About Authors
CHETNA MODI1*, VINIT MODI2, SHWETA RAY2, PIYUSH NARIYA2, VARSHA GADHVI2
1Professor, Department of Pharmaceutics, Anand Pharmacy College, Anand, Gujarat, India.
2Research Scholar, Department of Pharmaceutics, Anand Pharmacy College, Anand, Gujarat, India.
*chetnamodi306@gmail.com
ABSTRACT
Magnetic nanoparticles (MNPs) are gaining popularity due to their novel applications in targeted drug delivery systems. They are a form of nanoparticle that responds to magnetic fields. Such particles normally consist of two parts: a magnetic substance, which is frequently iron, and a nonmagnetic material. A useful chemical component and nickel or cobalt. Because of their unique magnetic properties, extremely low toxicity, and excellent biocompatibility, targeted drug/ gene delivery system is especially advantageous.Nanotechnology and molecular biology fields have emerged as novel approaches for cancer theranostics in recent decades. These systems are primarily concerned with the design and fabrication of engineered nanoparticles with multifunctional properties that address the limitations of conventional cancer diagnostics and therapeutics agents. Gene delivery methods efficiently present a gene of interest for its encoded protein to be expressed in a suitable host or host cell. The gene attached directly to the carrier is typically made up of magnetic iron oxide dispersed within a polymer matrix such as silica, polyvinyl alcohol (PVA), or dextran or encapsulated with a polymer or metallic shell. A new magnetic targeting technology could be used to target therapeutically armed monocytes or other forms of cellular gene delivery vehicles to tumors, overcoming the challenge of poor targeting in conventional cell-based gene therapy methods. MNPs’ broad potential in targeted drug administration is based on their unique characteristics, magnetism, and ease of manipulation using an external magnetic field (EMF), which guides drug-carrying MNPs straight to the specific location.
Reference Id: PHARMATUTOR-ART-3007
INTRODUCTION
Magnetic nanoparticles are controlled by magnetic fields. Such particles typically have two components: a magnetic material, which is often iron, nickel, or cobalt, and a chemical component with functionality. While nanoparticles have a diameter of less than one micrometer (usually 1-100 nanometers), microbeads have a diameter of 0.5-500 micrometers. Magnetic nanobeads with a diameter of 50-200 nanometers are magnetic nanoparticle clusters formed of a number of individual magnetic nanoparticles.(Mørup, Hansen, and Frandsen 2019) Magnetic nanoparticles have recently been the focus of much research due to their appealing properties that could see potential use in catalysis including nanomaterial-based catalysts, biomedicine, tissue-specific targeting, microfluidics, magnetic resonance imaging, magnetic particle imaging, data storage, environmental remediation, nanofluids, optical filters, defect sensor.(Omid Veiseh, Jonathan Gunn 2011)
Nanotechnologies are widely used in the medical and pharmaceutical fields, namely for the detection of diseases, controlled medication delivery, biosensors, tissue engineering, and other applications. Drug delivery nanoparticles must, above all else, be biodegradable and biocompatible. Recent articles mention a wide range of nanosystems for these goals but MNPsa significant class of nanoscale materials with the potential to completely transform clinical diagnostic and therapeutic methodsare of particular interest. As indicated in a study by Corchero and Villaverde, various applications of MNPs are also being extensively researched. These include magnetically improved transfection, magnetically aided gene therapy, magnetically induced hyperthermia, and magnetic-force-based tissue engineering.(Schneider-Futschik and Reyes-Ortega 2021)
To identify the targeted cells, such as cancer cells, more specialised targeting systems are created. This can be done by combining a suitable ligand with the nanoparticle, which has a particular binding activity for the target cells (Figure 1). Additionally, nanoparticles offer a surface on which to attach several therapeutic agents, increasing the concentration of both therapeutic and diagnostic agents at the diseased location. Controlling the size of nanoparticles (>3-5 nm) can also change the active molecule's concentration and behaviour (Table 1).
Because of the control over particle size and the surface coating with the stealth ligand, they can evade the body's immune system and circulate in circulation for a longer length of time.(Cicha et al. 2017)
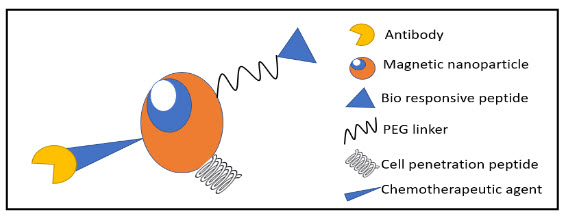
Figure 1 Schematic diagram representing the functionalization of magnetic nanoparticles with bio-responsive peptide, peg linker, chemotherapeutic agent, antibody, and cell-penetrating peptide
Through local or systemic blood circulation, targeted drug/gene delivery refers to the transfer of drugs/genes via nanocarriers to organs, tissues, and cells. This enables the drugs/genes to directly act on the targeted illness locations and have therapeutic effects. By increasing therapeutic molecular activity at certain places while lowering hazardous side effects at healthy sites, this method of selective delivery keeps the systemic effect to a minimum.(Mody et al. 2010; Vatta, Sanderson, and Koch 2006)
Table 1 Techniques and their advantage and Disadvantages
|
TECHNIQUE |
ADVANTAGES |
DISADVANTAGES |
NANOPARTICLES |
|
Ultrasound |
Simple and non-invasive Because nonionizing radiation is being released, there is no radiation risk. comparatively less costly than other currently accessible imaging methods |
Resolution of images is often limited Reflected very strongly on passing from tissue to gas, or vice versa Does not pass well through bone Attenuation can reduce the resolution of the image |
Fe2O3, Gd2O3 |
|
CT |
Detection of even minute variations between bodily components, and a large field of view ability to produce body cross-sectional pictures |
Radiation-based contrast agents required for improved soft tissue contrast Non-specificity of the tissue |
nano-sized silver and gold particles cellular contrast Multimodal nanoparticles for imaging
|
|
PET |
Able to visualise physiological and metabolic processes |
Radiations Several tumours exhibit a low FDG affinity. FDG uptake is common in various benign cells in addition to tumour cells. The most problematic issue is motion artefact. Images have a lower resolution when compared to CT or MRI. As a result, lesions have poor localisation. Interpretation is really difficult. the costliest method |
Particles that are radioactively tagged or conjugated with any organic moiety that contains 19F are preferred above those that are 64Cu, 62Cu, 82Rb, and 68Ga. |
|
MRI |
improved resolution able to display anatomical details utilises no ionising radiation |
Costly to use cannot be used to individuals who have metallic implants, such as pacemakers. |
Although next generation multimodal imaging agents are also taken into consideration for clinical studies, iron oxide nanoparticles are still the most often employed. |
TYPES OF MAGNETIC NANOPARTICLES
Oxides : ferrites
The most researched magnetic nanoparticles to date are ferrite nanoparticles or iron oxide nanoparticles (iron oxides with the crystal structure of magnetite or magnetomite). Since they only display their magnetic behaviour when an external magnetic field is applied, superparamagnetic ferrite particles, which are smaller than 128 nm, avoid self-agglomeration (Figure 2). By carefully arranging many individual superparamagnetic nanoparticles into superparamagnetic nanoparticle clusters, such as magnetic nanobeads, the magnetic moment of ferrite nanoparticles may be significantly boosted.Remanence returns to zero when the external magnetic field is turned off. Similar to non-magnetic oxide nanoparticles, ferrite nanoparticles' surfaces are frequently altered with surfactants, silica, silicones, or derivatives of phosphoric acid to improve their stability in solutions.(Mørup, Hansen, and Frandsen 2019)
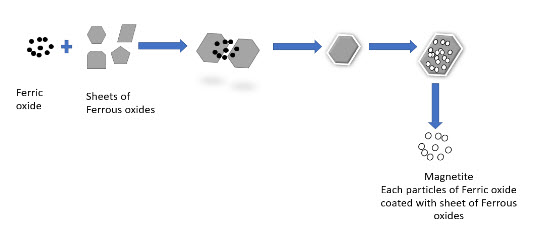
Figure 2 Flow chart of ferric oxide use in MNPs procedure
Ferrites with a shell
Maghemite or magnetite magnetic nanoparticles often do not allow for strong covalent interactions with functionalization molecules due to their relatively inert surface. However, by adding a layer of silica to the surface of the magnetic nanoparticles, their reactivity may be increased. Organo-silane molecules and the silica shell may form covalent bonds that make it simple to add different surface functional groups to the silica shell. The functionalized silica shell can also be covalently linked to various fluorescent dye molecules. In comparison to metallic nanoparticles, ferrite nanoparticle clusters with narrow size distribution composed of superparamagnetic oxide nanoparticles (80 maghemite superparamagnetic nanoparticles per bead) have a number of benefits.(Mørup, Hansen, and Frandsen 2019)
• Higher chemical resistance (crucial for biomedical applications)
• Restricted size range (crucial for biomedical applications)
• Since they don't magnetically agglomerate, they have higher colloidal stability.
• The size of the nanoparticle cluster can adjust the magnetic moment.
• Kept superparamagnetic characteristics (independent of the nanoparticle cluster size)
• Simple covalent functionalization is made possible by the silica surface.
Metallic
Due to their stronger magnetic moments, metallic nanoparticles may be useful for some technological applications, whereas oxides (maghemite, magnetite) might be useful for biological applications. This means that metallic nanoparticles may be produced smaller than their oxide counterparts at the same time. On the other hand, metallic nanoparticles have the significant drawback of being pyrophoric and to varying degrees reactive to oxidising chemicals. This makes them harder to handle and encourages unintended side effects, which makes them less suitable for biological applications. Metallic particle colloids are also significantly more difficult to create.
Metallic with a shell
The metallic core of magnetic nanoparticles may be passivated by gentle oxidation, surfactants, polymers and precious metals. In an oxygen environment, Co nanoparticles form an anti-ferromagnetic CoO layer on the surface of the Co nanoparticle. Recently, work has explored the synthesis and exchange bias effect in these Co core CoO shell nanoparticles with a gold outer shell. Nanoparticles with a magnetic core consisting either of elementary Iron or Cobalt with a nonreactive shell made of graphene have been synthesized recently.(Mørup, Hansen, and Frandsen 2019)
When contrasted to ferrite or elemental nanoparticles, the benefits are:
• Increased magnetization
• Increased stability in basic and acidic solutions as well as with organic solvents
• Chemical reactions on the graphene surface using techniques for carbon nanotubes
PREPARATION METHOD OF MAGNETIC NANOPARTICLES
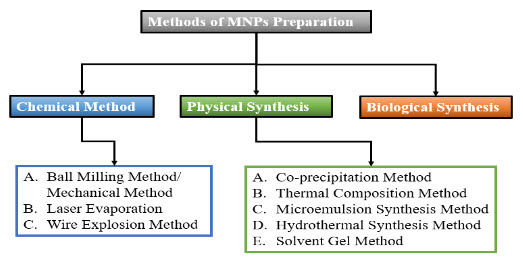
Figure 3 Methods of MNPs Preparations
The development of various methods for the synthesis of MNPs has been the subject of intense research during the last 10 years.(Ali et al. 2021)
To produce MNPs with the necessary size, shape, stability, and biocompatibility, many synthetic techniques are utilized. In order to create MNPs, the most popular techniques are ball milling, coprecipitation, thermal decomposition, hydrothermal, microemulsion, sol-gel, and biological approaches. Figure 3 provides a visual representation of MNPs created using diverse methods.(Ali et al. 2021)(Lu, Salabas, and Schüth 2007)
Physical Methods
The physical techniques use both top-down and bottom-up strategies. Through high intensity ball milling, the bulk materials are reduced to nanoparticle size in the top-down method. Mechanical crushing makes it challenging to get the proper form and size of NPs. In contrast to the top-down strategy, the bottom-up method may produce well-dispersed and small nano scaled microscopic particles. Laser evaporation is an illustration of a bottom-up strategy. MNPs are also created using different physical techniques such as the wire explosion method and the inert-gas condensation method. Three physical processesball milling, laser evaporation, and wire explosionwill be covered in this review.(Ali et al. 2021)
A. Ball Milling Method/Mechanical Method
A top-down method of creating MNPs from bulk material is by ball milling. The mechanical grinding of course-textured, rather than fine-textured, particles, is a straightforward and practical procedure. It was invented for the first time in 1970. The operation is fairly straightforward; the raw materials are contained in a little hollow cylindrical jar with plenty of steel balls acting as the grinding medium. Steel balls continuously colliding with solid materials impart kinetic energy to the solids, resulting in powder that is nano- or micron-sized. The key variables influencing the creation of nano/micro size crystals are the ball to powder ratio, ball size, vibration speed, and milling duration.
B. Laser Evaporation
In a bottom-up method called laser evaporation, nanoparticles are created by condensation from the liquid or gaseous phase. The simple process of laser evaporation, also known as laser ablation, uses a high intensity laser to produce MNPs. Iron oxide MNPs may also be made using this approach. In this procedure, coarse-textured raw materials (in the m or mm size ranges) are chosen and evaporated through the laser beams focus. The material is positioned at the bottom of a cell that is filled with a liquid solution, and the laser beam is focused on it.A laser beam is used to irradiate the substance in a solution. The creation of nanoparticles is caused by the rapid condensation and nucleation that results from cooling down the material's vapours in the gas phase. This process is less costly than wet chemistry procedures and does not require expensive chemicals or result in hazardous waste.
C. Wire Explosion Method
The wire explosion method is a brand-new physiochemical method that produces MNPs in a secure and hygienic manner. This procedure is a one-step, highly productive one that doesn't call for any extra processes like NP separation from solution or byproduct re-treatment. Iron oxide MNPs were previously made using this technique to remove arsenic from water. Making less polluted nanopowders with this method is safe for the environment and uses less energy. This approach does not result in monodispersed NPs
Chemical Methods
The many bottom-up processes used in chemical synthesis are diverse. Below is a detailed explanation of a few popular techniques that are frequently used to create MNPs.
A. Coprecipitation Method
The most popular technique for creating MNPs with regulated size and magnetic characteristics is coprecipitation. It is frequently used in biomedical applications and involves the use of less toxic materials and techniques. When we require a lot of nanocrystals, the coprecipitation method of making MNPs is highly practical and simple. This process is frequently used to create NPs with regulated sizes and desirable magnetic characteristics. To create MNPs, various metal ions are dissolved in a solvent. Ferric chloride (FeCl3), manganese (II) chloride (MnCl2), and sodium hydroxide (NaOH) salts were used as the metal ions, and the result was nanoparticles of manganese ferrite (MnFe2O4).(Ali et al. 2021; Kalubowilage, Janik, and Bossmann 2019; Kudr et al. 2017). With the addition of NaOH, Fe3+ and Mg2+ ions may combine to create MgFe2SO4 nanocrystals. Similar to this, Fe2+ and Fe3+ ions are coprecipitated to produce Fe3O4 NPs in another work. Different variables can alter the composition of MNPs, particle size, and shape during coprecipitation, including pH, metal ions, and their concentrations, salt type, and reaction temperature. It is quite easy to create MNPs using coprecipitation, which results in evenly disseminated small-size NPs. Additionally, this approach is popular because to its simplicity, however it can occasionally be challenging to regulate the form of MNPs by coprecipitation.(Chomoucka et al. 2010; Etheridge 2013; Mody et al. 2010; Mou et al. 2015)
B. Thermal Decomposition Method
In this technique, monodispersed NPs are made under high temperature using organometallic precursors. This technique produces MNPs with great crystallinity, regulated size, and well-defined form. To create MNPs of the necessary size and form, the breakdown of organometallic precursors is carried out in the presence of organic surfactants. Fatty acids, hexadecyl amine, and oleic acid are among the stabilising substances employed in the manufacture of MNPs. The stabilisersutilized in the breakdown process have the ability to slow down the nucleation of NPs, which regulate the growth of MNPS and aid in the production of a spherical shape and desired size of less than 30 nm.(Cicha et al. 2017; Mody et al. 2010; Mou et al. 2015)This method was said to yield magnetically active iron composites and Fe3O4 nanocrystals. Metal NPs are produced via the thermal breakdown of the zero-valent metal precursor Fe (CO)5, although oxidation may also result in the production of high-quality iron oxide MNPs.We have previously created monodispersed iron oxide MNPs with sizes ranging from 6 to 20 nm by decomposing Fe (CO)5.This technique has been cited as one of the best ways to make MNPs on a broad scale that are homogenous in size. The creation of hazardous organic-soluble solvents is a danger factor for this approach, which restricts its use in the biomedical industry. For the synthesis of smaller magnetic particles, thermal composition is more advantageous than coprecipitation.(Chomoucka et al. 2010; Etheridge 2013)
C. Microemulsion Synthesis Method
Surfactants and occasionally co-surfactants are used to create turbid systems of lipophilic and hydrophilic phases in microemulsions. This system of water, oil, and amphiphile is transparent and isotropic. In this procedure, water is magnetically agitated at room temperature while oil and a surfactant are combined.(Mody et al. 2010)
Microemulsions come in three different varieties:
1) oil in water (O/W), which is an aqueous phase with some oil droplets,
2) Water in oil (W/O), which is a mixture of oil as the dominating phase and some water droplets, and
3) An equal amount of both oil and water are present. For instance, in a microemulsion of the w/o type, a surfactant coated the droplets of water in an organic solvent, lowering the size of the MNPs.
The type of surfactant employed determines the size and form of MNPs produced with this approach. The w/o kind of microemulsion, which uses two microdroplets, one with a metal percussor and the other with a precipitating agent, was utilised to create several iron oxide MNPs. This procedure was used to create MNPS that had a silica covering and were afterwards changed with amino, which was helpful for separating Tumour cells. The MNPs created by microemulsion are sparse and evenly distributed.(Zhang et al. 2017)
D. Hydrothermal Synthesis Method
This technique is used to make NPs in an aqueous solution at high temperatures and pressures. One of the effective solution reaction-based methods for producing MNPs at high pressure and temperature is hydrothermal synthesis, also known as solvothermal synthesis. MNPs are created during the hydrothermal process through a hydrolysis and oxidation reaction. The degree of mineral solubility in the water affects how crystals form. Through the use of this technique, uniform-sized particles of different magnetic nanomaterials were produced. For instance, 15 nm-sized Fe3O4 nanoparticles with a spherical form were synthesized and used in tumour MRI.Similar to this, 25 nm-sized Chitosan-coated Fe3O4 NPs were created and used in the immobilization of enzymes. Synthesized MNPs' shape and crystallinity rely on how well the solvent, time, pressure, and temperature are mixed. Comparing this procedure to the microemulsion method will help you produce more NPs. However, because this process requires high temperatures and pressure, it must be done carefully and with specialized machinery. Due to its benefits of creating NPs with a desired form, size, high crystallinity, and constant composition, the hydrothermal approach is favored over the sol-gel and other methods.(Ali et al. 2021; Avval et al. 2020; Zhang et al. 2017)
E. Sol-Gel Method
The whole chemistry of this procedure involves the hydrolysis and polycondensation of metal alkoxides to create gels at room temperature. To create a sol or colloidal solution, metallic salts are dissolved in water or other solvents and uniformly disseminated. By stirring and raising the temperature, the van der Waals forces between the particles take place and the interaction between particles grows. Finally, gel is formed as a result of drying the solution and removing the solvent from the combination. The creation of iron oxide and silica-coated MNPs may be accomplished using this technique.(Cicha et al. 2017; Mody et al. 2010; Shen, Li, and Qiao 2018; Vatta, Sanderson, and Koch 2006)
This approach yields very pure solid materials with good crystallinity and tunability. To get pure MNPs, the technique must sometimes be repeated because of contamination brought on by byproduct reactions. Its effectiveness in producing scattered NPs is limited by the formation of three-dimensional oxide networks. This method's prolonged reaction time and use of hazardous organic solvents are further drawbacks.(Avval et al. 2020; Chomoucka et al. 2010; Kalubowilage, Janik, and Bossmann 2019; Kudr et al. 2017)
Biological Synthesis Method
The process of creating MNPs by biological synthesis, which employs living things like plants and microbes, is widely established (fungi, viruses, bacteria, and actinomycetes). The MNPs created using this technology have a high degree of biocompatibility and have practical applications in the biomedical industry. The effectiveness, environmental friendliness, and clean procedure of this technology are advantages. However, its low NP dispersion is a drawback.The process of creating MNPs by biological synthesis, which employs living things like plants and microbes, is widely established (fungi, viruses, bacteria, and actinomycetes). The MNPs created using this technology have a high degree of biocompatibility and have practical applications in the biomedical industry. The effectiveness, environmental friendliness, and clean procedure of this technology are advantages. Its modest NP dispersion, however, is a disadvantage.
Nitrate reductase activity, shuttle electron quinones, and a mixed mechanism were the three hypothesized processes.However, it is difficult to understand the procedure in order to prepare MNPs. As a catalyst for the Suzuki-Miyaura reaction and photocatalysis, Fe3O4 magnetic material produced biologically was employed (Figure 4). It is still necessary to look at several methodological drawbacks, such as yield and MNPs dispersion.(Dhavale et al. 2018)
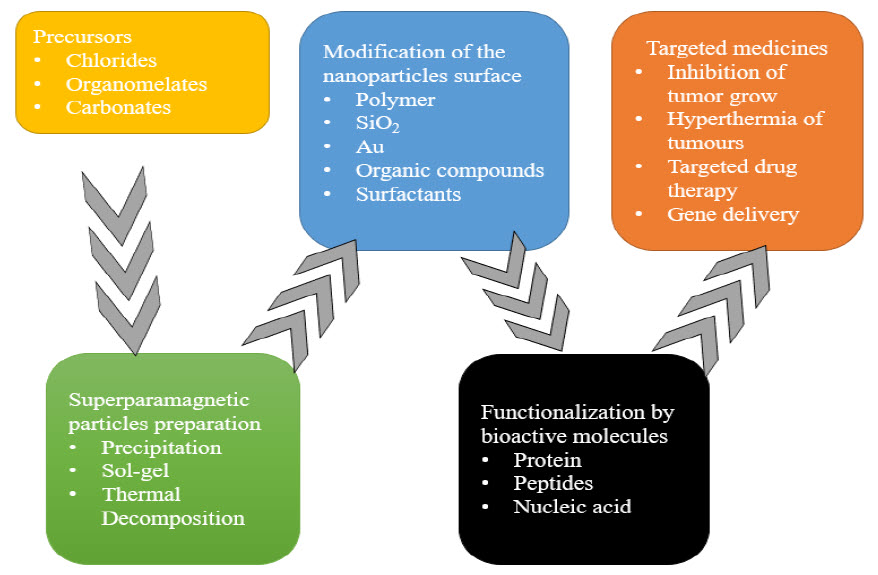
Figure 4 Process of magnetic nanoparticles preparation as nanocarrier
Table 2 Comparison of different synthesis methods
|
SYNTHESIS METHODS |
MERITS |
DEMERITS |
|
Ball milling method |
Easy to use, produces fine powder |
Pollution of the product |
|
Laser evaporation |
Low cost of experiments, no chemical use, and non-polluting output |
Costly laser systems that consume a lot of energy |
|
Wire explosion method |
environmentally sound, ethical, and very productive |
A little amount of product contamination could happen. |
|
Coprecipitation |
Simple and abundant |
contaminants, time-consuming |
|
Thermal decomposition |
a manageable size and a good yield |
toxic substances |
|
Microemulsion synthesis |
a stable thermodynamic system |
low output |
|
Hydrothermal/ Solvothermal |
enough crystallinity |
Needs high pressure and temperature |
|
Sol-gel method |
high crystallinity and purity |
longer-lasting, hazardous organic solvents |
|
Sono-chemical reaction |
narrow size distribution, saturation magnetization, and high crystallinity |
Mechanism still needs to be further understood. |
|
Microwave |
accelerated, quick crystallization kinetics |
homogeneous heating causes homogenous nucleation. |
|
Chemical reduction |
Simple and secure |
environmental damage |
|
Chemical vapor deposition |
wide variety of material manufacture |
low output and impurities |
|
Arc discharge |
simple and affordable |
Hard to regulate particle size |
|
Laser pyrolysis |
Rapid cooling and intense localised heating |
Expensive |
|
Combustion synthesis |
quick, easy, and low cast |
produce impurities |
|
Annealing |
Controllable chemical composition and particle size |
produce impurities |
|
Biological method |
Clean, effective, and environmentally friendly |
Poor NP dispersion |
MATERIAL USE IN PREPARATION OF MNPs
Mono component Magnetic Nanostructures
Fe, Ni, Co-Based Magnetic Nanostructures: -
MNPs of a particular kind called iron NPs have particular magnetic characteristics. the most thoroughly investigated NPs in the realm of nanomedicine due to their magnetic properties and exceptional biocompatibility. They are reasonably priced, ecologically safe, and have high physical and chemical stability. Previously, iron carbonyl [Fe (CO)5] was broken down to create monostructure Fe NPs in the presence of oleic acid (OA). Because iron NPs are sensitive to oxygen, iron NPs were made using a simple aqueous phase synthesis technique that included poly (N-vinylpyrrolidone), which is helpful in preventing oxidation of the metal surface.By reducing Ni (acac)3 in the presence of hexadecyl amine (HDA), monodisperse nickel (Ni) NPs with an average particle size of 3.7 nm were created (Hou and Gao, 2003). Cobalt (Co) NPs with a size range of 2–6 nm was created using a bulky trialkyl phosphine reducing agent, whereas NPs with a size range of 7–11 nm were created using a less bulky form of the surfactant. This demonstrates the coordination of the surfactant trialkyl phosphine with neutral metal surface sites.(Kalubowilage, Janik, and Bossmann 2019)
Metal Alloys Magnetic Nanostructure: -
Metal alloy NPs with super magnetic features are very promising. Due to their great chemical stability and magnetic crystallinity, iron-palladium (FePd) and iron-platinum (FePt) are examples of metal alloy nanostructures. FePt NPs are excellent for usage in biomedical applications and may be made via vacuum deposition or solution-phase synthesis. Utilizing tributyl phosphine and adamantine carboxylic acid as stabilisers, FePd NPs were made using the organic phase thermal decomposition technique at room temperature. With an adjustable size range of 11–16 nm, the FePd NPs exhibit super magnetic characteristics. In the past, face-centred cubic monodisperse FePd NPs were made by reducing Pt (acac)2 and breaking down Fe (CO)5 using a wet chemical method. However, it has a tendency to aggregate when converted to fct (face-centred tetragonal). By thermal annealing, the fcc-Fe3O4 NPs with Mg coating was converted to fct-FePt NPs. The layer of magnesium oxide (MgO) prevented NPs from aggregation. FePt NPs with gold coating were synthesized by reducing Pt (acac)2 and decomposing Fe (CO)5 in octyl ether solvent.(Chomoucka et al. 2010; Cicha et al. 2017; Dhavale et al. 2018; Etheridge 2013; Katz 2019; Kudr et al. 2017; Mørup, Hansen, and Frandsen 2019; Mou et al. 2015; Nikiforov and Filinova 2009; Omid Veiseh, Jonathan Gunn 2011; Schneider-Futschik and Reyes-Ortega 2021; Shen, Li, and Qiao 2018; Yang et al. 2012; Zhang et al. 2017)
Metal Oxide Magnetic Nanostructures: -
Due to their magnetic properties and chemical durability, metal oxide nanoparticles (NPs) have recently attracted a lot of attention. One of them, Fe3O4, has demonstrated a good prospective use in magnetic separation and biomedicine. They are made using a straightforward procedure that primarily relies on Fe complexes in alkaline conditions. The solvent and surfactant can be adjusted to get the desired size. In order to create Fe3+ "OOC," oleyl amine, a surfactant, was in contact with the metal ions. Because oleyl amine serves as a capping agent, particle development is constrained throughout this process. When an oleyl amine surfactant is employed to adjust the energy of the particle surface to encourage development in a certain direction, several forms of iron oxides, such as octahedral Fe3O4 NPs and Fe3O4 nano prisms, may be produced.The quantity of surfactant utilised can regulate the direction of particle development. Additionally, thermal annealing of FeO NPs can cause them to change into iron oxides (Fe3O4). Cobalt oxides were produced using comparable methods. Cobalt nitrate [Co (NO3)3] generated nanoplatelets (NPLs) of cobalt (II) hydroxide [Co (OH)2] during a hydrothermal process in the presence of PVP, which may further lead to cobalt oxide (CoO) NPLs.(Katz 2019)
Metal Carbides Magnetic Nanostructures: -
Iron carbides have amazing magnetic characteristics and stability, however (Fe5C2, Fe3C, and Fe2C). Because of the difficulties in synthesising them and regulating the size and shape, they are rarely researched. Fe(CO)5 was broken down in the presence of octadecyl amine to yield NPs of Fe5C2. To create Fe5C2, iron NPs with excellent crystalline structure were carbonised. When carbide NPs were created, bromide was added to adjust the surface energy, but the mechanism behind this addition was unknown. This method produced Fe5C2 NPs with a 20 nm size that had an amorphous shell. In order to create iron/carbide NPs with various crystallinity configurations, a synthetic chemical method was created.These iron carbides have mild ferromagnetic characteristics, which indicated that different types of iron carbides may arise depending on the synthetic processes used to create them. The penetration of carbon content by selective absorption may be impacted, particularly when halide ions are present.(Ulbrich et al. 2016)
Multicomponent Magnetic Nanostructures
Heterostructure Magnetic Nanostructures: -
The MNPs with heterostructures are made up of a variety of parts, including a magnetic component and other parts, and as a whole, they display unique features. These many parts together to form a multicomponent magnetic structure offer cutting-edge features. Through a seed-mediated process, mono-components were employed as seeds to make MNPs. Studies are being done on core@shell heterostructures, which are made up of several parts encased in shells that have synergistic effects. For instance, Fe3O4@Au@Ag NPs with adjustable characteristics were created.FePt-based heterostructures, such as FePt-Au, have received much research due to their outstanding magnetism. Au is renowned for its numerous uses in the areas of biology and catalysis. Au NPs development over FePt NRs was seen in the produced FePt-Au heterostructure nanowires (HNWs). By managing Au complexes, FePt-Au structures that can be tanned may be created. To create FePt-Au heterostructure NPs, a similar procedure was used.
Exchange Couple Magnetic Nanostructures: -
To satisfy the demands of a higher energy barrier product, the impact of an exchange-coupled between a hard and soft origin magnetic phase is crucial. It will act as a single phase when soft origin magnetic phase particles are smaller in size than hard origin magnetic phase particles. According to theoretical calculations, exchange coupling may be efficient if the size is less than twice the thickness of the hard-magnetic phase. However, during synthesis, this is possible under extremely controlled circumstances. The method of chemical synthesis offers a thorough grasp of how the magnetic moment forms at the nanoscale.(Majidi et al. 2016)Coating hard magnetic cores with soft magnetic shells, which can boost their magnetization, is an efficient way to produce an exchange coupling effect.(Malhotra et al. 2020; Mody et al. 2010)
CHARACTERIZATION OF MNPS
To evaluate their physicochemical characteristics, the MNPs are examined using a variety of equipment. The display of various physicochemical features of NPs depends significantly on their size. Their characteristics can be altered by even a little change in their nanoscale dimension. Atomic Force Microscopy (AFM), Energy Dispersive X-ray Diffraction (EDXD), Scanning Electron Microscopy (SEM), Fourier Transform Infrared (FT-IR) Spectroscopy, UV Spectrophotometer, Transmission Electron Microscopy (TEM), and Mossbauer Spectroscopy are a few of the tools used for their characterization (MS).(18–20,23–25)
1. Size and Surface Morphology
The form and size of MNPs can alter their physicochemical characteristics. Surface area, size, and particle dispersion are measured using the Brunauer Emmet Teller (BET) and Dynamic Light Scattering (DLS) procedures. AFM, TEM/HRTEM, SEM/FESEM, and other methods can be used to analyse the surface morphology of MNPs. The photos we obtain using these tools offer us a general notion of their size and form, from which we may compute their diameter. Surface roughness, step height, and location of scattered particles are all measured using the AFM method. Information on the composition, shape, and size of NPs may be found via TEM. SEM provides information on sample composition and surface topography.
2. Elemental Mapping/Composition
Elemental composition and surface morphology can be determined using a variety of equipment, including EDS/EDXD, XRF (X-ray fluorescence), TEM, SEM, and XPS (X-ray photoelectron spectroscopy). The elemental composition of NPs is also studied using the instruments Atomic Absorption Spectrophotometry (AAS) and Inductively Coupled Plasma Mass Spectroscopy (ICP-MS). However, when the NPs are in solid form and must be dissolved in acids or bases before usage, AAS cannot detect the elemental composition. Through XRF, it is possible to ascertain a sample's elemental makeup. In comparison to other techniques, the sample preparation for XRF analysis is simple, quick, and safe. It is capable of detecting up to 100 ppb of an element (parts per billion). It is a non-destructive technique for using X-rays to analyse solid samples.
3. Bonding
Type and Organization The structure and bonding properties of MNPs are studied using a variety of methods. FT-IR, XAS (X-ray absorption spectroscopy), TGA (thermogravimetric analysis), XPS, and RS are the methods used (Raman spectroscopy). The XPS is appropriate for the surface configuration of NPs because it shows the mechanisms of reactions that occur on the surface of MNPs and gives information on the structure and speciation of elements. The oxidation state, particle binding energy, and the bonding between organic and inorganic materials may all be determined using FT-IR and XPS. FT-IR
4. Magnetism
The creation of MNPs via various synthetic techniques affects their magnetic properties. The MNPs, whose sizes span from nano to micro scales, exhibit the superparamagnetic feature. These NPs exhibit magnetic sensitivity and can interact with external magnetic fields when they are exposed to them. However, there is no magnetism seen when there is no external magnetic field. This characteristic of MNPs can enable them to be crucial components of regulated treatment and focused drug delivery.(kianfar 2021; Mørup, Hansen, and Frandsen 2019; Shen, Li, and Qiao 2018; Zhang et al. 2017)A variety of approaches with varying levels of sensitivity and information quality are used to quantify the magnetic of NPs. The net magnetization is measured using methods such as superconducting quantum interference device (SQUID) magnetometry and vibrating sample magnetometry (VSM). When examining materials in various states, such as thin films, crystals, powders, liquids, and gases, the SQUID is helpful. Both the VSM and the SQUID are extremely sensitive devices, with the VSM's sensitivity being 10-6 emu and the SQUID's being up to 10-10 emu.(Avval et al. 2020)
APPLICATIONS OF MAGNETIC NANO PARTICLES
The MNPs have attracted a lot of interest in the last 10 years due to their promising outcomes in several sectors. The use of MNPs is made more promising by their super-magnetic characteristics, distinctive size, shape, high surface area and volume ratio, and biocompatibility. These characteristics have drew many scholars from other domains to it. We have outlined the uses of MNPs in this review in a number of well-known industries, including biomedicine, biosensing, environment, agriculture, and catalysis. Following is a quick explanation of MNPs possible uses in certain sectors.(Avval et al. 2020).
A. Biomedicine
Due to their many physical and chemical characteristics, simplicity in manufacture, stability, and biocompatibility, MNPs have lately become widely employed in several biological applications. MNPs may communicate with outside magnetic fields. MNPs may also change the magnetic fields nearby, which improves magnetic resonance imaging (MRI). Different types of force and torque are produced and generated by the externally applied magnetic field at dipoles, leading to energy loss through translation, rotation, and dissipation. Such phenomena have a wide range of uses, including cell separation/biomarker, magnetic drug delivery, magneto-mechanical activation of cell surface receptors, biomedical imaging, bacterial Theranostics, triggering drug release, and hyperthermia. As a result of their use in several applications, MNPs are made from a variety of materials with differing physical and magnetic properties. However, one must take into account their possible biocompatibility and toxicity when doing biomedical research.(Katz 2019; Majidi et al. 2016; Omid Veiseh, Jonathan Gunn 2011)
B. Cancer Theranostics
Nanobiotechnology and molecular biology have recently emerged as cutting-edge methods for cancer Theranostics. These systems primarily entail the creation of tailored nanoparticles (NPs) with multifunctional features that solve the shortcomings of traditional cancer therapeutics and diagnostics (Figure 5). Nanoscale imaging probes for early cancer detection and cancer development can be created using NPs (Goel et al., 2017). The improved infiltration and retention (EPR: Electron Paramagnetic Resonance) impact of NPs is also being used to efficiently transport anticancer drugs, genes, or proteins to the targeted tumour locations.
Despite being encouraging, novel nanomaterials must still be developed quickly in order to meet the demands of developing cancer theranostics applications. This makes MNPs the perfect choice for applications such as cancer hyperthermia treatment, MRI, biosensing, and targeted drug administration because of their distinctive physicochemical qualities and super magnetic characteristics.(Chomoucka et al. 2010; Kalubowilage, Janik, and Bossmann 2019; Kudr et al. 2017).
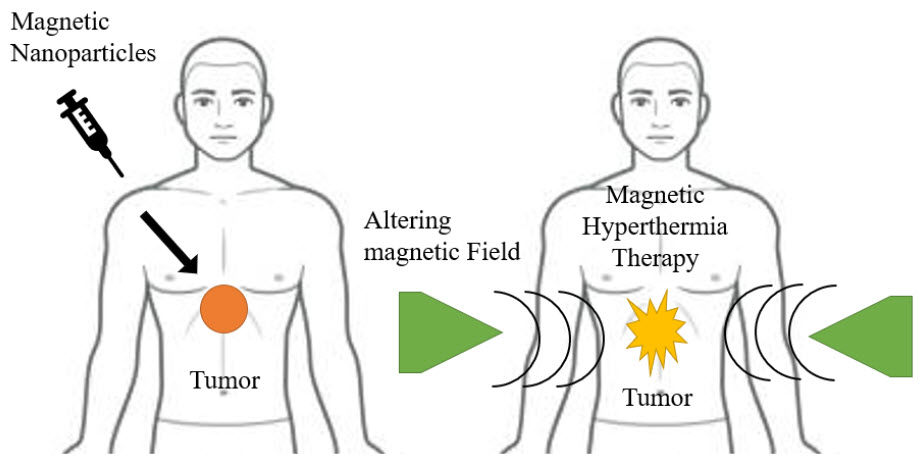
Figure 5 Cancer therapy
A hyperthermia therapy raises the localised tissue temperature by the induction of heat, causing permanent thermal ablation of pathogenic targets. A novel method of treating cancer called magnetic hyperthermia uses an external alternating magnetic field (AMF) to heat up MNP suspensions within the body. Because of lower pH and less thermotolerance in the malignant microenvironment, cancerous cells are more susceptible to hyperthermia than normal cells.
This method is seen to be a promising one for the treatment of cancer since it produces localised hysteric heat that is focused and causes less heating of surrounding tissues. The ability of MNPs-based hyperthermia treatment to penetrate deep tissue and target cancer cells with precision while sparing healthy tissue damage is by far its most significant benefit. The MNPs-based hyperthermia facilitates intracellular hyperthermia by directly heating cancer cells for therapeutic purposes. By attaching cell-targeted ligands to MNPs for precise targeting, the intracellular hyperthermia modality can be improved yet more. The effectiveness of treating cancer is significantly increased by this targeted and selective heat.(Chomoucka et al. 2010; Kalubowilage, Janik, and Bossmann 2019; Kudr et al. 2017; Naik, Khale, and Kanekar 2014; Salamat-Miller, Chittchang, and Johnston 2005)
These benefits have led to the recent transition of MNPs-based hyperthermia therapy for tumour elimination from laboratory studies to clinical trials. The MNPs can be utilised to increase chemotherapy by magnetic field-mediated hyperthermia as well as for drug administration and controlled release up exposure to the external field. Superparamagnetic La0.7Sr0.3MnO3 nanoparticles (SPMNPs) were functionalized with oleic acid-polyethylene glycol (PEG) to create a polymeric micelle structure that has a high loading capacity for the anticancer medication doxorubicin (DOX), which is meant to transport to cancer cells.(Avval et al. 2020; De Jong and Borm 2008; Mody et al. 2010; Yang et al. 2012)
According to the authors, SPMNPs not only improved medication delivery but also boosted cancer cells through a synergistic magnetic hyperthermia mechanism. SPMNPs that were drug-loaded enhanced the chemotherapeutic effect after being triggered by exogenous AMF. Furthermore, by only altering the AMF frequency, heat production and drug release may be seen for on-demand synergistic hyperthermia/chemotherapy.
C. Gene Therapy
In order for a gene of interest to express its encoded protein in the proper host or host cell, gene delivery techniques effectively convey the gene.In order for a gene of interest to express its encoded protein in the proper host or host cell, gene delivery techniques effectively convey the gene.(Majidi et al. 2016; Omid Veiseh, Jonathan Gunn 2011; Shen, Li, and Qiao 2018). With magnetofection, the gene is firmly affixed to the carrier. These carriers typically consist of a magnetic iron-oxide that is either enclosed in a polymer or metallic shell or disseminated inside a polymer matrix such as silica, polyvinyl alcohol (PVA), or dextran. An appropriate carrier system, such as virosomes, cationic liposomes, and nanoparticles, is needed to deliver genes to cells in order to improve cell internalisation and protect the DNA molecule from nuclease enzymatic degradation in order to obtain a large-sized nucleic acid molecule, the cytoplasm, or even the nucleus.Nanoparticles might be thought of as strong prospects for therapeutic uses because of a number of factors, including the following: They have the same size range as proteins, vast surface areas, the capacity to connect to several surface functional groups, adjustable absorption and release capabilities, as well as surface properties and particle size. Four primary types are used in gene delivery: inorganic nanoparticles, polymer-based nanoparticles, lipid-based nanoparticles, and hybrid nanoparticles. MNPs are inorganic nanoparticles that are frequently used as carriers for the transfer of genes. They are not vulnerable to microbial assault, and they exhibit high storage stability, according to earlier study data. Targeted distribution based on magnetism was originally described in 1978.(Avval et al. 2020; Chomoucka et al. 2010; J. Dobson 2006; Kalubowilage, Janik, and Bossmann 2019; Kudr et al. 2017; Schneider-Futschik and Reyes-Ortega 2021; Yang et al. 2012)
However, there is significant promise for using gene therapy with methods like to those used for medication delivery. The method must be modified for these applications to take nucleic acids' size and charge into consideration. There are three main gene delivery methods used today: nucleic acid electroporation, nucleic acid transfection, and viral vectors. Different systems perform differently. It has been shown that viral vectors can convey genes extremely well, but they may also introduce viral vector nucleic acid sequences into the host genome, which might have unfavourable effects, such as the inappropriate expression of harmful genes. Miyashita and colleagues advocated for the use of MNPs to improve the performance of the cell-fusion vector for the hemagglutinating virus Japan envelope (HAJ-E).(Ayubi et al. 2019; Mody et al. 2010; Vatta, Sanderson, and Koch 2006)
They discovered that even with less HAJ-E and no evidence of toxicity, transfection in vitro in BHK21 cells was enhanced by combining protamine sulphate (PS)-coated MNPs with HAJ-E. However, the exterior surface of the particles must first be modified to facilitate attachment of the target molecules in order for MNPs to function as effective carriers for DNA or pharmaceutical medications (Figure 6). Utilizing cleavable linkers or electrostatic interactions between the therapeutic agent and the particle surface are two techniques for attaching molecules to the surface of the particles. Natural polymers like proteins and carbohydrates, synthetic organic polymers like polyethylene glycol, PVA, and poly-L-lactic acid, and gold can all be used to coat MNPs. In in vitro magnetofection, the particles are often coated with polyethyleneimine (PEI), which uses charge interactions to affix DNA to the particle's surface.(KAKKAR et al. 2020; Majidi et al. 2016; Mou et al. 2015; Omid Veiseh, Jonathan Gunn 2011; Shen, Li, and Qiao 2018)
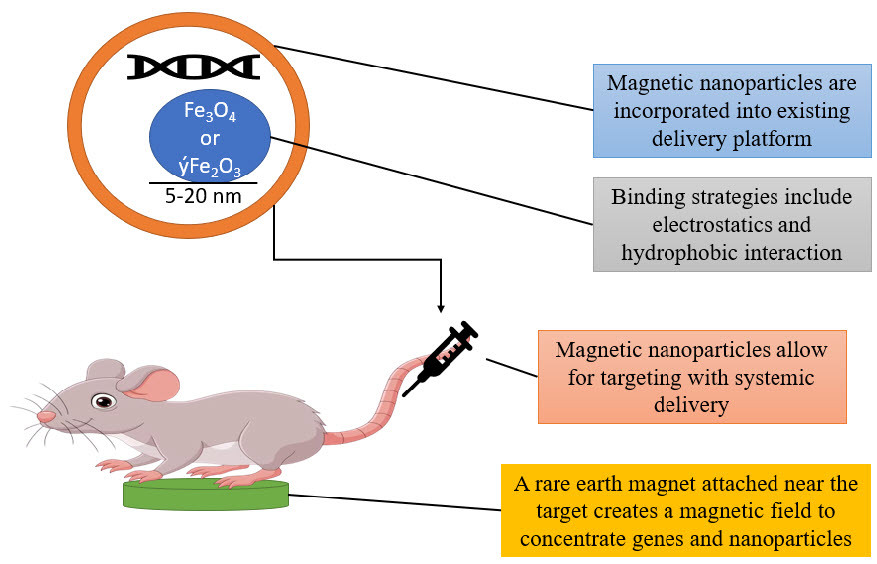
Figure 6 gene therapy in mice
Cathryn Mahi, Barry Byrne, and colleagues coated the adeno-associated virus (AAV) expressing Green Fluorescent Protein (GDP) to the surface of MNPs using a cleavable heparin sulphate linker in the first research to demonstrate targeted delivery of DNA using MNPs.(21–30,4)
REFERENCES
1. Ali, Arbab et al. 2021. “Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications.” Frontiers in Chemistry 9(July): 1–25.
2. Arosio, Paolo. 2021. “Applications and Properties of Magnetic Nanoparticles.” Nanomaterials 11(5): 11–13.
3. Avval, Zhila Mohajeri et al. 2020. “Introduction of Magnetic and Supermagnetic Nanoparticles in New Approach of Targeting Drug Delivery and Cancer Therapy Application.” Drug Metabolism Reviews 52(1): 157–84. https://doi.org/10.1080/03602532.2019.1697282.
4. Ayubi, Morteza et al. 2019. “Magnetic Nanoparticles Decorated with PEGylated Curcumin as Dual Targeted Drug Delivery: Synthesis, Toxicity and Biocompatibility Study.” Materials Science and Engineering C 104(May): 109810. https://doi.org/10.1016/j.msec.2019.109810.
5. Chomoucka, Jana et al. 2010. “Magnetic Nanoparticles and Targeted Drug Delivering.” Pharmacological Research 62(2): 144–49. http://dx.doi.org/10.1016/j.phrs.2010.01.014.
6. Cicha, Iwona et al. 2017. “Magnetic Nanoparticles for Medical Applications.” MACROMOL MDPI 12(8): 825–29.
7. Dhavale, R. P. et al. 2018. “Monolayer Grafting of Aminosilane on Magnetic Nanoparticles: An Efficient Approach for Targeted Drug Delivery System.” Journal of Colloid and Interface Science 529: 415–25. https://doi.org/10.1016/j.jcis.2018.06.006.
8. Dobson, J. 2006. “Gene Therapy Progress and Prospects: Magnetic Nanoparticle-Based Gene Delivery.” Gene Therapy 13(4): 283–87.
9. Dobson, Jon. 2006. “Magnetic Nanoparticles for Drug Delivery.” wiley interscience 494(March): 477–94.
10. Dubuisson, Jean, Aurore Fehlmann, and Patrick Petignat. 2015. “Management of Presumed Benign Giant Ovarian Cysts: A Minimally Invasive Technique Using the Alexis Laparoscopic System.” Journal of Minimally Invasive Gynecology 22(4): 540.
11. Etheridge, Michael L. 2013. “Understanding the Benefits and Limitations of Magnetic Nanoparticle Heating for Improved Applications in Cancer Hyperthermia and Biomaterial Cryopreservation.” PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY 16(22): 119–28.
12. Gul, Saima et al. 2019. “A Comprehensive Review of Magnetic Nanomaterials Modern Day Theranostics.” Frontiers in Materials 6(July): 1–15.
13. De Jong, Wim H., and Paul J.A. Borm. 2008. “Drug Delivery and Nanoparticles: Applications and Hazards.” International Journal of Nanomedicine 3(2): 133–49.
14. KAKKAR, VASUNDHRA, SHAHID UD DIN WANI, SURYA PRAKASH GAUTAM, and ZULFKAR LATIEF QADRIE. 2020. “Role of Microspheres in Novel Drug Delivery Systems: Preparation Methods and Applications.” International Journal of Current Pharmaceutical Research 12(3): 10–15.
15. Kalubowilage, Madumali, Katharine Janik, and Stefan H. Bossmann. 2019. “Magnetic Nanomaterials for Magnetically-Aided Drug Delivery and Hyperthermia.” Applied Sciences (Switzerland) 9(14).
16. Katz, Evgeny. 2019. “Synthesis, Properties and Applications of Magnetic Nanoparticles and Nanowires—a Brief Introduction.” Magnetochemistry 5(4).
17. kianfar, Ehsan. 2021. “Magnetic Nanoparticles in Targeted Drug Delivery: A Review.” Journal of Superconductivity and Novel Magnetism 34(7): 1709–35. https://doi.org/10.1007/s10948-021-05932-9.
18. Kudr, Jiri et al. 2017. “Magnetic Nanoparticles: From Design and Synthesis to Real World Applications.” Nanomaterials 7(9).
19. Lu, An Hui, E. L. Salabas, and Ferdi Schüth. 2007. “Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application.” Angewandte Chemie - International Edition 46(8): 1222–44.
20. Majidi, Sima et al. 2016. “Magnetic Nanoparticles: Applications in Gene Delivery and Gene Therapy.” Artificial Cells, Nanomedicine and Biotechnology 44(4): 1186–93.
21. Malhotra, Nemi et al. 2020. “Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review.” Molecules MDPI 25(14): 1–26.
22. Mody, VickyV, Rodney Siwale, Ajay Singh, and HardikR Mody. 2010. “Introduction to Metallic Nanoparticles.” Journal of Pharmacy and Bioallied Sciences 2(4): 282.
23. Mørup, Steen, Mikkel F. Hansen, and Cathrine Frandsen. 2019. “Magnetic Nanoparticles.” Comprehensive Nanoscience and Nanotechnology 1–5: 89–140.
24. Mou, Xianbo, Zeeshan Ali, Song Li, and Nongyue He. 2015. “Applications of Magnetic Nanoparticles in Targeted Drug Delivery System.” Journal of Nanoscience and Nanotechnology 15(1): 54–62.
25. Naik, Tarjani S, Anubha Khale, and Hema Kanekar. 2014. “Evauation of Mouth Dissolving Films: Physical and Chemical Methods.” nternational Journal of Pharmaceutical and Phytopharmacological Research 4(1): 62–65.
26. Nikiforov, Vladimir N., and Elena Yu Filinova. 2009. “Biomedical Applications of Magnetic Nanoparticles.” Magnetic Nanoparticles (November 2018): 393–455.
27. Omid Veiseh, Jonathan Gunn, and Miqin Zhang. 2011. “Design and Fabrication of Magnetic Nanoparticles for Targeted Drug Delivery and Imaging Omid.” NIH Public Access 62(3): 284–304.
28. Salamat-Miller, Nazila, Montakarn Chittchang, and Thomas P. Johnston. 2005. “The Use of Mucoadhesive Polymers in Buccal Drug Delivery.” Advanced Drug Delivery Reviews 57(11): 1666–91.
29. Schneider-Futschik, Elena K., and Felisa Reyes-Ortega. 2021. “Advantages and Disadvantages of Using Magnetic Nanoparticles for the Treatment of Complicated Ocular Disorders.” Pharmaceutics 13(8): 1–16.
30. Shen, Lazhen, Bei Li, and Yongsheng Qiao. 2018. “Fe3O4 Nanoparticles in Targeted Drug/Gene Delivery Systems.” Materials MDPI 11(2): 1–29.
31. Ulbrich, Karel et al. 2016. “Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies.” Chemical Reviews 116(9): 5338–5431.
32. Vatta, Laura L., Ron D. Sanderson, and Klaus R. Koch. 2006. “Magnetic Nanoparticles: Properties and Potential Applications.” Pure and Applied Chemistry 78(9): 1793–1801.
33. Williams, Harry M. 2017. “The Application of Magnetic Nanoparticles in the Treatment and Monitoring of Cancer and Infectious Diseases.” Bioscience Horizons: The International Journal of Student Research 10: 1–10.
34. Yang, Hung Wei et al. 2012. “Potential of Magnetic Nanoparticles for Targeted Drug Delivery.” Nanotechnology, Science and Applications 5(1): 73–86.
35. Zhang, Ting et al. 2017. “Preparation of Magnetic Nanoparticles via a Chemically Induced Transition: Role of Treating Solution’s Temperature.” Nanomaterials 7(8).









