactions with mucin20.
Initial contact time
The initial contact time between mucoadhesive and the mucus layer determines the extent of swelling and the interpenetration of polymer chains. Along with the initial pressure, the initial contact time can dramatically effect the performance of a system. The mucoadhesive strength increases as the initial contact time increases. Although longer initial contact time should be based on the tissue viability. In case of mucoadhesives that need to be polymerized at the application sites, the initial contact time is critical. It is easily controlled when mucoadhesives are applied to exposed areas such as eyes, nose or mouth21.
Selection of the model substrate surface
The handling and treatment of biological substrates during the testing of mucoadhesives is an important factor, since physical and biological changes may occur in the mucus gels or tissues under the experimental conditions. The viability of the biological substrate should be confirmed by examining properties such as permeability, electrophysiology or histology. Such studies may be necessary before and after performing the in vitro tests using tissues.
Buccal bioadhesives drug delivery systems
With a better understanding of the mechanisms of bioadhesives dosage forms have been reported. Because of the presence of a smooth and relatively immobile for placement of a bioadhesive dosage form, the buccal region appears to be more suitable for sustained delivery of therapeutic agents using a bioadhesives system.
Since there is a limit to the size of the bioadhesives dosage form, only a limited amount of drug can be used in these systems. In general, any drug with a daily requirement of 25 mg or less is suitable for buccal delivery. Drugs with short biological half-lives, requiring a sustained effect, poor permeability, sensitivity to enzymatic degradation and poor solubility may be successfully delivered via a bioadhesive oral delivery system. Relevant bioadhesive dosage forms in the buccal cavity include adhesive tablets, adhesive gels, adhesive patches, and adhesive ointments.
Adhesive tablets
Unlike conventional tablets, bioadhesive tablets allow drinking and speaking without major discomfort. Triamcinolone acetonide has been formulated as bioadhesive tablets for the treatment of aphtous stomatitis as a small, thin and double layered. Schor et al, developed nitroglycerine bioadhesive tablet, susadrin for agina pectoris22.
Adhesive gels
Bioadhesive gels may be used to deliver the drug via the buccal mucosa with the possibility of prolonging residence time and improve bioavailability. Polyacrylic acid and polymethacrylate have been used as gel forming polymers.
Adhesive patches
Bioadhesive patches may range from simple erodible and nonerodible adhesive disks to laminated systems. Designed to provide either unidirectional or bidirectional release of the drug.
Adhesive ointment
Bioadhesive ointment have not been investigated as extensively as tablets and patches. A case of prompt and dramatic improvement following buccal application of half an inch of 2% nitroglycerine ointment was reported in a woman with acute myocardial infrations. Mucoadhesive liposomal ointment containing triamcinolone acetonide was also tested23.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Physiological variables
Mucin turnover: The natural turnover of mucin molecules from the mucus layer is important for at least two reasons. First the mucin turnover is expected to limit the residence time of the mucoadhesives on the mucus layer. No matter, how high the mucoadhesive strength, mucoadhesives are detached from the surface due to mucin turnover. However the turnover rate may be different in the presence of mucoadhesives. Second, mucin turnover results in substantial amounts of soluble mucin molecules. These molecules interact with mucoadhesives to the tissue surface.
Disease states : The physiochemical properties of the mucus are known to change during disease conditions such as common cold, gastric ulcers, ulcerative colitis, cystic fibrosis, bacterial and fungal infections of the female reproductive tract and inflammatory conditions of the eye24.
METHODS USED TO STUDY BIOADESION
Several test method have been reported in literature for studying bioadhesion. These tests are necessary not only to screen a large number of candidate mucoadhesives, but also to study their mechanisms. These tests are also important during the design and development of a bioadhesive controlled release system as they ensure compatibility, physical and mechanical stability, surface analysis and bioadhesive bond strength.
The test methods can broadly be classified into two major categories:
1. In-vitro/Ex vivo methods
2. In-vivo methods
Evaluation
Methods of Bioadhesive Determination
Several methods have been reported in the literature for measuring mucoadhesive strength. Some of them are given as under :
(a). In Vitro Methods
Most in vitro methods are based on the measurement of either shear or tensile stress. One technique reported by uses a Wilhelmy plate method25. In this method, the plates are coated with a polymer to be tested and immersed in a temperature- controlled mucus solution. The force required to pull the plate out of the solution is determined.
Robinson et al, developed a fluorescence probe technique using cell cultures, which indirectly measures the binding between a polymer and epithelial cells.
An in vitro measurement system using a stainless steel sieve as an adhesive surface is described for assessing adhesivity of mucoadhesive tablet containing 60% of carbomer- 94126.
These methods usually measure the force required to break the adhesive bond between a model membrane and the test polymers. The instruments usually employed are modified balances or tensile testers. Modified tensiometer. The adhesive as a function of the pressure and the contact time. Any mucoadhesive system is adhesive to fingers, since most mucoadhesives are non-specific and not mucin specific. Like mucin, the skin has many hydroxyl groups. Although the thumb test may not be conclusive, it provides useful information on mucoadhesive potential.
Adhesion number
With a mucoadhesive in the form of small particles, the adhesion number can be used as a parameter for mucoadhesion. The determination of adhesion strength for small particles would be difficult. The adhesion number is typically represented by the following equations.
Na = (N/No) X 100
Release Studies
Characterization of Drug Release
Two methods have been used to characterize drug release from patches one is simple dissolution using a modified paddle method. Special flasks containing 100 ml of the dissolution medium were used. A second method uses a diffusion cell for determining drug release and considered an improvement over dissolution in that only one face of the patch is in contact with the medium via a hydrated hydrogel, a situation that more closely mimics the moist surface of the buccal cavity. There was a correlation between drug release and patch hydration suggesting that swelling is an important mechanism27.
Buprenorphine was released from the patches in a linear fashion over approximately 10 hours, By 24 hours, nearly 75% of the drug had been released. However, the microenvironment properties of the patch, for example, pH might be affected by the interaction between the active drug and the polymers, implying that the release profile could change from drug to drug.
(a). In Vitro Methods
Beaker method
The dosage from in this method is made to adhere at the bottom of the beaker containing the medium and stirred uniformly using overhead stirrer. Volume of the medium used in the literature for the study varies from 50-500 ml and the stirrer speed from 60-300 rpm.
Dissolution apparatus
Standard USP or BP dissolution apparatus have been to study in vitro release profiles using both rotating elements paddle and basket. Dissolution medium for the study varied from 100-500 ml and speed of rotation from 50-100 rpm.
Other methods
Other methods involve plexiglass sample blocks placed in flasks30, agar gel method, Valia-Chien cell, USP2 dissolution apparatus, etc.
Although a number of methods have been reported, the ideal method would be one where sink condition is maintained and dissolution time vitro simulates dissolution time in vivo.
(b). In Vivo Methods
The most desirable in vivo approach is to perform experiments in human volunteers or patients. However, it is very difficult to begin with this approach, because of the difficulties of cost, time, toxicity of drug and ethical considerations. Therefore, animal models are being usually used for this purpose.
The most important and difficult aspect of the experimental design is the choice of animal species. Animal models such as dog, cat, rabbit, rat and sheep have been used to determine the oral mucosal absorption characteristics of drugs.
Very few, and certainly no extensive in vivo (animal) in vivo (human) correlation have been reported, which would allow us, to compare the oral mucosal absorption characteristics of a particular animal with those of its human counter part. However, the methods used in in vivo studies are absorption cells and perfusion cells.
Disc methods
These methods have advantage that the absorption across a defined oral cavity mucosa can be studied. A polytef disc with a diameter of approximately 3.5 cm and height of 1 cm was used in a study. The disc had a central depressions depth of 4 mm. A water soaked filter paper disc was placed in the depression and known amount of drug spread onto it. Once the drug had dissolved the device was placed onto defined oral mucosal surface and maintained in place for 5 min. After removal, a non impregnated disc was used to wipe the oral mucosa, the discs combined and analysed26.
Disc techniques allow investigators to study drug loss across a fixed area defined oral cavity membrane. Major limitation of the technique include adherence of the disc to the membrane, leakage of drug form the disc and interference from salivary secretions.
Perfusion cells for animal studies
Veillard et al, developed the perfusion cell, which was made from a medical grade silicon polymer. The cell had a volume of 0.075 cm3 and exposed area of 0.25 cm2. Barshun et al, constructed a pliable cell made of a hydrophilic vinyl polysiloxane polymer which had an internal volume of 1 ml and allowed a 1.8 cm2 area of buccal membrane to be perfused. The design also incorporated sealing lip to prevent leaks. Ranthbone reported a buccal perfusion cell design constructed from inflexible material such as nylon or Teflon. Buccal perfusion cells of the types mentioned above offer fixed (known) interfacial areas over which transfer can take place into a defined oral cavity membrane.
(c). Human Techniques
Animal models play an important role in the development of an oral mucosal drug delivery system, but these models are only appropriate to use for the screening of a series of compounds, investigating the mechanisms and usefulness of permeation enhancers or evaluating a set of formulations, if one is certain that the route of penetration, the structure and the composition of permeation barrier for both the drug and excipients are an exact mimic of its human counterpart27.
Until a suitable animal model is found whose absorption characteristics correlate well with its human counterpart and in which the route of penetration, structure and composition of the permeation barrier are considered, an exact mimic of human oral mucosa, the continued refinement of human methodologies is of utmost importance. Accurate measurement of the oral mucosal absorption characteristics of drugs in man is needed for the rational design of a delivery system28.
Non absorbable marker compound
To account dir non absorbable losses that might occur due to swallowing of drug during buccal absorption test, use of non absorbable markers have been suggested. Insulin, 125I-labelled polyvinlypyrrolidone, polyethylene glycol and phenol red have been to assess the extent of drug loss arising from non-absorbable sources during a 5 min buccal absorption test.
Pre-test wash out
In order to cleanse mouth and adjust pH prior to buccal absorption test, mouth was rinsed by subjects for 10-30s, prior to a test with 20 ml of the buffer used in the test.
Post-test rising
Immediately after a buccal absorption test, subjects rinsed their mouth with an aliquot of fresh drug-free buffer or distilled water for a short period of time. Beckett & Triggs employed a 10 ml, 10 rinse, whereas others have used a 10ml, 5 rinse (20 ml, 30 rinse, and 5 ml, 3 rinse. It is clear that the post-test swirling period, although required, should involve small volumes and be restricted to short periods of time. This is particularly important with drugs that can readily return to the oral cavity from the membranes.
Kinetics of drug absorption
In order to estimate the transfer kinetics of a given drug the buccal absorption test requires repeated swirling over different time periods upto a maximum of 15 minutes, a process which can take davx for the mapping of a drug’s kinetic profile. In this respect, Tucker, reported a technique which enable kinetic data to be collected in a single 15 min trial. The method validated using verapamil involved multiple samples being withdrawn form the mouth throughout the duration of test.
Monitoring of drug appearance in blood
Drug loss from the oral cavity duri9ng buccal absorption test may lead to entry of drug in systemic circulation. Studies measuring drug loss from the oral cavity and apperance in the blood are sparse in literature. Younes et al used a 5 min buccal absorption test to investigate the absorption of flurbiprofen. Blood samples were collected upto 48hr post dosing.
REFERENCE
1. Bodde, H.E., De Vries, M.E., and Junginger, H.E., Mucoadhesive polymers for the buccal delivery of peptides, structure-adhesiveness relationships, J. Control. Rel., 13:225-231, 1990.
2. Gandhi, R.E. and Robinson, J.R., Bioadhesion in drug delivery, Ind. J. Pharm. Sci., 50:145-152, 1988.
3. Harris, D. and Robinson, J.R., Drug delivery via the mucous membranes of the oral cavity, J. Pharm. Sci., 81:1-10, 1992. Reproduced with permission of the American Pharmaceutical Association.
4. Wertz, P.W. and Squier, C.A., Cellular and molecular basis of barrier function in oral epithelium, Crit. Rev. Ther. Drug Carr. Sys., 8:237-269, 1991.
5. Squier, C.A., Cox, P., and Wertz, P.W., Lipid content and water permeability of skin and oral mucosa, The J. Invest. Dermat., 96:123-126, 1991.
6. Squier, C.A. and Wertz, P.W. Structure and function of the oral mucosa and implications for drug delivery, in eds. M.J. Rathbone, Oral Mucosal Drug Delivery, Marcel Dekker, Inc., New York, New York, 1-26, 1996.
7. Galey, W.R., Lonsdale, H.K., and Nacht, S., The in vitro permeability of skin and buccal mucosa to selected drugs and tritiated water, J. Invest. Dermat., 67:713-717, 1976.
8. Gandhi, R.B. and Robinson, J.R., Oral cavity as a site for bioadhesive drug delivery, Adv. Drug Del. Rev., 13:43-74, 1994.
9. Squier, C.A. and Hall, B.K., The permeability of mammalian non-keratinized oral epithelia to horseraddish peroxidase applied in vivo and in vitro, Arch. Oral Biol., 29:45-50, 1984.
10. Ishida, M., Nambu, N., and Nagai, T., Mucosal dosage form of lidocaine for toothache using hydroxypropyl cellulose and carbopol, Chem. Pharm. Bull., 30:980-984, 1982.
11. Elkayam, R., Friedman, M., Stabholz, A., Soskolne, A.w., Sela, M.N., and Golub, L., Sustained release device containing minocycline for local treatment of periodontal disease, J. Control. Rel., 7:231-236, 1988.
12. Samaranayake, L. and Ferguson, M., Delivery of antifungal agents to the oral cavity, Adv. Drug Del. Rev., 13:161-179, 1994.
13. Nagai, T. and Machida, Y., Mucosal adhesive dosage forms, Pharm. Int., 196-200, 1985.
Aungst, B.J. and Rogers, N.J., Comparison of the effects of various transmucosal absorption promoters on buccal insulin delivery, Int. J. Pharm., 53:227-235, 1989.
14. Siegel, I.A. and Gordon, H.P., Surfactant-induced increase of permeability of rat oral mucosa to non-electolytes in vivo, Arch. Oral Biol., 30:43-47, 1985.
15. Shojaei, A.H. and Li, X., In vitro permeation of acyclovir through porcine buccal mucosa, Proceedings of International Symposium on Controlled Release of Bioactive Materials, 23:507-508, 1996.
16. Shojaei, A.H. and Li, X., Determination of transport route of acyclovir across buccal mucosa, Proceed. Int. Symp. Control. Rel. Bioact. Mater., 24:427-428, 1997.
17. Manganaro, A.M. and Wertz, P.W., The effects of permeabilizers on the in vitro penetration of propranolol through porcine buccal epithelium, Mil. Med., 161:669-672, 1996.
18. Gandhi, R. and Robinson, J., Mechanisms of penetration enhancement for transbuccal delivery of salicylic acid, Int. J. Pharm., 85:129-140, 1992.
19. Wolany, G.J.M., Munzer, J., Rummelt, A., and Merkle, H.P., Buccal absorption of Sandostatin (octreotide) in conscious beagle dogs, Proceed. Intern. Symp. Control. Rel. Bioact. Mater., 17:224-225, 1990.
20. Kurosaki, Y., Hisaichi, S., Hamada, C., Nakayama, T., and Kimura, T., Effects of surfactants on the absorption of salicylic acid from hamster cheek pouch as a model of keratinized oral mucosa, Int. J. Pharm., 47:13-19, 1988.
21. Ishida, M., Machida, Y., Nambu, N., and Nagai, T., New mucosal dosage form of insulin, Chem. Pharm. Bull., 29:810-816, 1981.
22. Sanzgiri, Y.D., Topp, E.M., Benedetti, L., and Stella, V.J., Evaluation of mucoadhesive properties of hyaluronic caid benzyl esters, Int. J. Pharm., 107:91-97, 1994.
23. Lehr, C.M., Bouwstra, J.A., Schact, E.H., and Junginger, H.E., In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers, Int. J. Pharm., 78:43-48, 1992
24. Park, K. and Robinson, J.R., Bioadhesive polymers as platforms for oral-controlled drug delivery: method to study bioadhesion, Int. J. Pharm., 19:107-127, 1984.
25. Nagai, T. and Machida, Y., Buccal delivery systems using hydrogels, Adv. Drug Del. Rev., 11:179-191, 1993.
26. Nagai and Machida, Y., Buccal delivery systems , Adv. Drug Del. Rev., 11:187-194, 1993.
27. Parikh Bhavik et. al. Design and evaluation of buccal patches of valsartan IJPI’s Journal of Pharmaceutics and Cosmetology, IJPI’s Journal of Pharmaceutics and Cosmetology; 2011; (1); 50-55.
28. Rohit Chaudhary et. al. Formulation, Development and In-Vitro Evaluation of Mucoadhesive Buccal Patches Of Methotrexate International Journal of Pharma Sciences and Research (IJPSR), 1, (9), 2010, 357-365.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









